Abstract
Little information is available on the intra-individual variability of oxidative stress biomarkers in healthy individuals and even less in the context of the menstrual cycle. The objective of this study was to characterize the analytical and biological variability of a panel of 21 markers of oxidative damage, antioxidant defence and micronutrients in nine healthy, regularly menstruating women aged 18–44 years. Analyses included measurement of lipid peroxidation, antioxidant enzymes and antioxidant vitamins. Blood specimens were collected, processed and stored using standardized procedures on days 2, 7, 12, 13, 14, 18, 22 and 28 in one cycle for each subject. Replicate analyses of markers were performed and two-way nested random effects ANOVA was used to describe analytical, intra-individual and inter-individual variability. No statistically significant differences at α = 0.05, or temporal effects across the menstrual cycle were observed. Analytical variability was the smallest component of variance for all variables. The ICC among replicates ranged from 0.80 to 0.98. Imprecision based on quality control materials ranged from 1 to 11%. The critical differences between serial results varied greatly between assays ranging from 6 to 216% of the mean level. These results provide important initial information on the variability of biomarkers of oxidative stress, antioxidant defence and micronutrients across the menstrual cycle.
Keywords: Oxidative stress, antioxidants, biological variation, menstrual cycle
Introduction
Oxidative stress (OS) is an imbalance between free radicals and reactive oxygen species (ROS) and protective radical scavenging antioxidants (AOX) resulting from either an overproduction of ROS or a deficit in AOX protection (Terada 2006). Biomarkers of OS are increasingly being evaluated in experimental, clinical and epidemiological studies and have been implicated in the pathogenesis of numerous diseases including atherosclerosis (Olinski et al. 2002), cancer (Olinski et al. 2002), diabetes (Dandona 2002), respiratory disease (Paredi et al. 2002) and others (Multhaup & Masters 1999, Mathur et al. 2002, Soory 2002, Spector 2000, Agar & Durham 2003, Basu 2003, Dawson & Dawson 2003, Kruidenier et al. 2003), including conditions associated with premature birth (Matsubasa et al. 2002).
Many methods for the measurement of OS have been proposed and several reviews have been written (Morrow et al. 1992, Spiteller & Spiteller 1997, Davies et al. 1999, De Zwart et al. 1999, Hensley et al. 2000, Collins et al. 2004, Pratico et al. 2004). However, there is no current consensus on which methods are the most useful, reliable, accurate or specific for different types of oxidative insults (Pryor 1999). Despite successful application of these markers in some studies, laboratory variability has been a concern during the evaluation of published studies. Little information is available on the variability of OS markers in healthy individuals, especially as they vary across the menstrual cycle. Further in vivo studies assessing the variability and reliability of OS measures are needed (Pryor & Godber 1991).
There is increased interest in investigating the role of OS in female reproduction, including the aetiology of infertility and endometriosis (Agarwal et al. 2005). While the mechanisms that relate OS with female fertility are not completely understood, recent animal and human studies have suggested that OS may have an important role in spontaneous abortion (Samborskaia & Ferdman 1966, Jenkins et al. 2000), infertility in men and women (Rayman 2000, Xu et al. 2001), birth weight (Kim et al. 2005), follicular growth and development (Murray et al. 2001), endometriosis (Murphy et al. 1998) and regulation of endometrial angiogenesis (Maas et al. 2001). Investigation of the relationships between OS and female fertility and reproductive outcomes may be masked by normal menstrual cycle variation.
To evaluate the relation between OS and female fertility we selected a panel of biomarkers for longitudinal study across the menstrual cycle. As markers of oxidative damage we measured malondialdehyde (MDA) as thiobarbituric acid reactive substances (TBARS) and a high-performance liquid chromatography (HPLC) lipid peroxidation profile of linoleic acid and its hydroxy and hydroperoxy oxidation products including 13-hydroxy octadecadieneoic acid (13-HODE), 13-hydroperoxy octadecadieneoic acid (13-HpODE), 9-hydroxy octadecadieneoic acid (9-HODE) and 9-hydroperoxy octadecadieneoic acid (9-HpODE). As measures of antioxidant defence we measured the erythrocyte activity of superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione reductase (GR), the plasma activity of glutathione peroxidase (pGPx) and the serum activity of paraoxonase 1 (PON1). As measures of micronutrient antioxidants we measured an HPLC fat-soluble vitamin profile (retinol, α, γ, and δ tocopherol and carotenoids) and total vitamin C.
The aim of this pilot study was to characterize the components of biological and measurement variability for the 21 OS markers measured by the laboratory. This included evaluation of laboratory measurement error (i.e. analytical acceptability index), the ability to distinguish significant changes in biomarker levels within (i.e. critical difference) and between (i.e. intraclass correlation coefficient) subjects, the feasibility of employing reference ranges of ‘normal’ to characterize individuals in terms of populations (i.e. the index of individuality), as well as estimation of the number of specimens required for characterization of the homeostatic set point. These data will be used to set analytical quality specifications, as well as to select those biomarkers most appropriate, for consideration in a larger observational study investigating the effects of menstrual cycle hormones on OS.
Materials and methods
Participants and specimens
Specimens were collected from nine regularly menstruating, premenopausal, female volunteers. Major exclusion criteria included: current use of oral contraceptives, vitamins, supplements, and prescription or over-the-counter medications; pregnancy in the past 6 months or actively trying to conceive in the next 3 months; history of certain gynaecological disorders (including abnormal Pap smear) and/or surgery; positive screen (IgM) for Chlamydia infection at screening; measured body mass index (BMI; kg m−2) <18.5 or >35.0; consuming a restricted or supplemented diet; history of physician-diagnosed chronic illness; or recent infection or X-ray exposure.
Fasting blood and urine specimens were collected between 7:00 and 8:30 a.m. on (approximately) days 2, 7, 12, 13, 14, 18, 22 and 27 of a 28-day cycle, to correspond to menstruation, early and late follicular phase, ovulation and early and late luteal phase. Fertility monitors (Clear Blue™) were used beginning on day 6 of the menstrual cycle to assist in timing of specimen collection. Collection and handling protocols were designed to minimize variability in pre-analytical factors (Murphy et al. 2000). Briefly, each participant observed 10 min of seated resting in order to allow fluid shifts to equilibrate. Phlebotomy tourniquets were applied for less than 1 min and removed immediately upon establishing blood flow. Tubes were protected from light, placed on ice and delivered to the processing laboratory within 15 min. All specimens were centrifuged at 4−8°C, portioned into multiple aliquots containing sufficient volume to provide for duplicate analysis for each assay and frozen within 90 min of phlebotomy.
Laboratory methods
Unless otherwise noted all chemicals were from Sigma Chemical Co. (St Louis, MO, USA). All laboratory measurements (144 determinations) were made in a single analytical batch using one set of reagents. For the HPLC linoleic acid peroxidation profile simultaneous measurement of all 144 determinations was impossible due to time requirements; therefore all specimens from one participant were measured together in nine consecutive sets.
HPLC lipid peroxidation profiles
Total lipid-derived linoleic acid, 13-HODE, 9-HODE, 13HpODE and 9-HpODE were determined simultaneously by HPLC with diode array detection (Browne & Armstrong 2000). The HPLC instrumentation consisted of a Shimadzu LC-10Avp pump, FCV 10 flow control valve, DGU14 degassing unit, SPD-M10A photodiode array, SIL-7A autosampler and a PC equipped with EZ Start chromatography data software (Shimadzu Scientific Instruments, Inc., Columbia, MD, USA). All solvents were HPLC grade from Fischer Scientific (Fair Lawn, NJ, USA). Linoleic acid, 13-HpODE, 9-HpODE, 13-HODE and 9-HODE, and 5-hydroxy-eicosahexaenoic acid methyl ester (internal standard) were purchased from Cayman Chemical Co. (AnnArbor, MI, USA). For analysis, 150 μL of the prepared sample was injected onto a SupelcoSil LC-18 (25 cm × 4.6 mm I.D., 5 μm particle size, ODS column (Supelco, Inc., Bellafonte, PA, USA) and eluted isocratically with a mobile phase of acetonitril/0.1% acetic acid/tetrahydrofuran (41:41:18 v/v/v) at a flow rate of 1.3 ml min−1. The column eluent was monitored by the photodiode array from 200–300 nm.
Thiobarbituric acid reacting substances
TBARS were measured using OxiTech reagent kits from ZeptoMetrix Corp. (Buffalo, NY, USA) and expressed in nmol ml−1 MDA equivalents (Armstrong & Browne 1994). TBARS pigment was measured at excitation of 535 nm and emission of 552 nm on a RF-5000U spectrofluorometer (Shimadzu Scientific Instruments Inc.).
Antioxidant enzymes
Kinetic enzyme assays were adapted to the Cobas Fara II autoanalyzer (Roche Diagnostic Systems, Inc., Basel, Switzerland) (Pippenger et al. 1998). Plasma glutathione peroxidase (PGPx), erythrocyte glutathione peroxidase (EGPx) and erythrocyte glutathione reductase (EGSHR) were performed using OxiTek reagent kits from ZeptoMetrix (Buffalo, NY, USA). Erythrocyte superoxide dismutase (ESOD) activity was determined by the inhibition of the oxidation of cytochrome C by xanthine/xanthine oxidase under standard conditions. One unit of SOD activity was defined as the amount of enzyme which induced a 50% inhibition of the reaction. Calibration curves of% inhibition vs SOD activity were generated using purified SOD. Erythrocyte enzyme activities were normalized per gram or milligram of haemoglobin (Hb). The activity of human serum paraoxonase was measured as both arylesterase activity (PONa) and paraoxonase activity (PONp) (Browne et al. 2007). Diethyl p-nitrophenyl phosphate (paraoxon), 98.0%, was obtained from Chem Service Inc. (Westchester, PA, USA). PONa activity was measured as the rate of formation of phenol using 4 mmol l−1 phenylacetete in 20 mM TRIS–HCl, pH 8.0, with 1.0 mM CaCl2. PONp activity was determined by the rate of formation of p-nitrophenol using 1 mmol l−1 paraoxon as the substrate in 50 mmol l−1 glycine buffer, pH 10.5, with 1.0 mmol l−1 CaCl2. Water blanks were used to correct for non-enzymatic hydrolysis.
Fat-soluble antioxidant vitamin profiles
Retinol, vitamin E (α, γ and δ tocopherol) and carotenoids (β-carotene, β-cryptoxanthin, lycopene and lutein/zeaxanthin) were measured in serum using HPLC (Browne & Armstrong 1998). Vitamin-A-alcohol (>95% cis-retinol) was purchased from Fluka Chemical Co. (Milwaukee, WI, USA). Lutein, zeaxanthin and β-cryptoxanthin were purchased from Indofine Pure Biochemicals (Hillsborough, NJ, USA). Results are expressed in μg ml−1. Lutein and zeaxanthin were quantified together as a single peak and reported as a total lutein/zeaxanthin.
Total vitamin C
Total ascorbic acid was determined by the dinitrophenylhydrazine (DNPH) method (Chalmers & McWhinney 1986). Samples for analysis were stabilized immediately following phlebotomy and centrifugation by adding 0.5 ml of heparin plasma to 2.0 ml of 6% MPA and centrifuging at 3000g for 10 min. Clear supernatant was decanted and frozen at −80°C for analysis. The absorbance of each DNPH derivatized sample was determined at 520 nm on a Shimadzu 160U spectrophotometer (Shimadzu Scientific Instruments, Inc.).
Quality control
As quality control (QC) material is not commercially available for many of the assays, ‘in-house’ controls were generated. These specimens were used to estimate method performance characteristics and assess imprecision. Intra-assay reproducibility was calculated from 20 determinations in the same run. Long-term interassay reproducibility and control ranges were generated by running five samples per day over a period of 20 days. The mean±two standard deviations was then calculated as the acceptable range for control materials which were analyzed in duplicate with all patient determinations. Performance was monitored using Levy–Jennings quality control plots. Daily quality and analytical run acceptability were evaluated according to Westgard multirule charts (Westgard et al. 1974). Day to day reproducibility was evaluated by direct limit Cusum Plots (Westgard et al. 1977). Fat-soluble vitamin control specimens consisted of 500 μl aliquots of a serum pool stored at −80°C in amber, 1.0 ml cryovials. Standard reference material (SRM) 968C1 and 968C2 from the National Institute of Standards and Technology (NIST) were included in all analytical runs, providing three-level quality control. Further laboratory quality assurance was maintained through participation in the Micronutrient Measurement Quality Assurance Program round-robin proficiency testing from NIST.
For HPLC lipid peroxidation profiles, TBARS and PGPx control specimens consisted of aliquots of an EDTA plasma pool stored at −80°C in amber, 1.0 ml cryovials in the presence of 10 μM indomethacin and 0.005% butylated hydroxyl toluene as antioxidant preservatives. Total ascorbic acid control specimens were prepared by adding dithiothreitol to heparinized plasma. A final concentration of 1 g l–1 has been shown to prevent the oxidative loss of vitamin C and samples are stable for at least 1 year at −80°C (Margolis & Duewer 1996). Antioxidant enzyme control material was provided by ZeptoMetrix Corp. as lyophilized, human blood plasma and human erythrocyte lysates.
Statistical analysis
Statistical analyses were conducted using SAS version 9.01 (SAS Institute Inc., Cary, NC, USA). An unabridged statistical description is included as a supplemental file. Distributions were considered for each of the 21 analytes, stratified by day and log-transformed where this improved the approximation to a normal. Two-way nested random effects ANOVA was used to describe the single menstrual cycle variability for each analyte. An exchangeable correlation matrix, which assumes constant correlation coefficient between any two error terms within the same subject, was specified for error terms to accommodate the clustered nature of the data. Models were specified as:
where Yijk described the analyte value for the kth replicate (k = 1, 2), on the jth day (j = 1, 2, 3, . . ., 8), for the ith subject (I = 1, 2, 3, . . ., 9); μ the grand mean for the analyte; (sub)i the random effect of the ith subject; (day)j(i) the random effect of the jth day nested in the ith subject; and ek(ji) the kth replicate nested in the jth day and the ith subject (Littell et al. 1998). Random effects and errors were assumed to be normally distributed with mean zero and a constant variance σ2.
The proportion of the total variance attributable to each effect was estimated by dividing the components of variance by the total variance where corresponds to to (day)j(i) and to ek(ij). The total variance for an analyte was defined as . The index of individuality (II), was calculated as (Fraser & Harris 1989). Coefficients of variation (CVs) for each analyte were calculated from the components of variance specified as ; where represents a variance component and xl an analyte mean. The analytical acceptability index (AI), was calculated as CVA/CVW where <0.25 is optimal performance, <0.5 is desirable performance and <0.75 is minimal performance (Cotlove et al. 1970).
Homogeneity of within-subject variance, across subjects, was evaluated using the index of heterogeneity (IH) defined as CVW+CVA/(√2/(n – 1)) × 100, with rejection of the homogeneity assumption where IH >1.35. The critical difference between serial results (CD), was estimated as 2.77 × √(CVW)2+(CVA)2 under the homogeneity of variances assumption (Fraser & Harris 1989). The minimum number of specimens, K, required to reduce the index of individuality to that observed for PONp (i.e. smallest observed in the current study) was defined as K>(IMean/IPONp)2. The intraclass correlation coefficient (ICC), defined as , describes the proportion of variability in observed values due to biological variation (Lachin 2004) across the range of measured values.
Results
The demographic characteristics of the study participants are listed in Table I. The distribution and number of measurements available for each marker are shown in Table II. The maximum number of specimens (9 participants × 8 days × 2 replicates = 144) were available for vitamin C (Vit C), pGPx and TBARS. For HPLC vitamins 142 replicates were available due to two HPLC autosampler instrumental errors (one on day 7 for one subject and day 27 for another). Two replicates were similarly missing for linoleic acid and lipid peroxide analyses (two participants on day 12). Among the HPLC analytes, 14 samples from two participants had visibly detectable analyte peaks which were below the limit of quantification (set by chromatographic peak width and threshold). In these cases, values were generated via manual forced peak integration (11 values for 9-HODE, two for 9-HpODE and one for 13-HpODE only). An identical procedure was employed for the δ-tocopherol measures with values below the limit of quantification. This strategy preserved sample size while avoiding the effect of censoring (Schisterman et al. 2006). One participant had both replicates unavailable for the day 7 serum AOX enzymes analyses and another had both replicates unavailable for the day 2 erythrocyte AOX enzymes analyses.
Table I.
Characteristics of the study participants (n = 9).
| Characteristic | |
|---|---|
| Age (years) | |
| Mean | 38.5±4.7 |
| Min–max | 30.9–44.8 |
| Cycle length (days) | |
| Mean | 28.4±1.3 |
| Min–max | 26–30 |
| Height (cm) | |
| Mean | 165.1±8.9 |
| Min–max | 149.2–179.5 |
| Weight (kg) | |
| Mean | 71.1±18.5 |
| Min–max | 53.7–111.9 |
| Body mass index (kg cm−2) | |
| Mean | 25.9±5.1 |
| Min–max | 20.9–34.7 |
| Race, n (%) | |
| White | 8 (88.9) |
| African-American | 1 (11.1) |
| Education | |
| High school graduate | 0 |
| College, no degree | 2 (22.2) |
| Associate’s degree | 3 (33.3) |
| Bachelor’s degree | 4 (44.4) |
Table II.
Number of repeated measurements available for each marker in nine study participants.
| Range
|
||||
|---|---|---|---|---|
| Total replicates | Mean value | Min | Max | |
| Vitamins and micronutrients | ||||
| Retinol (μg ml−1) | 142 | 0.39 | 0.230 | 0.530 |
| δ-Tocopherol (μg ml−1) | 142 | 0.03 | NQ | 0.172 |
| γ-Tocopherol (μg ml−1) | 142 | 2.17 | 0.76 | 3.2 |
| α-Tocopherol (μg ml−1) | 142 | 10.69 | 7.06 | 17.01 |
| Lutein (μg ml−1) | 142 | 0.14 | 0.07 | 0.20 |
| β-Cryptoxanthin (μg ml−1) | 142 | 0.11 | 0.044 | 0.261 |
| β-Carotene (μg ml−1) | 142 | 0.28 | 0.079 | 0.897 |
| Lycopene (μg ml−1) | 142 | 0.59 | 0.312 | 1.131 |
| Vitamin C (mg dl−1) | 144 | 1.97 | 1.047 | 3.344 |
| Lipids and lipid peroxides | ||||
| TBARS (μmol l−1) | 144 | 1.46 | 1.061 | 2.096 |
| 13-HODE (μmol l−1) | 142 | 0.15 | 0.034 | 0.561 |
| 9-HODE (μmol l−1) | 142 | 0.16 | NQ | 0.413 |
| 13-HpODE (μmol l−1) | 142 | 0.26 | NQ | 1.507 |
| 9-HpODE (μmol l−1) | 142 | 0.35 | NQ | 2.199 |
| Linoleic (μg ml−1) | 142 | 233.7 | 107.7 | 350.1 |
| Plasma AOX enzymes | ||||
| pGPx (U l−1) | 144 | 850.36 | 616.4 | 1130.5 |
| Serum AOX enzymes | ||||
| PONa (kU l−1) | 140 | 69.28 | 51.4 | 249.3 |
| PONp (U l−1) | 140 | 294.21 | 117 | 711 |
| Erythrocyte AOX enzymes | ||||
| eSOD (U g−1 Hgb) | 140 | 7.93 | 5409 | 14816 |
| eGSHR (U g−1 Hgb) | 140 | 7.53 | 4.92 | 12.40 |
| eGPx (U g−1 Hgb) | 140 | 57.8 | 35.8 | 99.0 |
PONa, PON1 arylesteraseactivity; PONp, PON1 paraoxonase activity; eGSHR, erythrocyte glutathione reductase activity; pGPx, plasma glutathione peroxidase activity; eGPx, erythrocyte glutathione peroxidase activity; eSOD, erythrocyte superoxide dismutase activity; TBARS, thiobarbituric acid reactive substances; 9-HODE, 9 hydroxy octadecadieneoic acid; 13-HODE, 13 hydroxy octadecadieneoic acid; 13-HpODE, 13 hydroperoxy octadecadieneoic acid; 9-HpODE, 9 hydroperoxy octadecadieneoic acid.
NQ, not quantified (below limit of quantification).
Analytical performance
The QC performance for all assays is shown in Table III and list the mean, within-run and between-run CV. Markers are divided into groups based on the QC material that was used. For fat-soluble vitamins, all within- and between-run CVs were below 10.0% with the exception of δ-tocopherol where levels of the ‘in-house’ and SRM 968C1 QC materials were below the limit of quantification of our system for this analyte (0.005 μg ml−1). Lipid and lipid peroxide imprecision ranged from 4.7 to 11.2%. Although measured simultaneously, lower imprecision was noted for linoleic acid owing to inherently higher concentration. Plasma TBARS demonstrated imprecision between 8.0 and 9.0%. Serum PONa and PONp determinations demonstrated CVs <2.0% across a broad range of enzyme activity as did PGPx with CVs <5.0%. Erythrocyte antioxidant enzymes eSOD, eGPx and eGSHR demonstrated considerably higher between-run relative to within-run imprecision. In-house QC materials demonstrated no statistically significant changes over the study period and are in our experience are stable for at least 18 months when stored at −80°C (data not shown).
Table III.
Study variable means, within-run and between-run imprecision based on quality control materials expressed as percentage coefficient of variation (%CV).
| Mean | Within%CV (between%CV) | Mean | Within%CV (between%CV) | Mean | Within%CV (between%CV) | |
|---|---|---|---|---|---|---|
| Vitamins and micronutrients | SRM 968 C1 | SRM 968 C2 | In-house QC | |||
| Retinol (μg ml−1) | 0.74 | 3.01 (6.09) | 0.44 | 2.96 (5.93) | 0.52 | 6.17 (6.03) |
| δ-Tocopherol (μg ml−1) | 0.05 | 21.23 (40.78) | 0.58 | 8.85 (14.27) | 0.03 | 71.85 (61.90) |
| γ-Tocopherol (μg ml−1) | 3.71 | 2.15 (5.49) | 1.65 | 2.29 (5.26) | 1.96 | 4.55 (7.54) |
| α-Tocopherol (μg ml−1) | 6.88 | 2.34 (4.49) | 14.96 | 2.51 (5.52) | 13.52 | 4.99 (5.83) |
| Lutein (μg ml−1) | 0.07 | 5.55 (5.93) | 0.08 | 5.00 (9.16) | 0.11 | 6.55 (7.75) |
| β-Cryptoxanthin (μg ml−1) | 0.06 | 2.89 (8.00) | 0.03 | 9.68 (9.69) | 0.07 | 5.95 (9.13) |
| β-Carotene (μg ml−1) | 0.14 | 6.52 (7.53) | 0.35 | 6.66 (8.74) | 0.17 | 5.27 (9.86) |
| Lycopene (μg ml−1) | 0.35 | 5.59 (8.25) | 0.48 | 5.99 (8.72) | 0.43 | 7.63 (9.02) |
| Heparin/DTT plasma | ||||||
| Vitamin C* (mg dl−1) | 1.12 | 6.88 (9.60) | ||||
| Lipids and lipid peroxides | EDTA plasma QC1 | EDTA plasma QC2 | ||||
| TBARS (μmol l−1) | 2.0 | 8.10 (8.29) | 1.10 | 8.20 (8.90) | ||
| 13-HODE (μmol l−1) | 0.105 | 9.20 (11.20) | ||||
| 9-HODE (μmol l−1) | 0.120 | 9.00 (9.50) | ||||
| 13-HpODE (μmol l−1) | 0.037 | 8.00 (10.50) | ||||
| 9-HpODE (μmol l−1) | 0.053 | 10.40 (10.30) | ||||
| Linoleic (μg ml−1) | 225 | 4.70 (4.40) | ||||
| Plasma antioxidant enzymes | EDTA plasma QC1 | EDTA plasma QC2 | ||||
| pGPx (U l−1) | 474 | 3.30 (4.58) | ||||
| Serum antioxidant enzymes | Serum QC1 | Serum QC2 | ||||
| PONa (kU l−1) | 106.7 | 1.20 (1.20) | 124.3 | 0.90 (1.10) | ||
| PONp (U l−1) | 115.1 | 1.40 (1.60) | 457.2 | 1.20 (1.30) | ||
| Erythrocyte antioxidant enzymes | Erythrocyte lysate QC | |||||
| eSOD (U mg−1 Hgb) | 4.74 | 4.64 (8.02) | ||||
| eGSHR (U g−1 Hgb) | 3.57 | 3.65 (10.64) | ||||
| eGPx (U g−1 Hgb) | 36.38 | 5.01 (7.92) |
For definitions see footnote for Table II.
Biological variability
The mean and standard deviation of variable levels for the study relative to the mean and standard deviation of levels for each participant are depicted in Appendix Figures 1-3. Prior to analysis, a log transformation was applied to α-tocopherol, β-cryptoxanthin, β-carotene, lycopene, Vit-C, TBARS, 13-HpODE, linolenlic and linoleic acids, SOD, GSHR to improve approximation to a normal distribution. Only six of nine study participants had levels of δ-tocopherol in the quantifiable range limiting variability estimates for this micronutrient. The components of variance, the ICC among replicate analyses and the calculated values for analytical, intra-individual and inter-individual%CVs are shown in Table IV. In general, analytical variability was small relative to both intra- and inter-individual variability, comprising less than 10% of total variability for all analytes except 13-HpODE which was 12.35%. The ICCs are greater than 0.9 for all analytes except δ-tocopherol which was 0.76.
Table IV.
Components of variance, ICCs among replicate analyses, and calculated values for inter-individual (B), intra-individual (W), and analytical (A) coefficients of variation (CV) for study analytes.
| Analyte | ICC | 95%lo | 95%Hi | CVB | CVW | CVA | |||
|---|---|---|---|---|---|---|---|---|---|
| Vitamins and micronutrients | |||||||||
| Retinol | 90.96 | 6.11 | 2.93 | 0.98 | 0.95 | 0.98 | 21.26 | 5.51 | 3.82 |
| δ-Tocopherol | 16.50 | 78.33 | 5.17 | 0.76 | 0.65 | 0.84 | 52.01 | 113.31 | 29.11 |
| γ-Tocopherol | 67.62 | 31.58 | 3.00 | 0.99 | 0.98 | 0.99 | 27.59 | 18.85 | 3.00 |
| α-Tocopherol* | 73.36 | 24.33 | 2.31 | 0.97 | 0.95 | 0.98 | 6.65 | 3.83 | 1.18 |
| Lutein | 80.71 | 16.60 | 2.70 | 0.97 | 0.95 | 0.98 | 22.35 | 10.14 | 4.09 |
| β-Cryptoxanthine* | 92.72 | 6.84 | 0.44 | 1.00 | 0.99 | 1.00 | 19.73 | 5.36 | 1.36 |
| β-Carotene* | 93.52 | 4.18 | 2.30 | 0.98 | 0.96 | 0.98 | 48.36 | 10.23 | 7.58 |
| Lycopene* | 67.93 | 27.93 | 4.14 | 0.94 | 0.91 | 0.96 | 47.90 | 30.72 | 11.82 |
| Vitamin C* | 51.31 | 44.09 | 4.59 | 0.92 | 0.87 | 0.95 | 25.75 | 23.87 | 7.71 |
| Lipids and lipid peroxides | |||||||||
| TBARS* | 49.83 | 38.80 | 11.37 | 0.81 | 0.72 | 0.88 | 30.19 | 26.64 | 14.42 |
| 13-HODE | 14.23 | 84.43 | 1.34 | 0.91 | 0.87 | 0.95 | 21.95 | 53.45 | 6.73 |
| 9-HODE† | 38.38 | 60.56 | 1.07 | 0.97 | 0.96 | 0.98 | 32.76 | 41.16 | 5.46 |
| 13-HPODE*† | 33.49 | 54.16 | 12.35 | 0.97 | 0.96 | 0.98 | 31.92 | 40.59 | 19.38 |
| 9- HPODE† | 34.14 | 64.39 | 1.47 | 0.96 | 0.93 | 0.97 | 56.14 | 77.10 | 11.67 |
| Linoleic acid* | 88.60 | 5.45 | 5.95 | 0.94 | 0.90 | 0.96 | 0.13 | 0.03 | 0.03 |
| Plasma AOX enzymes | |||||||||
| PGPX | 70.38 | 26.23 | 3.39 | 0.95 | 0.93 | 0.97 | 11.45 | 6.99 | 2.51 |
| Serum AOX enzymes | |||||||||
| PONa | 82.80 | 15.35 | 1.85 | 0.98 | 0.95 | 0.98 | 33.61 | 14.47 | 5.03 |
| PONp* | 94.44 | 4.56 | 1.00 | 0.99 | 0.98 | 0.99 | 9.33 | 2.05 | 0.96 |
| Erythrocyte AOX enzymes | |||||||||
| eSOD* | 7.49 | 91.82 | 0.69 | 0.92 | 0.87 | 0.95 | 0.61 | 2.14 | 0.19 |
| EGSHR* | 45.48 | 48.43 | 6.09 | 0.88 | 0.82 | 0.92 | 6.22 | 6.42 | 2.27 |
| eGPX* | 80.05 | 16.85 | 3.10 | 0.96 | 0.98 | 0.98 | 5.25 | 2.41 | 1.03 |
Log-transformed to improve the approximation to a normal.
Values reported below the limit of quantitiation. For definitions see footnote for Table II.
Table V shows the calculated parameters of biological variation AI, II, k, IH, and CD. A generally held criterion sets an AI threshold <0.5; however, performance of each analyte was evaluated according to ranks such that AI<0.25 was ‘optimal’, AI<0.5 was ‘desirable’, AI<0.75 was minimal, and AI≥0.75 was insufficient (Fraser et al. 1997). All study variables achieved at least minimal analytical acceptability except for linoleic acid which failed to achieve at least minimal performance. Substantial variation in the individuality of analytes (i.e. II) was observed, ranging from 0.24 for PONp to 3.52 for SOD. The smaller the II,the greater the individuality and repeated measurements give little additional information. This is evidenced in the number of measurements needed to estimate the homeostatic set point (k) which correlates strongly with II and ranges from 1 (retinol, β-cryptoxanthin and PONp) to nearly 15 (eSOD) measurements required to achieve a 95% chance that the mean is within ±5% of the true value. Five variables (δ-tocopherol, 13-HODE, 13-HpODE and 9-HpODE) failed to meet homogeneity assumptions demonstrating IH values greater than 1.35 while 9-HODE was borderline at 1.28. The range of critical difference values varies greatly from less than 1% to greater than 200% between two successive serial values. The largest CD values are associated with the 13-HODE, 9-HODE, 13-HpODE and 9-HpODE where large, heterogeneous intra-individual variability results make it difficult to assess the significance of changes in serial results.
Table V.
The calculated parameters of biological variation including the analytical acceptability index (AI), index of individuality (II), index of heterogeneity (IH) and the critical difference between serial results (CD).
| Analyte | AI | II | IH | Analytical performance | CD | k |
|---|---|---|---|---|---|---|
| Vitamins and micronutrients | ||||||
| Retinol | 0.69 | 0.32 | 0.26 | Minimum | 18.565 | 1.3 |
| δ-Tocopherol | 0.26 | 2.25 | 3.90 | Desirable | 15.315 | 2.9 |
| γ-Tocopherol | 0.16 | 0.69 | 0.60 | Optimum | 52.878 | 9.3 |
| α-Tocopherol* | 0.31 | 0.60 | 0.14 | Desirable | 18.565 | 2.5 |
| Lutein | 0.40 | 0.49 | 0.39 | Desirable | 30.272 | 2.0 |
| β-Cryptoxanthine* | 0.25 | 0.28 | 0.18 | Desirable | 35.263 | 1.2 |
| β-Carotene* | 0.74 | 1.92 | 0.49 | Minimum | 2.459 | 7.9 |
| Lycopene* | 0.38 | 0.69 | 1.17 | Desirable | 91.174 | 2.8 |
| Vitamin C | 0.32 | 0.97 | 0.87 | Desirable | 69.49 | 4.0 |
| Lipids and lipid peroxides | ||||||
| TBARS* | 0.54 | 1.00 | 1.12 | Minimum | 83.907 | 4.1 |
| 13-HODE | 0.13 | 2.45 | 1.65 | Optimum | 149.230 | 3.7 |
| 9-HODE† | 0.13 | 1.27 | 1.28 | Optimum | 115.008 | 5.2 |
| 13-HPODE*† | 0.48 | 1.41 | 1.64 | Desirable | 124.603 | 5.8 |
| 9-HPODE† | 0.15 | 1.39 | 2.43 | Optimum | 216.004 | 5.7 |
| Linoleic acid* | 1.04 | 0.36 | 0.00 | Insufficient | 0.132 | 1.5 |
| Plasma AOX enzymes | ||||||
| pGPX | 0.36 | 0.65 | 0.26 | Desirable | 20.573 | 2.7 |
| Serum AOX enzymes | ||||||
| PONa | 0.69 | 0.32 | 0.53 | Minimum | 42.437 | 1.9 |
| PONp* | 0.47 | 0.24 | 0.08 | Desirable | 6.27 | 1.0 |
| Erythrocyte AOX enzymes | ||||||
| eSOD* | 0.09 | 3.52 | 0.06 | Optimum | 5.961 | 14.5 |
| eGSHR* | 0.35 | 1.09 | 0.24 | Desirable | 18.854 | 4.5 |
| eGPX* | 0.43 | 0.50 | 0.09 | Desirable | 7.256 | 2.1 |
Log-transformed to improve the approximation to a normal.
Values reported below the limit of quantitiation. For definitions see footnote for Table II.
No statistically significant differences, at α = 0.05, in temporal effects across the menstrual cycle (i.e. ‘day’), were observed. The lack of findings may be due to the small sample size of the current study which may have insufficient power to detect significant differences over the time period studied. A larger study should be done to evaluate these possible effects.
Discussion
Data on biological variation of biomarkers associated with OS are generally lacking, in relation to the menstrual cycle in particular. Limited studies have been conducted to address these methodological issues (Kadiiska et al. 2000, 2005a,b, Kato et al. 2006) and there is still controversy over which biomarkers to use. It has been suggested that multiple biomarkers of OS should be utilized for research purposes until more sensitive and specific assays are developed (Chait 1994). The current consensus in laboratory medicine is that quality specifications should be based on the parameters of biological variation (Fraser & Hyltoft Petersen 1999, Hyltoft Petersen et al. 2002). In this study we generated biological variability data for a panel of biomarkers that are commonly measured in the laboratory when assessing OS. Using these data on components of biological variation, we have derived desirable quality specifications, indexed the usefulness of population-based reference ranges and estimated the level of changes in serial results obtained in an individual needed to find statistical significance. Although the number of participants investigated was small, a comparison of studies on biological variation demonstrates that estimates of intra-individual and inter-individual variation are similar regardless of the number of individuals studied (Fraser 1992, Sebastian-Gimbaro 1997, Ricos et al. 2004).
Lipid peroxidation (LPO) is a prominent manifestation of oxidative damage and a number of markers of this process have been developed. We chose to estimate MDA by TBARS reaction. MDA by highly specific GC-NICI-MS has been advocated as a useful marker of lipid peroxidation (Hyltoft Petersen et al. 2002); however; the instrumentation is costly and not widely available. Indirect estimation of MDA can be made by reaction with thiobarbituric acid which forms a fluorescent adduct with MDA. Although criticized for its specificity it is still the most commonly employed biomarker of LPO because of its simplicity (Kadiiska et al. 2005b) and has been shown to correlate well with GC-MS (Liu et al. 1997). Despite its wide application this is, to our knowledge, the first attempt to characterize the components of variability of this assay during the menstrual cycle. One previous study of a reference sample group (Nielsen et al. 1997) estimated reference limits for the group to be between 0.36 and 1.24 μM l−1 with intra-individual CVs of 6–30%. The analytical performance of this assay is minimally acceptable (CV ≈ 8% and AI = 0.54) and has a high degree of intra-individual variability necessitating replicate analysis to estimate the true plasma level which must nearly double to detect significantly different serial results.
In addition to TBARS we have employed an HPLC technique for the direct determination of linoleic acid peroxidation. Linoleic acid (LA; octadecadieneoic acid, ODE) is the most abundant PUFAs in human blood plasma and its LPO products predominate (Spiteller & Spiteller 1997, Browne & Armstrong 2000). HpODEs are rapidly reduced in biological surroundings to corresponding hydroxy acids (HODEs) which are secondary products of LPO but are significantly more stable than HpODEs (Browne & Armstrong 2000). Linoleic acid peroxidation products are of high physiological relevance and in some diseases characterized by inflammation or cell injury are present at 10–100 times higher concentration compared with healthy individuals and been advocated to be excellent biomarkers of oxidative stress (Spiteller & Spiteller 1997).
The analytical performance of the HPLC measurement of each HODE and HpODE was at least analytically desirable (AI<0.48) and demonstrated a CV of less than 5% based on external QC material. Our estimates of intra-individual variation for lipid peroxidation products range from 26 to 77% which is not largely dissimilar to those reported for F2 isoprostanes of about 42% (Helmersson & Basu 2001) indicating that there is considerable day-to-day biological variation in LPO. Factors that might cause this variability include differences in the formation and metabolism of these compounds depending on various endogenous regulating factors, i.e. enzymes and β-oxidation systems. Other reasons could be variability of endogenous defence mechanisms against OS and inflammation.
There is limited information with regard to fluctuations in antioxidant enzyme in the context of menstrual cycle with the most consistent finding has been increased pGPx and eGPx activity with increasing oestrogen levels (Massafra et al. 1998, 2000, Serviddio et al. 2002, Ha & Smith 2003). Antioxidant enzymes play an important role in inactivation and removal of toxic ROS. Besides individual differences in gene expression, antioxidant enzyme activities depend on other endogenous and/or environmental factors since within-subject variation is greater than analytical imprecision. The antioxidant activities in erythrocytes and plasma do not necessarily reflect levels of antioxidant defence in the whole organism since antioxidant enzyme activities vary among tissues.
Our results indicate that for PON1, pGPx, eGPx and GSHR, variation between individuals are generally larger than variation within-subject, suggesting enzyme activities in blood may reflect differences in antioxidant defence. The analytical performance for all assays is at least desirable, the analytical imprecision is less than 5%, and our estimate of GPx, GSHR and SOD within-subject variation is comparable to previous reports (Guemouri et al. 1991, Andersen et al. 1997). A previous estimate of the biological variability in PON 1 paraoxonase and arylestarase activities, during a 4-week time period in a larger and more diverse group of men and women, demonstrated comparable analytical variance but smaller intra-individual and larger inter-individual variance than those reported here (Browne et al. 2007).
The components of variation for fat-soluble vitamins in plasma reported in the literature (Talwar et al. 2005) are similar to our values, suggesting that our measurements represent reliable estimates of biological variation. Furthermore, there is no indication of increased variability of these vitamins during the menstrual cycle in our study. Other reports describing the variation of carotenoid and tocopherols levels during the menstrual have produced conflicting results. With regard to carotenoids, Rock et al. (1995) reported that α-carotene and lutein were increased in the mid-leuteal phase and the early follicular phase respectively, in young women, only if uncorrected for total cholesterol. Other carotenoids did not vary across the menstrual cycle, whether corrected or uncorrected for total cholesterol. Tangney et al. (1991) and Palan et al. (2006) also found no variation in carotenoids across the menstrual cycle. In contrast, in a controlled dietary study, individual plasma carotenoid concentrations increased from 8 to 29% at different phases of the menstrual cycle compared with menses (Forman et al. 1996). With regard to tocopherols, several reports have demonstrated a non-significant fluctuation in plasma α-tocopherol levels during the menstrual cycle. Lanza et al. (1998) reported 12% lower levels at menses than during luteal phase, Reimer et al. (2005) reported 12% lower levels during follicular phase compared with the luteal phase and Palan et al. (2006) observed 28% lower levels in the follicular versus luteal phase. It should be noted however that these observations were made under controlled dietary conditions which were not a component of several studies failing to demonstrate menstrual cycle variation and may explain the inability of the present study to detect these changes. Finally, it has been suggested that concentrations of these fat-soluble vitamins vary depending on the amount of lipid present and should be standardized per amount of cholesterol or total lipid. In at least one report this normalization did not affect the parameters of biological variation (Maes et al. 1996). Since a major goal of the present study was to characterize analytical variability, we did not standardize fat-soluble vitamin levels to cholesterol in an effort to avoid the introduction of bias (Schisterman et al. 2005) and additional analytical and biological variability inherent to this procedure.
Acknowledgments
This study was supported by an intramural contract with the National Institute of Child Health and Human Development: Contract # ADB-N01-HD-4-3394.
Appendix
Graphs of the grand mean and standard deviation of study variable levels for all study subjects (All) and the mean and standard deviation of levels for each of nine individual subjects (1–9).
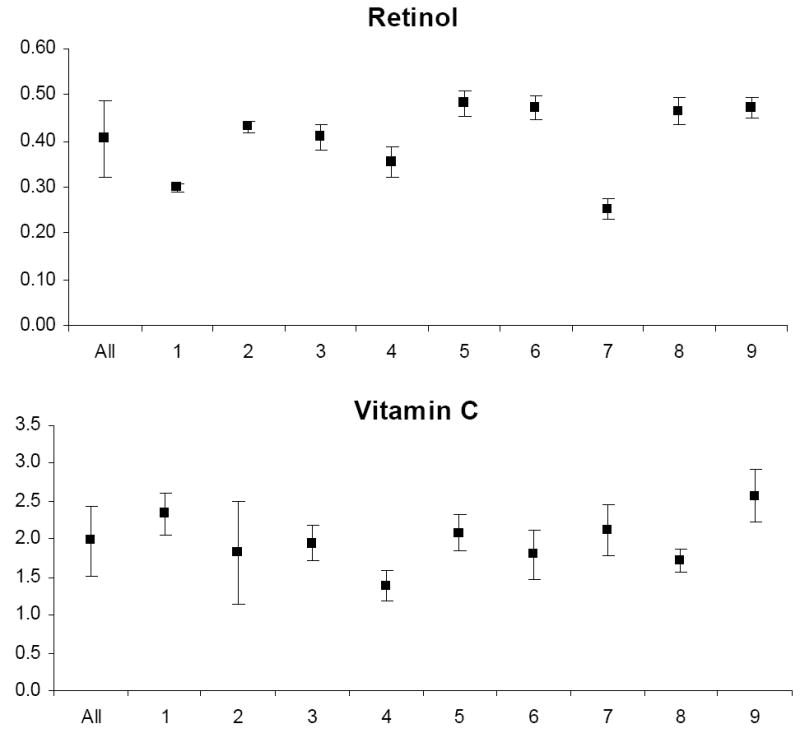
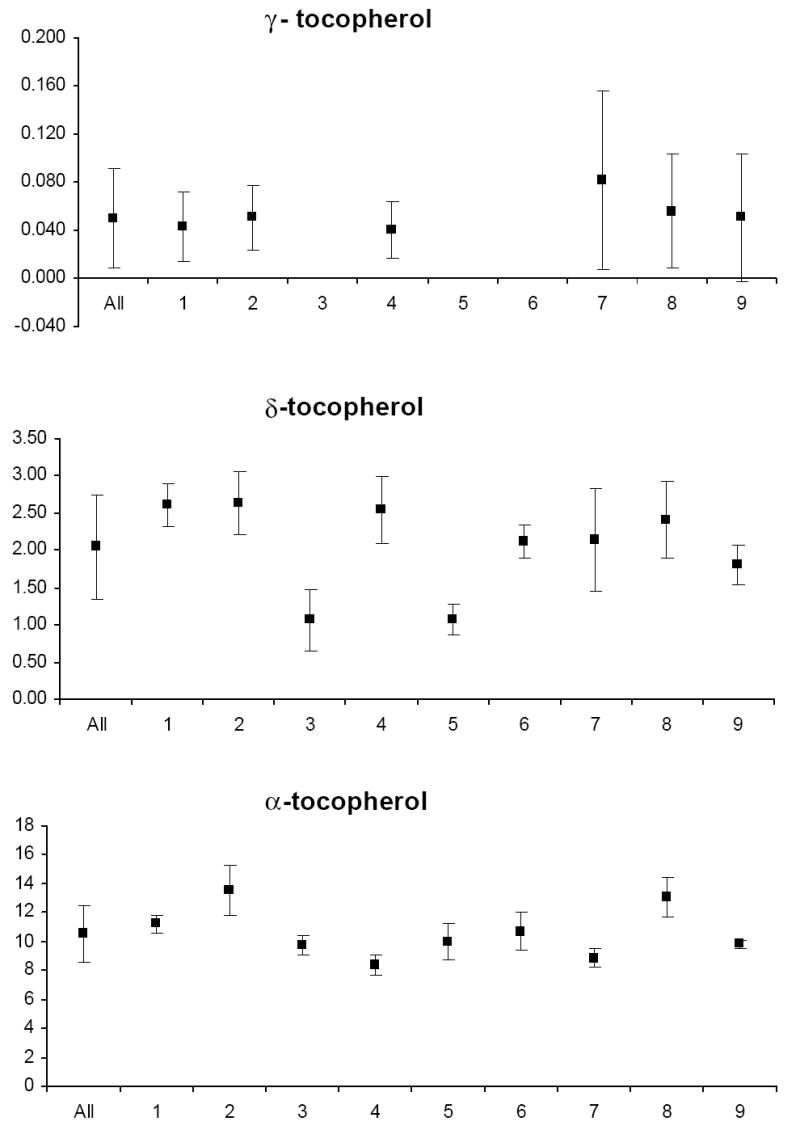
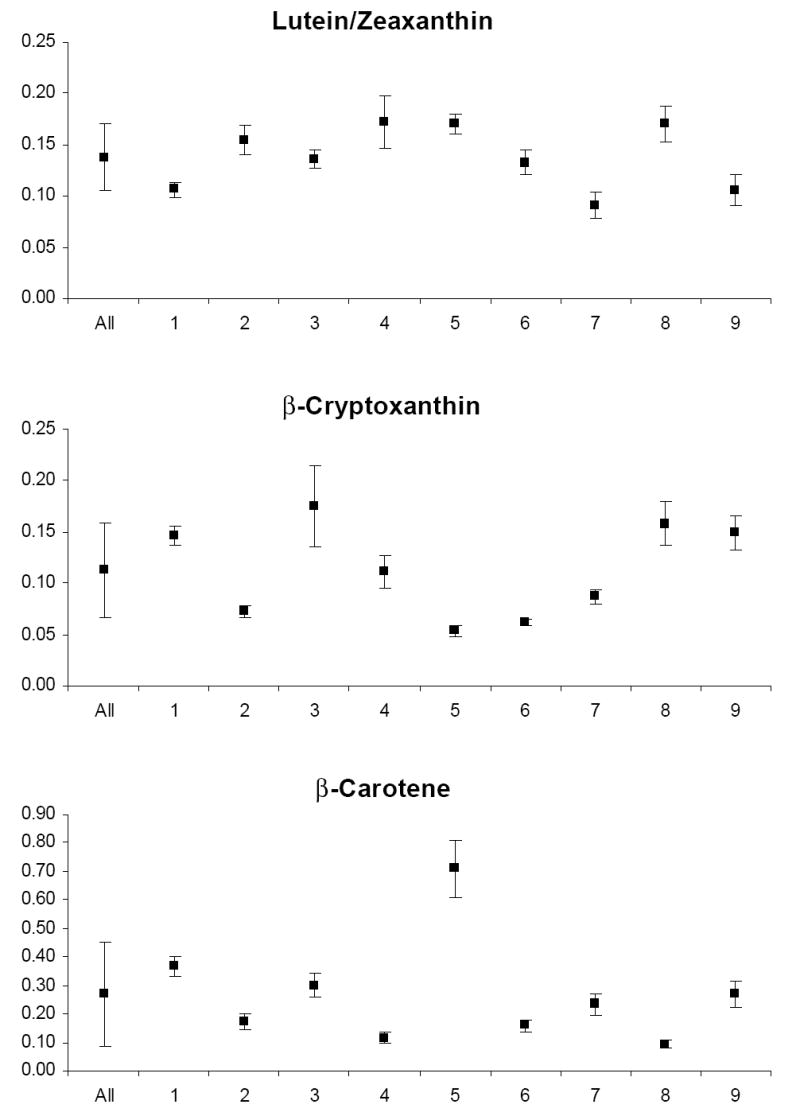
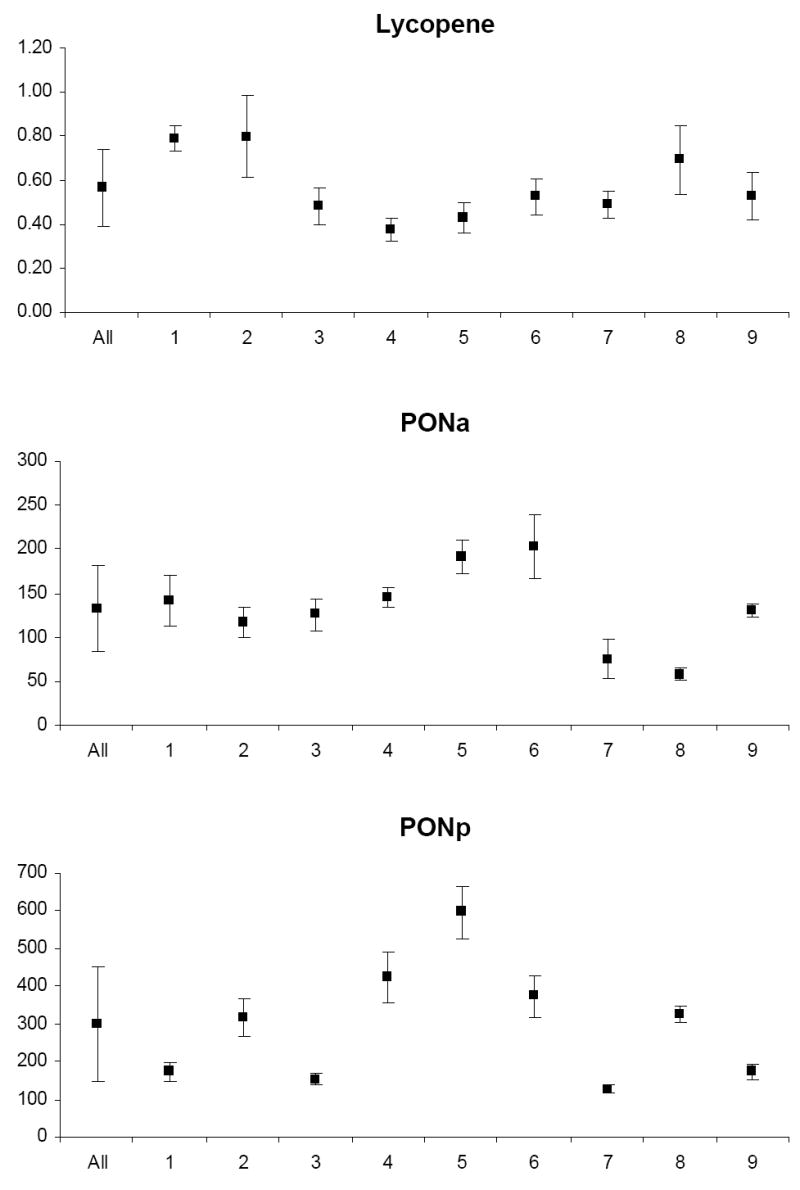
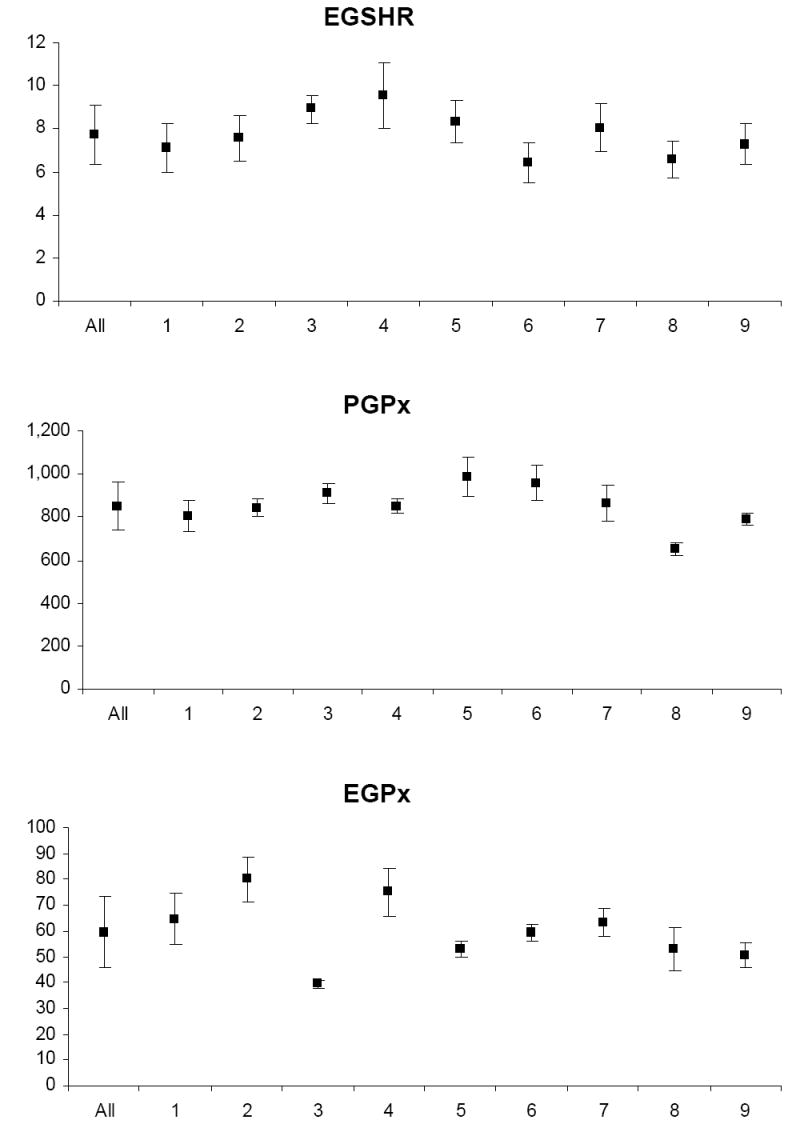
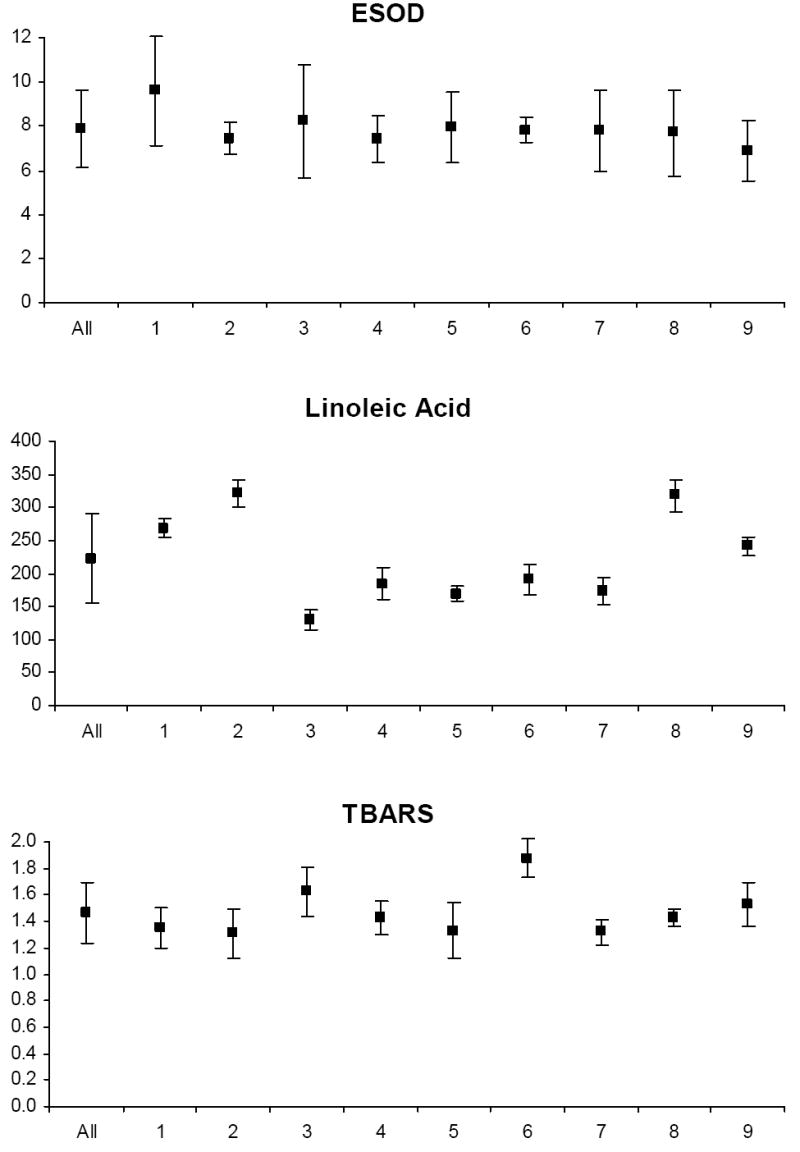
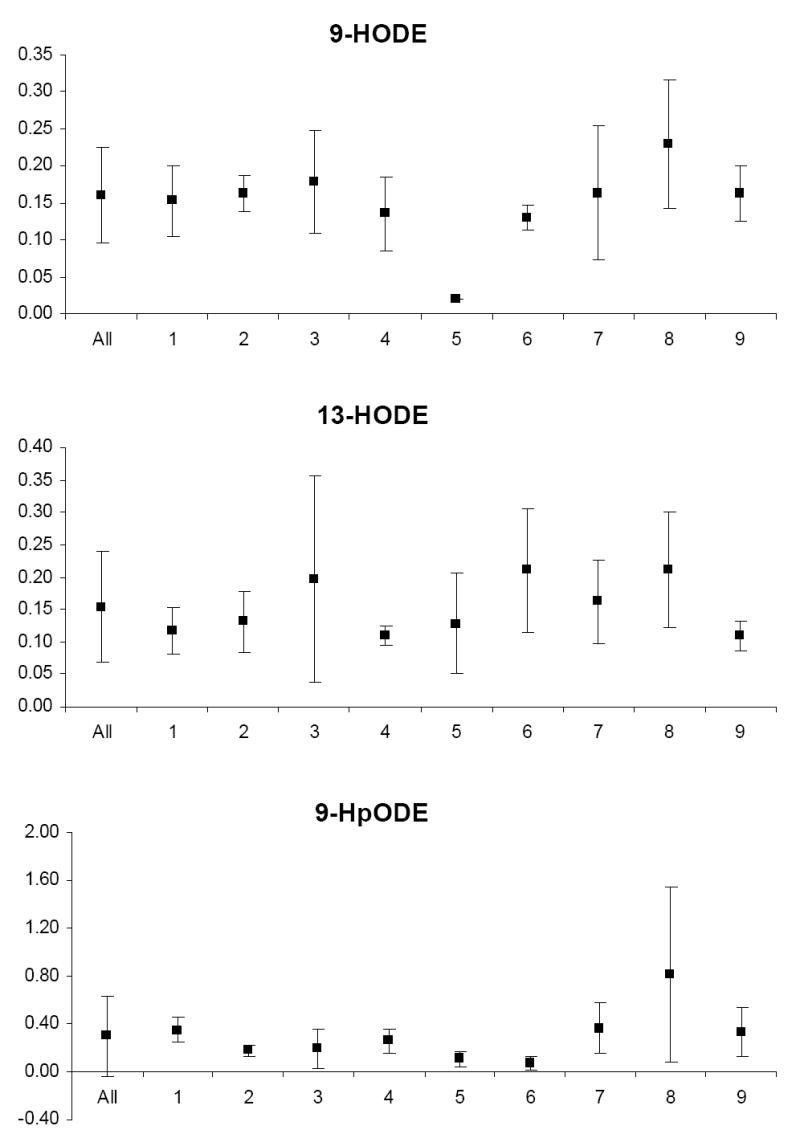
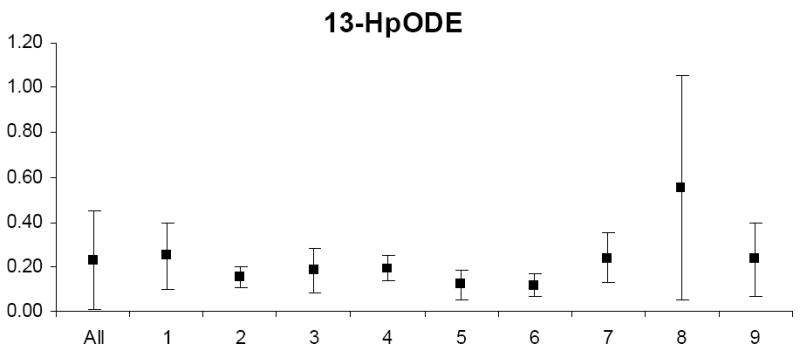
PONa, PON1 arylesteraseactivity; PONp, PON1 paraoxonase activity; EGSHR, erythrocyte glutathione reductase activity; PGPx, plasma glutathione peroxidase activity; EGPx, erythrocyte glutathione peroxidase activity and ESOD, erythrocyte superoxide dismutase activity; TBARS, thiobarbituric acid reactive substances; 9-HODE, 9 hydroxy octadecadieneoic acid; 13-HODE, 13 hydroxy octadecadieneoic acid; 13-HpODE, 13 hydroperoxy octadecadieneoic acid and 9-HpODE, 9 hydroperoxy octadecadieneoic acid.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- Agar J, Durham H. Relevance of oxidative injury in the pathogenesis of motor neuron diseases. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. 2003;4:232–242. doi: 10.1080/14660820310011278. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reproductive Biology & Endocrinology. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen HR, Nielsen JB, Nielsen F, Grandjean P. Antioxidative enzyme activities in human erythrocytes. Clinical Chemistry. 1997;43:562–568. [PubMed] [Google Scholar]
- Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. In: Armstrong D, editor. Free Radicals in Diagnostic Medicine, Advances in Experimental Medicine & Biology. Vol. 366. New York: Plenum Press; 1994. pp. 43–58. [DOI] [PubMed] [Google Scholar]
- Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189:113–127. doi: 10.1016/s0300-483x(03)00157-4. [DOI] [PubMed] [Google Scholar]
- Browne RW, Armstrong D. Simultaneous determination of serum retinol, tocopherols and carotenoids by high pressure liquid chromatography. Methods in Molecular Biology. 1998;108:269–275. doi: 10.1385/0-89603-472-0:269. [DOI] [PubMed] [Google Scholar]
- Browne RW, Armstrong D. HPLC analysis of lipid derived polyunsaturated fatty acid peroxidation products in oxidatively modified human plasma. Clinical Chemistry. 2000;46:829–836. [PubMed] [Google Scholar]
- Browne RW, Koury ST, Marion SM, Wilding G, Muti P, Trevisan M. Accuracy and biological variation of human serum paraoxonase 1 activity and polymorphism (Q192R) by kinetic enzyme assay. Clinical Chemistry. 2007;53:310–317. doi: 10.1373/clinchem.2006.074559. [DOI] [PubMed] [Google Scholar]
- Chait A. Low-density lipoprotein oxidation and the pathogenesis of atherosclerosis. Western Journal of Medicine. 1994;160:183–184. [PMC free article] [PubMed] [Google Scholar]
- Chalmers AH, McWhinney BC. Two spectrophotometric methods compared for measuring low concentrations of ascorbate in plasma and urine. Clinical Chemistry. 1986;32:1412–1413. [PubMed] [Google Scholar]
- Collins AR, Cadet J, Moller L, Poulsen HE, Vina J. Are we sure we know how to measure 8-oxo-7, 8-dihydroguanine in DNA from human cells? Archives of Biochemistry & Biophysics. 2004;423:57–65. doi: 10.1016/j.abb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Cotlove E, Harris EK, Williams GZ. Biological and analytic components of variation in long-term studies of serum constituents in normal subjects. 3. Physiological and medical implications. Clinical Chemistry. 1970;16:1028–32. [PubMed] [Google Scholar]
- Dandona P. Endothelium, inflammation, and diabetes. Current Diabetes Reports. 2002;2:311–315. doi: 10.1007/s11892-002-0019-0. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Fu S, Wang H, Dean RT. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radical Biology & Medicine. 1999;27:1151–1163. doi: 10.1016/s0891-5849(99)00206-3. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- De Zwart LL, Meerman JHN, Commandeur JNM, Vermeulen NPE. Biomarkers of free radical damage: applications in experimental animals and in humans. Free Radical Biology & Medicine. 1999;26:202–226. doi: 10.1016/s0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- Forman MR, Beecher GR, Muesing R, Lanza E, Olson B, Campbell WS, McAdam P, Raymond E, Schulman JD, Graubard BI. The fluctuation of plasma carotenoid concentrations by phase of the menstrual cycle: a controlled diet study. American Journal of Clinical Nutrition. 1996;64:559–565. doi: 10.1093/ajcn/64.4.559. [DOI] [PubMed] [Google Scholar]
- Fraser CG. Biological variation in clinical chemistry. an update: collected data, 1988–1991. Archives of Pathology & Laboratory Medicine. 1992;116:916–923. [PubMed] [Google Scholar]
- Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Critical Reviews in Clinical & Laboratory Science. 1989;27:409–437. doi: 10.3109/10408368909106595. [DOI] [PubMed] [Google Scholar]
- Fraser CG, Hyltoft PP, Libeer JC, Ricos C. Proposals for setting generally applicable quality goals solely based on biology. Annals of Clinical Biochemistry. 1997;34:8–12. doi: 10.1177/000456329703400103. [DOI] [PubMed] [Google Scholar]
- Fraser CG, Hyltoft Petersen P. Analytical performance characteristics should be judged against objective quality specifications. Clinical Chemistry. 1999;45:321–323. [PubMed] [Google Scholar]
- Guemouri L, Artur Y, Herbeth B, Jeandel C, Cuny G, Siest G. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clinical Chemistry. 1991;37:1932–1937. [PubMed] [Google Scholar]
- Ha EJ, Smith AM. Plasma selenium and plasma and erythrocyte glutathione peroxidase activity increase with estrogen during the menstrual cycle. Journal of the American College of Nutrition. 2003;22:43–51. doi: 10.1080/07315724.2003.10719274. [DOI] [PubMed] [Google Scholar]
- Helmersson J, Basu S. F(2)-isoprostane and prostaglandin F(2 alpha)metabolite excretion rate and day to day variation in healthy humans. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2001;65:99–102. doi: 10.1054/plef.2001.0295. [DOI] [PubMed] [Google Scholar]
- Hensley K, Williamson KS, Floyd RA. Measurement of 3-nitrotyrosine and 5-nitro-γ-tocopherol by high-performance liquid chromatography with electrochemical detection. Free Radical Biology & Medicine. 2000;28:520–528. doi: 10.1016/s0891-5849(00)00155-6. [DOI] [PubMed] [Google Scholar]
- Hyltoft Petersen P, Fraser CG, Jorgensen L, Brandslund I, Stahl M, Gowans E, et al. Combination of analytical quality specifications based on biological within- and between-subject variation. Annals of Clinical Biochemistry. 2002;39:543–550. doi: 10.1177/000456320203900601. [DOI] [PubMed] [Google Scholar]
- Jenkins C, Wilson R, Roberts J, Miller H, McKillop JH, Walker JJ. Antioxidants: their role in pregnancy and miscarriage. Antioxidants & Redox Signaling. 2000;2:623–628. doi: 10.1089/15230860050192369. [DOI] [PubMed] [Google Scholar]
- Kadiiska MB, Gladen BC, Baird DD, Graham LB, Parker CE, Ames BN, et al. Biomarkers of oxidative stress study III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radical Biology & Medicine. 2005a;38:711–718. doi: 10.1016/j.freeradbiomed.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radical Biology & Medicine. 2005b;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Kadiiska MB, Gladen BC, Baird DD, Dikalova AE, Sohal RS, Hatch GE, et al. Biomarkers of oxidative stress study: are plasma antioxidants markers of CCl(4) poisoning? Free Radical Biology & Medicine. 2000;28:838–845. doi: 10.1016/s0891-5849(00)00198-2. [DOI] [PubMed] [Google Scholar]
- Kato I, Ren J, Heilbrun LK, Djuric Z. Intra- and inter-individual variability in measurements of biomarkers for oxidative damage in vivo: Nutrition and Breast Health Study. Biomarkers. 2006;11:143–52. doi: 10.1080/13547500600565693. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Hong YC, Lee KH, Park HJ, Park EA, Moon HS, et al. Oxidative stress in pregnant women and birth weight reduction. Reproductive Toxicology. 2005;19:487–492. doi: 10.1016/j.reprotox.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. Journal of Pathology. 2003;201:28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- Lachin JM. The role of measurement reliability in clinical trials. Clinical Trials. 2004;1:553–66. doi: 10.1191/1740774504cn057oa. [DOI] [PubMed] [Google Scholar]
- Lanza E, Forman MR, Johnson EJ, Muesing RA, Graubard BI, Beecher GR. alpha-Tocopherol concentrations in plasma but not in lipoproteins fluctuate during the menstrual cycle in healthy premenopausal women. Journal of Nutrition. 1998;128:1150–1155. doi: 10.1093/jn/128.7.1150. [DOI] [PubMed] [Google Scholar]
- Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. Journal of Animal Science. 1998;76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- Liu J, Yeo HC, Doniger SJ, Ames BN. Assay of aldehydes from lipid peroxidation: gas chromatography-mass spectrometry compared to thiobarbituric acid. Analytical Biochemistry. 1997;245:161–166. doi: 10.1006/abio.1996.9990. [DOI] [PubMed] [Google Scholar]
- Maas JW, Groothuis PG, Dunselman GA, de Goeij AF, Struyker Boudier HA, et al. Endometrial angiogenesis throughout the human menstrual cycle. Human Reproduction. 2001;16:1557–1561. doi: 10.1093/humrep/16.8.1557. [DOI] [PubMed] [Google Scholar]
- Maes M, Weeckx S, Wauters A, Neels H, Scharpe S, Verkerk R, et al. Biological variability in serum vitamin E concentrations: relation to serum lipids. Clinical Chemistry. 1996;42:1824–1831. [PubMed] [Google Scholar]
- Margolis SA, Duewer DL. Measurement of ascorbic acid in human plasma and serum: stability, intralaboratory repeatability, and interlaboratory reproducibility. Clinical Chemistry. 1996;42:1257–1262. [PubMed] [Google Scholar]
- Massafra C, De Felice C, Gioia D, Buonocore G. Variations in erythrocyte antioxidant glutathione peroxidase activity during the menstrual cycle. Clinical Endocrinology. 1998;49:63–67. doi: 10.1046/j.1365-2265.1998.00441.x. [DOI] [PubMed] [Google Scholar]
- Massafra C, Gioia D, De Felice C, Picciolini E, De Leo V, Bonifazi M, et al. Effects of estrogens and androgens on erythrocyte antioxidant superoxide dismutase, catalase and glutathione peroxidase activities during the menstrual cycle. Journal of Endocrinology. 2000;167:447–452. doi: 10.1677/joe.0.1670447. [DOI] [PubMed] [Google Scholar]
- Mathur S, Devaraj S, Jialal I. Accelerated atherosclerosis, dyslipidemia, and oxidative stress in end-stage renal disease. Current Opinion in Nephrology and Hypertension. 2002;11:141–147. doi: 10.1097/00041552-200203000-00003. [DOI] [PubMed] [Google Scholar]
- Matsubasa T, Uchino T, Karashima S, Kondo Y, Maruyama K, Tanimura M, et al. Oxidative stress in very low birth weight infants as measured by urinary 8-OHdG. Free Radical Research. 2002;36:189–193. doi: 10.1080/10715760290006510. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proceedings of the National Academy of Sciences. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhaup G, Masters CL. Metal binding and radical generation of proteins in human neurological diseases and aging. Metal Ions in Biological Systems. 1999;36:365–387. [PubMed] [Google Scholar]
- Murphy JM, Browne RW, Hill L, Bolelli GF, Abagnato C, Berrino F, et al. Effects of transportation and delay in processing on the stability of nutritional and metabolic biomarkers. Nutrition & Cancer. 2000;37:155–160. doi: 10.1207/S15327914NC372_6. [DOI] [PubMed] [Google Scholar]
- Murphy AA, Santanam N, Parthasarathy S. Endometriosis: a disease of oxidative stress? Seminars in Reproductive Endocrinology. 1998;16:263–273. doi: 10.1055/s-2007-1016286. [DOI] [PubMed] [Google Scholar]
- Murray AA, Molinek MD, Baker SJ, Kojima FN, Smith MF, Hillier SG, et al. Role of ascorbic acid in promoting follicle integrity and survival in intact mouse ovarian follicles in vitro. Reproduction. 2001;121:89–96. doi: 10.1530/rep.0.1210089. [DOI] [PubMed] [Google Scholar]
- Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clinical Chemistry. 1997;43:1209–1214. [PubMed] [Google Scholar]
- Olinski R, Gackowski D, Foksinski M, Rozalski R, Roszkowski K, Jaruga P. Oxidative DNA damage: assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. Free Radical Biology & Medicine. 2002;33:192–200. doi: 10.1016/s0891-5849(02)00878-x. [DOI] [PubMed] [Google Scholar]
- Palan PR, Magneson AT, Castillo M, Dunne J, Mikhail MS. Effects of menstrual cycle and oral contraceptive use on serum levels of lipid-soluble antioxidants. American Journal of Obstetrics & Gynecology. 2006;194:e35–8. doi: 10.1016/j.ajog.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Paredi P, Kharitonov SA, Barnes PJ. Analysis of expired air for oxidation products. American Journal of Respiratory and Critical Care Medicine. 2002;166:S31–S37. doi: 10.1164/rccm.2206012. [DOI] [PubMed] [Google Scholar]
- Pippenger CE, Browne RW, Armstrong D. Regulatory antioxidant enzymes. Methods in Molecular Biology. 1998;108:299–313. doi: 10.1385/0-89603-472-0:299. [DOI] [PubMed] [Google Scholar]
- Pratico D, Rokach J, Lawson J, Fitzgerald GA. F2-isoprostanes as indices of lipid peroxidation in inflammatory diseases. Chemistry & Physics Lipids. 2004;128:165–171. doi: 10.1016/j.chemphyslip.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Pryor WA. Oxidative stress status: OSS, BOSS and “Wild Bill” Donovan. Free Radical Biology & Medicine. 1999;27:1135–1136. doi: 10.1016/s0891-5849(99)00213-0. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Godber SS. Noninvasive measures of oxidative stress status in humans. Free Radical Biology & Medicine. 1991;10:177–184. doi: 10.1016/0891-5849(91)90073-c. [DOI] [PubMed] [Google Scholar]
- Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- Reimer RA, Debert CT, House JL, Poulin MJ. Dietary and metabolic differences in pre versus postmenopausal women taking or not taking hormone replacement therapy. Physiology & Behavior. 2005;84:303–312. doi: 10.1016/j.physbeh.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Ricos C, Garcia-Lariol J-V, Alvarez V, Caval F, Domenechl M, Hernandez A, et al. Westgard QC: 2004. [2007 January 12]. Biological variation database, and quality specifications for imprecision, bias and total error (desirable and minimum): The 2004 update. Available: http://www.westgard.com/guest26.htm via the INTERNET. [Google Scholar]
- Rock CL, Demitrack MA, Rosenwald EN, Brown MB. Carotenoids and menstrual cycle phase in young women. Cancer Epidemiology. Biomarkers & Prevention. 1995;4:283–288. [PubMed] [Google Scholar]
- Samborskaia E, Ferdman T. Mechanism of abortion induced by ascorbic acid. Biulletin Eksperimentalnoi Biologii I Meditsiny. 1966;62:96–98. [PubMed] [Google Scholar]
- Schisterman EF, Vexler A, Whitcomb BW, Liu A. The limitations due to exposure detection limits for regression models. American Journal of Epidemiology. 2006;163:374–383. doi: 10.1093/aje/kwj039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman E, Whitcomb B, Louis G, Louis T. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environmental Health Perspectives. 2005;113:853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian-Gimbaro MA. Intra- and inter-individual biological variability data bank. European Journal of Clinical Chemistry and Clinical Biochemistry. 1997;35:845–852. [PubMed] [Google Scholar]
- Serviddio G, Loverro G, Vicino M, Prigigallo F, Grattagliano I, Altomare E, et al. Modulation of endometrial redox balance during the menstrual cycle: relation with sex hormones. The Journal of Clinical Endocrinology and Metabolism. 2002;87:2843–2848. doi: 10.1210/jcem.87.6.8543. [DOI] [PubMed] [Google Scholar]
- Soory M. Hormone mediation of immune responses in the progression of diabetes, rheumatoid arthritis and periodontal diseases. Current Drug Targets. Immune. Endocrine and Metabolic Disorders. 2002;2:13–25. [PubMed] [Google Scholar]
- Spector A. Review: oxidative stress and disease. Journal of Ocular Pharmacology and Therapeutics. 2000;16:193–201. doi: 10.1089/jop.2000.16.193. [DOI] [PubMed] [Google Scholar]
- Spiteller P, Spiteller G. 9-Hydroxy-10,12-octadecadieneoic acid (9HODE) and 13-Hydroxy-9,11-octadecadieneoic acid (13HODE): excellent markers for lipid peroxidation. Chemistry & Physics Lipids. 1997;89:131–139. [Google Scholar]
- Talwar DK, Azharuddin MK, Williamson C, Teoh YP, McMillan DC, St J, O’Reilly D. Biological variation of vitamins in blood of healthy individuals. Clinical Chemistry. 2005;51:2145–2150. doi: 10.1373/clinchem.2005.056374. [DOI] [PubMed] [Google Scholar]
- Tangney C, Brownie C, Wu SM. Impact of menstrual periodicity on serum lipid levels and estimates of dietary intakes. Journal of the American College of Nutrition. 1991;10:107–113. doi: 10.1080/07315724.1991.10718133. [DOI] [PubMed] [Google Scholar]
- Terada LS. Specificity in reactive oxidant signaling: think globally, act locally. Journal of Cell Biology. 2006;174:615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgard J, Carey R, Wold S. Criteria for judging precision and accuracy in method development and evaluation. Clinical Chemistry. 1974;20:825–833. [PubMed] [Google Scholar]
- Westgard J, Groth T, Aronsson T. Combined Shewhart–cusum control chart for improved quality control in clinical chemistry. Clinical Chemistry. 1977;23:1881–1887. [PubMed] [Google Scholar]
- Xu D, Ong C, Shen H. The associations between concentration of selenium in semen and sperm parameters as well as oxidative DNA damage in human sperm. Chinese Journal of Preventive Medicine. 2001;35:394–396. [PubMed] [Google Scholar]


