Abstract
Noninvasive, transient, and local image-guided blood-brain barrier disruption (BBBD) has been demonstrated with focused ultrasound exposure in animal models. Most studies have combined low pressure amplitude and low time average acoustic power burst sonications with intra-vascular injection of pre-formed micro-bubbles to produce BBBD without damage to the neurons. The BBB has been shown to be healed within a few hours after the exposure. The combination of focused ultrasound beams with MR image guidance allows precise anatomical targeting as demonstrated by the delivery of several marker molecules in different animal models. This method may in the future have a significant impact on the diagnosis and treatment of central nervous system (CNS) disorders. Most notably, the delivery of the chemotherapy agents liposomal Doxorubicin and Herceptin has been shown in a rat model.
Keywords: Blood-brain barrier, brain, targeted drug delivery, image-guided therapy, drug delivery, HIFU, focused ultrasound, ultrasound
Keywords: Ultrasonics, Magnetic Resonance Imaging, Drug Delivery Systems, Ultrasound Contrast Agents, Blood-Brain Barrier
1. Introduction
The endothelial cells of the central nervous system (CNS) are unique cells that are tightly fused to each other by intercellular attachments known as “tight junctions” [1,2]. In addition to the endothelium the blood -brain barrier (BBB) is formed by astrocytes, neurons, microglia and pericytes. The endothelial cells have low pinocytic activity and thus form a barrier between the blood and the cells in the CNS [3]. The factors that determine penetration of substances from the blood to the CNS are lipid solubility, molecular size and charge, and whether or not they can utilize any carriers in the endothelial cells. Thus the BBB limits the passage of most molecules (over 95% of potential therapeutically useful molecules) from circulation into the brain parenchyma, precluding their use in the treatment or diagnosis in the CNS [1,2,4,5]. There are two ways to enhance propagation through the barrier: Chemical modification of the drugs, or the use of other carriers such as aminoacid and peptide carriers can increase transport through the BBB. The tight junctions (TJs) can also be opened temporarily by an intra-arterial injection of certain chemicals such as mannitol or other hyper-osmotic solutions. It assumed that these agents cause the endothelial cells to shrink, resulting in a disruption of the TJs that lasts for a few hours [6]. Both the above methods produce diffuse BBBD within the entire tissue volume supplied by the injected artery branch [1],[2] and they cannot be used to selectively target a small CNS volume. This type of localized BBB disruption currently can be accomplished clinically only by direct invasive injection of agents into the targeted region of the brain [2].
Local BBBD was observed after experimental exposure of animal brains to ultrasound [7], [8], [9] indicating that ultrasound may offer a local method for drug delivery. In this paper we will review the literature and our experience in using focused ultrasound for image-guided focal and transient BBBD.
2. Ultrasound exposure through the skull
A major limitation in the utilization of ultrasound for the disruption of the BBB has been the poor penetration of ultrasound through the skull, and for several decades it was believed that the skull bone had to be removed to perform ultrasound treatments in the brain [10–12]. Although focusing was attempted for overcoming the attenuation losses[13], the high speed of sound in the bone coupled with its dependence on the density of the bone further complicates ultrasound transmission through the skull. The distortion of the wave front by the heterogeneous bone makes focusing in the brain difficult with traditional single focus transducers [13]. Phased arrays with CT-derived phase and amplitude corrections have been proposed to overcome this problem [14,15]. Such methods have been able to focus ultrasound through human skulls [14] and create thermal lesions in rabbit [16,17] and sheep brains [18] in vivo, and a magnetic resonance imaging (MRI) guided system is currently in a clinical phase I trial for thermal ablation of malignant brain tumors [19]. Since the time averaged ultrasound power is at least two orders of magnitude larger for thermal ablation than for the BBBD, it is clear that such systems can be used in humans for BBBD. It has also been proposed that skull induced distortions could be limited by reducing the frequency of the sound beam, thus increasing the wavelength. Both experiments [13] and simulations [20] have shown that the use of low frequency (around 250–300 kHz) will allow focusing through human skull without patient-specific distortion corrections. There is also another route for using higher frequencies that allows more precise focusing without the need of CT-derived corrections. This method uses the transmission of shear waves that distort less than longitudinal waves [21].
3. Ultrasound methods for BBBD
3.1. Temperature effects
Although local BBBD was observed after exposure of animal brains to ultrasound [7], [8], [9], there were no experiments that deliberately tried to exploit this phenomenon for molecular delivery into the brain until Patrick et al.[22]proposed it for the delivery of chemotherapy agents into brain tumors. They demonstrated that the BBB is leaky around ultrasound-induced thermal lesions, but not if the exposures did not result in tissue coagulation. This thermally-induced BBBD has been further investigated, but it has always been associated with tissue damage[23]. However, in tissue cultures, elevated temperatures have been shown to induce reversible BBBD [24].
3.2. Cavitation effects
Although short, high-intensity ultrasound exposure above the cavitation threshold was found to produce BBBD, sometimes without brain tissue damage [25], this was not able to be reproduced consistently. Similar results were observed with pulsed, higher frequency (2 MHz) sonications [26] where the association with cavitation was not clear. There is also clinical evidence that sonications can disrupt the BBB [27]. In a clinical study, low frequency continuous wave ultrasound (approximately 300kHz) was used for blood clot lysis in combination with intravenous injection of tissue plasminogen activator (TPA). After some treatments, contrast-enhanced CT demonstrated that the BBB was disrupted in a remote location. It is not possible to determine exactly why the disruption occurred, but it is possible that the low frequency exposures in combination with the injected fluid caused cavitation. It is not clear if the BBBD was associated with tissue damage.
3.3 Micro-bubble aided BBBD
A few years ago, noninvasive and reversible disruption of the BBB was demonstrated using focused ultrasound bursts in conjunction with an ultrasound contrast agent [28]. Contrast agents that contain preformed micro-bubbles are now extensively investigated for many therapeutic applications (see review [29]). Since this method has been the only one to produce reversible BBBD without brain tissue damage, it has been extensively investigated by several research groups. These studies will be reviewed in the following pages.
3.3.1. Description of the method
This method for BBBD with ultrasound utilizes pre-formed micro-bubbles injected into the blood stream before the ultrasound burst exposures. The working hypothesis for this research was that since the micro-bubbles act as energy concentrators and are contained in the blood, the induced biological effects would be confined to the blood vessel walls, for the most part (figure 1)[28]. So far all of the animal experiments support this hypothesis.
Figure 1.
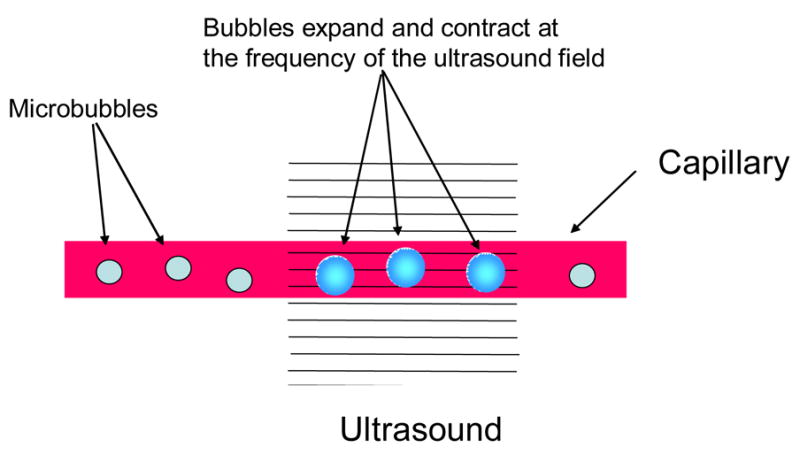
A diagram of the ultrasound induced BBBD.
The ultrasound exposures used in most experiments have been 10-ms bursts at low pressure amplitude (<1 MPa) repeated at the frequency of 1 Hz for the duration of 20–30 s. The micro-bubbles, developed for ultrasound imaging, contain gas (air or perfuorocarbon) encased in a shell, most often made of albumin or lipids, and have diameters between approximately 1 and 5 micrometers such that they pass through the capillary network. When the bubbles pass through the tissue volume exposed to ultrasound they expand and contract at the frequency of the propagating acoustic wave due to the cyclic pressure reductions and increases associated with the wave propagation. The bubble oscillations also cause the surrounding fluid to flow (microstreaming) thus creating large shear forces around the bubbles [30]. In addition, the bubbles are pushed by a radiation force [30,31] in the direction of wave propagation. Above a threshold, the bubble oscillations become so large that the inertia of the surrounding fluid causes the bubble to collapse, inducing high temperatures and pressures and resulting in a shock wave that propagates at supersonic speed radially from the collapse site [32,33]. If the bubbles collapse close to a vessel wall, they can create fluid jets that can puncture the wall [32–34]. As a result the bubbles absorb and concentrate energy from the ultrasound wave into a microscopic tissue volume reducing the ultrasound power levels by at least two orders of magnitude from that required to induce bio-effects without the bubbles [28]. The acoustic signals emitted by the micro-bubbles and their relationship to the BBBD and vascular damage has been investigated [35]. It was discovered that the bubble collapse demonstrated by wideband emission was associated with extravasations of red blood cells (RBCs). The results also showed that bubble oscillations, detectable from the emission spectrum, were present when BBBD without vascular damage was induced. Therefore, the acoustic signals may provide a method for exposure control.
The micro-bubbles used so far are standard, clinically proven, diagnostic ultrasound contrast agents. Optison ™ (GE Healthcare, Milwaukee,WI), containing bubbles of perfluorocarbon gas (Perflutren) with a human albumin shell, has been the most commonly used agent. According to the manufacturer, the bubbles have a mean diameter of 2–4.5 micrometers with a maximum diameter of 32 micrometers. The bubble concentration in the agent is between 5 and 8 × 108 bubbles/mL. Most of the animal experiments have been performed at the clinically recommended diagnostic dose of 0.2 ml of Optison/kg. As expected, experiments have shown that other ultrasound contrast agents containing pre-formed micro-bubbles can be used for BBBD [36,37] and thus the method is not specific to one agent.
Ultrasound contrast agents contain some large bubbles that are too large to pass through the capillary network. Since the contrast agent is injected IV, the large bubbles are filtered away by the lungs. However, if intra-arterial injection is needed, then large bubbles should be removed to avoid development of micro-embolizations. These large bubbles were most likely the cause for the BBB disruptions that were seen in experiments with rats with intra-arterial injection of Optison without sonications [38].
3.3.2. The mechanism of ultrasound induced BBBD
In order to determine the time course between the sonications and the disruption of the BBB, a series of mouse experiments was performed using in vivo multi-photon microscopy [39]. In these experiments two dyes of different molecular weights were injected IV into a mouse positioned in a microscopy system that allowed simultaneous ultrasound exposures. The results showed that the dye leakage occurred without extravasation of RBCs (which was also seen on one occasion) via two observed routes. First, micro-disruptions were observed where the dye rapidly leaked from a point on the vessel wall. This could be caused by bubble collapse with associated jet formation that punctured the vessel wall. These micro-disruptions happened more in arteries than veins and were associated with points of bifurcation. Second, the dye leaked slowly through apparently intact endothelium. The results demonstrated that the sonications were associated with an almost instantaneous constriction of at least some of the arteries and arterioles followed by the slow leakage of dye molecules through the vessels walls. The smaller (10kD MW)(Alexa Fluor 488, Molecular Probes, Eugene, OR, USA), molecules leaked at a higher rate than the larger (70 kD MW)(dextran-conjugated Texas Red, Molecular Probes, Eugene, OR, USA) molecules which showed a much lower intensity. The vessel diameter relaxed slowly after reaching a minimum during or just after the sonication. It is not known whether or not the constriction was the cause of the BBBD or if it was just an unrelated byproduct of the sonications. The most likely reason for the vessel constriction is mechanical stimulation induced by the radiation force caused by the sonication or by the micro-streaming associated with the bubble oscillations. One potential mechanism for the BBBD could be that the vasoconstriction may reduce the oxygen transfer temporarily but long enough to trigger ischemia related receptors. It is known that ischemia can compromise the BBB [40].
The ultrastructural changes that may be responsible for the BBBD were investigated using electron-microscopy [41,42]. The marker molecules used were immunoglobulin and horseradish peroxidase. These studies identified three main mechanisms: First, rupture of the blood vessel was associated with extravasations of the RBCs. Second, there was an occasional widening of the TJs. This was similar to what was shown by Mesiwala et al. [26] with high frequency ultrasound exposures without micro-bubbles. Third, it was observed that vacuoles were transporting marker molecules through the endothelial cells.
Another study evaluated the impact of the sonications on the molecular integrity of TJs after focused ultrasound disruption of the BBB. Using immunoelectron microscopy, the distribution of TJs-specific trans-membrane proteins occludin, claudin-1, claudin-5, and of sub-membranous ZO-1 was studied at 1, 2, 4, 6 and 24 h after sonications. The protein expression was quantified by counting immunosignals per micrometer of length of junctional clefts. The BBB disruption was demonstrated by the leakage of intravenously injected horseradish peroxidase (m.w. 40,000 Da) and lanthanum chloride (La3+ ~ 139 Da) at 1 h, 2 h and in a few vessels at 4 h after ultrasound application. These changes were paralleled by disintegration of the TJs complexes manifested by redistribution of molecular components and loss of immunosignals for occludin, claudin-5 and ZO-1, while claudin-1 seemed less involved. At 6 and 24 h after the sonication there was no leakage through paracellular clefts and the barrier function of the TJs was completely restored [43] (figure 3).
Figure 3.
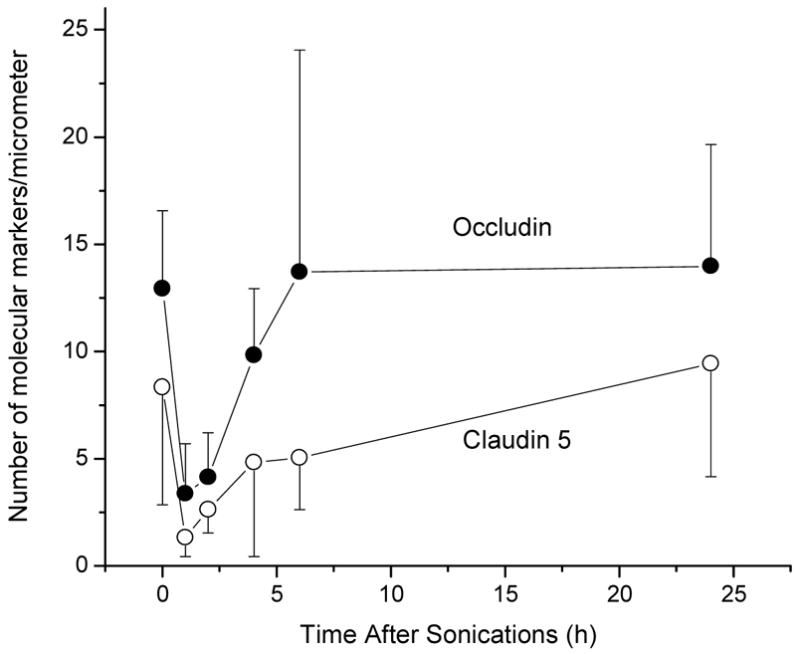
The number of TJ proteins Occludin and Claudin 5 as a function of time before and after sonications of rat brain through intact skull [43].
Further experiments were performed to investigate the trans-endothelial vesicular traffic (figure 4) after ultrasound exposure in the rabbit brain, using ultrastructural morphometry and horseradish peroxidase (HRP) as a tracer. The mean endothelial pinocytotic density (the number of HRP-containing vesicles per μm2 of the cell cytoplasm) was over an order of magnitude higher in the arterioles after sonication than in similar vessels in the unexposed control locations. Conversely, the sonications did not increase the pinocytotic density significantly in the capillaries and only a small number of HRP-positive vesicles were observed in the venules (Table 1.). Therefore the ultrasound exposure-induced trans-cytoplasmic traffic is important only in the pre-capillary microvessels [44].
Figure 4.
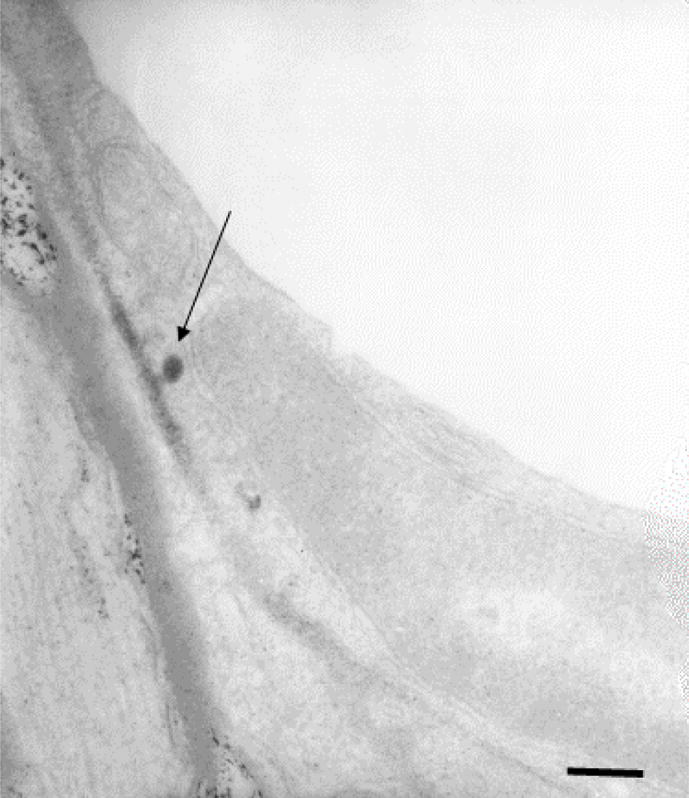
An electron microscopy image of horseradish peroxidase transport through the endothelium showing a vacuole emptying its content after transporting it through the endothelial cell [44]. These rat brain sonications were done through the intact skull.
Table 1.
Mean density (vesicles per μm2) of HRP-containing vesicles in the endothelial cells’ cytoplasm for control and sonicated brain vessels 1h after sonication (mean=/−SD) [44]
| Capillaries | Arterioles | Venules | |
|---|---|---|---|
| Controls | 0.7 ± 0.23 | 0.14 ± 0.03 | 0.17±0.12 |
| Sonicated at 0.69 MHz | 0.92± 0.25 | 1.63 ± 0.23*, † | 0.36±0.10 |
| Sonicated at 0.26 MHz | 1.05 ± 0.20 | 2.43 ± 0.27**,† | 0.55±0.17 |
P<0.05 compared with capillaries at same sonication
P<0.001 compared with capillaries at same sonication
P<0.001 compared with arterioles controls and to venules at same sonication
3.3.3. The influence of exposure parameters on the BBB disruption
The disruption of the BBB with focused ultrasound and micro-bubbles has been investigated extensively using standard commercial MRI agents such as gadopentetate dimeglumine (Magnevist, Berlex Laboratories Inc, Wayne, NJ) (928 MW) that does not penetrate through the BBB. These studies showed that focal disruption of the BBB is feasible at pressure amplitudes that do not induce necrosis, ischemia or apoptosis of the brain tissue (burst length = 10 ms, repetition frequency = 1 Hz, sonication duration= 20 s, rarefactional pressure amplitude = 0.4–0.5 MPa, and frequency =0.69 MHz) [42]. Figure 2 shows the focal enhancement induced in rabbit brain. The amount of contrast enhancement was found to be dependent on the pressure amplitude. However, at higher pressures, the exposures induced tissue damage (figure 5). The frequency of 1.7 MHz with the same burst sonications was associated with a few locations of extravasated RBCs that appeared not to have any long term effect on the brain tissue [45]. At lower frequencies the pressure threshold of the BBB disruption decreased and the number of extravasated RBCs decreased such that at the frequency of 250 kHz the BBB disruption was possible without any extravasated RBC [35]. The disruption of the BBB healed approximately in 6 h after the sonications and only a small signal enhancement was observed with contrast enhanced MRI (figure 6). The imaging studies performed at 2 – 5 days, and 4 weeks after the sonications revealed that the BBB was completely healed [42,45]. These studies were done with only one small molecule contrast agent and the BBBD may be different for different molecules. Similarly a light microscopy study demonstrated intact brain tissue and vasculature at these follow up time points. In order to determine the potential impact of the BBBD on the brain, they were examined with light microscopy and several histology stains. As stated earlier, the only tissue effect that was seen at the pressure amplitude levels close to the threshold of BBBD was occasional extravasations of RBCs. The number of extravasations decreased with decreasing ultrasound frequency and increased with increasing pressure amplitude. For example, at the 1.7 MHz approximately 5 % of the detected vessels had extravasated RBCs. These extravasations appeared to be absorbed by the tissue over the course of four weeks with no detectable adverse reaction. No apoptotic or ischemic tissue regions were observed at these exposure levels. However, when the pressure amplitude was increased, both ischemic and apoptotic cells were detected with the number increasing with the pressure amplitude. A further elevation in the pressure amplitude resulted in tissue necrosis and hemorrhage [46].
Figure 2.
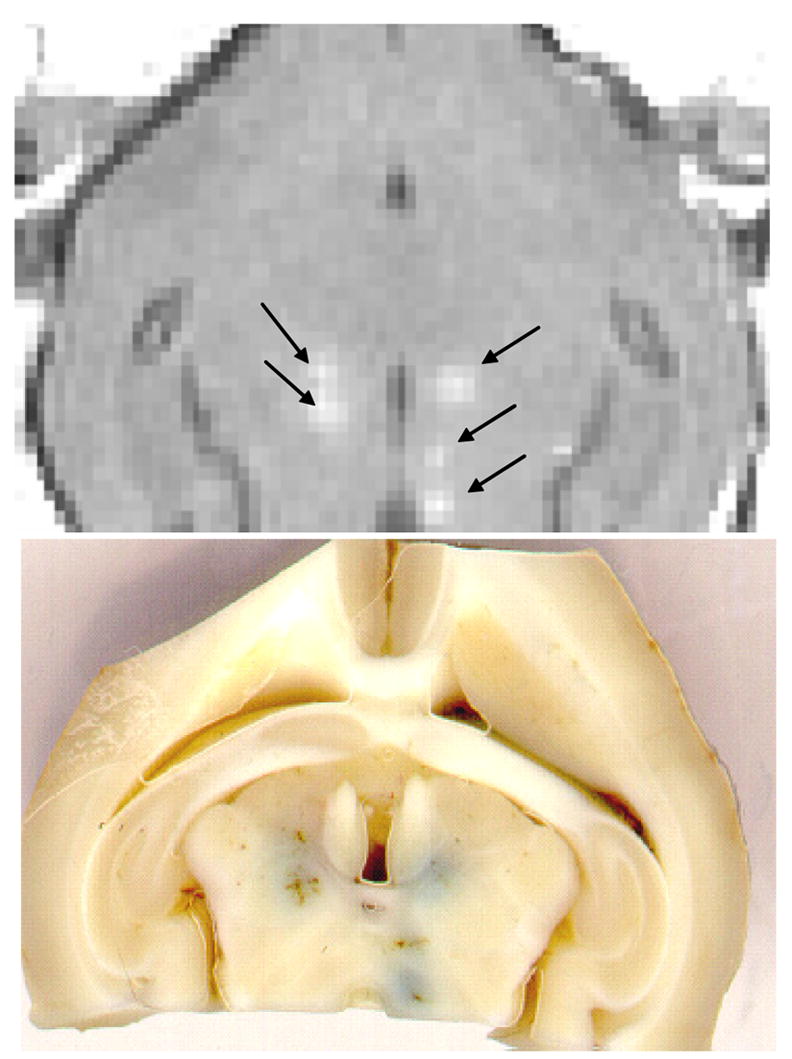
Top: An example of T1-weighted MR image of a rabbit brain obtained after five sonications (arrows) through a cranietomy and an injection of a bolus of gadolinium contrast agent. The image was acquired across the focus of the ultrasound beam and show local contrast enhancement at the sonicated locations. Bottom: The post mortem slice of the same brain showing trypan blue leakage at the sonicated location. The dye was injected a few minutes after the sonications.
Figure 5.
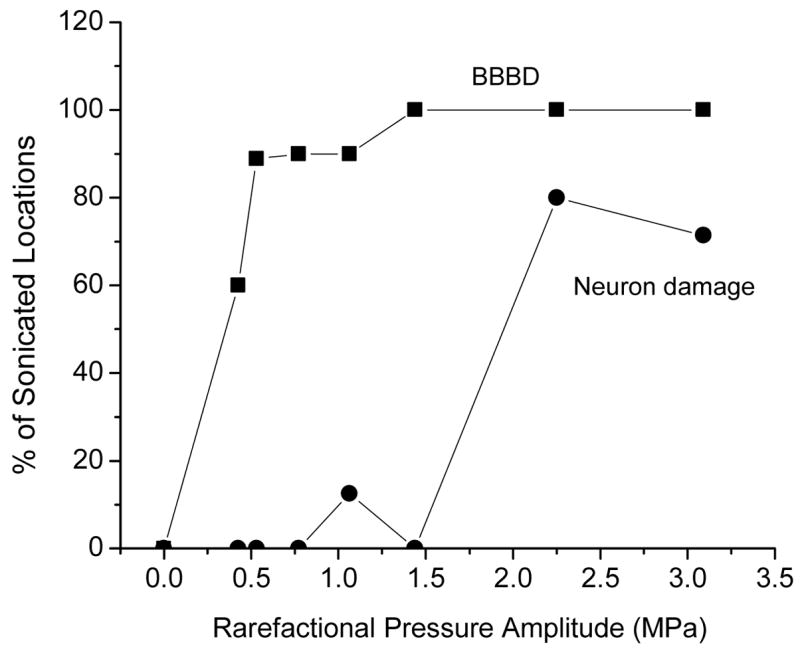
A. The percentage of sonicated locations in rabbit brain that showed contrast enhancement in T1-weighted MR images after sonication as a function the pressure amplitude during the 10 ms ultrasound bursts at the frequency of 0.68MHz. The sonications for this graph were done through a craniotomy window but the study demonstrated that equivalent response can be induced also through intact skull. The percentage of the locations that showed light microscopy evidence of neural damage is also plotted in the graph [42].
Figure 6.
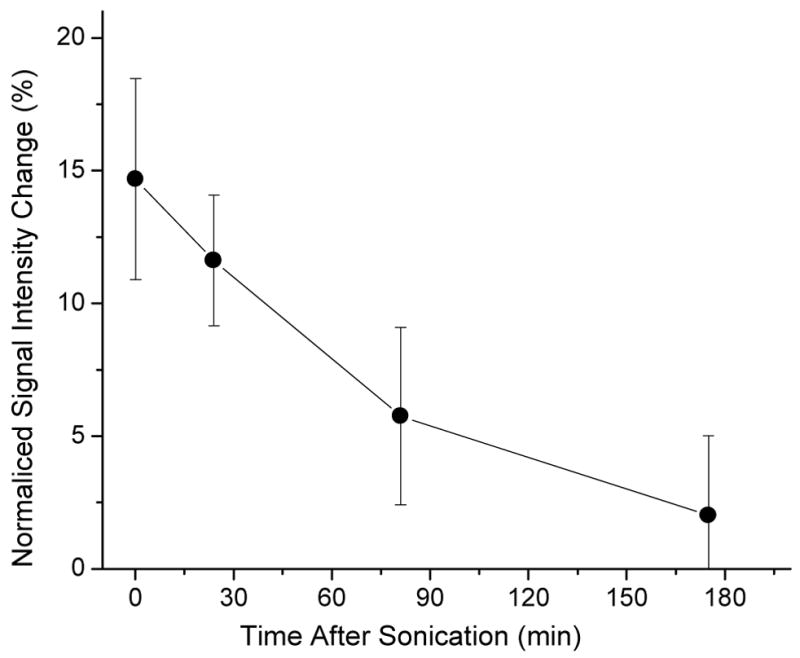
The normalized T1-signal intensity change after injection of gadopentetate dimeglumine contrast agent in rabbit brain as function of time after sonication when the contrast was injected demonstrating the healing of the BBBD [30]. The 10 ms burst sonications at the burst repetition frequency of 1 Hz and at a frequency of 0.26 MHz were done through intact skull.
Both the 50% threshold of the BBBD disruption detected by MRI contrast agent injection and the red blood cell extravasations were found to increase almost linearly as a function of frequency [47,48]. The threshold stayed constant at 10 and 100 ms bursts but increased when the burst length was reduced. An increase in the duration of the exposure has been shown to increase the amount of the contrast agent leaked across the BBB. The burst repetition frequency did not have a large impact on the BBBD, however, the higher repetition frequency of 10 Hz used by Choi et al [49] appeared to require significantly higher pressure amplitudes (approximately 2.5 MPa at the frequency of 1.5 MHz) perhaps indicating dependency on the burst repetition frequency. When exposures similar to Doppler (10 us bursts repeated at the frequency of 1 kHz) were used, the threshold for the BBBD was also higher and always associated with tissue damage [50]. This agrees with a clinical study demonstrating that transcranial Doppler studies (lower pressure amplitude) are not associated with the BBB disruption [51].
The studies investigating the impact of the concentration of the micro-bubbles on the BBBD are inconclusive. These studies were performed with the same burst sonications as stated above. Yang et al [37] demonstrated a linear increase in the BBBD with increased bubble quantity agreeing with Treat et al. [52] who demonstrated higher drug delivery with higher bubble concentration. Conversely, McDannold et al. [48] did not see a significant increase in the BBBD with increasing bubble quantity. It is logical that the BBBD should increase with the number of bubbles, and it may be that the large variation from experiment to experiment seen in the study of McDannold et al masked the trend in their study.
Many of the potential CNS therapies require large molecules and particles to be delivered through the BBB. In order to test the feasibility of such treatments, an intravascular MRI contrast agent MION (20 nm nanoparticulate contrast agent, MION-47, Center for Molecular Imaging Research, MGH, Boston, MA, USA) was used to study the feasibility of these treatments. The experiments showed that the leakage of MION was the largest when the agent was present in the blood during the sonications (burst length = 10 ms, PRF = 1 Hz, sonication duration = 20 s and frequency = 250 kHz) (figure 7). The MION delivery was much diminished when it was injected IV post sonication [30,42]. These experiments, together with the dual-photon and electron microscopy observations, demonstrated that large molecules and particles can be delivered through the BBB when the agent is in the blood during the sonication.
Figure 7.
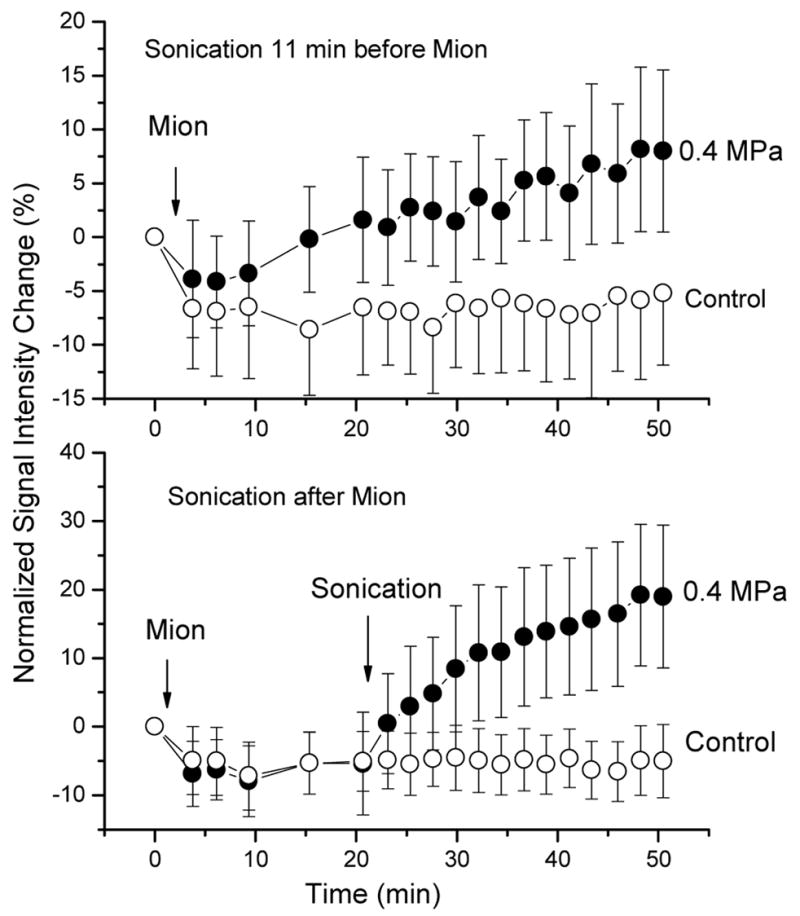
The signal intensity change in the sonicated and the unsonicated control brain locations as a function of time after sonication. The was sonication before (A) and after (B) the contrast agent (MION) injection.
3.3.4. Delivery of antibodies through BBBD
Many potential brain treatments could use antibody based approaches. For example, Herceptin, which is an anti-HER2 monoclonal antibody, could be used for some brain metastasis of breast cancer [53] and the anti-CD20 monoclonal antibody, Rituximab, for malignant lymphoma [54]. There is also evidence suggesting that antibodies against the Aβ may reverse cognitive deficits in early Alzheimer’s disease [55]. There are, however, major problems in using these promising agents in vivo in the CNS because the antibodies have a large molecular size, and thus, they are blocked by the BBB if administered into the circulation.
In order to study the feasibility of using ultrasound (burst length = 10 ms, PRF = 1 Hz, sonication duration = 20 s and frequency = 680 kHz) induced BBB disruption for antibody delivery, the first study [56] used a dopamine-4-receptor-targeting antibody to test the functionality of the antibody after its delivery through the BBB. Post-mortem antibody staining of brain sections of the exposed volume showed positive signals in the brain. When the sections were evaluated under the microscope, the signals detected were characteristic for location of the Dopamin D4 receptors in the hippocampus and small cells in the basal ganglia within the sonicated tissue volume. The staining was limited to the sonicated location with no obvious signal in the contra lateral site. The staining intensity correlated with the MRI contrast enhancement seen after the sonications, indicating that the MRI signal may be useful in quantifying and controlling the antibody delivery in the brain.
The second study investigated a delivery of the antibody-based therapy agent Herceptin with the same sonication parameters and demonstrated that it can be delivered through the BBB [57]. Although much more work needs to be done to show that adequate quantities of the antibody based agents can be delivered into the brain, the initial studies show promise.
3.3.5. Delivery of Chemotherapy agents through the BBB
Targeted BBBD could potentially aid in the delivery of chemotherapy agents in brain tumors [58]. The first study investigated the delivery of liposome-encapsulated doxorubicin which does not penetrate the BBB and demonstrated that increased concentrations were detected in the sonicated (burst length = 10 ms, PRF = 1 Hz, sonication duration = 120s and frequency = 1.5 or 1.7 MHz) locations of rat brains [52]. The sonicated locations showed significantly higher concentrations of doxorubicin than the contra-lateral side. The concentration of the drug in the brain tissue was observed to increase linearly with increasing micro-bubble concentration with approximately 1000 ng/g of tissue at clinical diagnostic micro-bubble concentration of 0.1 ml/kg. The measured doxorubicin concentrations should be adequate to produce a clinically significant response [59] if they can be produced in human tumors. Although the treatment looks promising, the effectiveness and side-effects of the treatment in an animal brain tumor model needs to be investigated before the clinical potential can be explored.
3.3.6. Gene delivery to the brain
Gene therapy could have significant potential in the treatment of various CNS diseases [60] but the delivery into the brain is limited by the large size of the agents. It is likely that the focused ultrasound method could be used to deliver gene vectors through the BBB. So far there is only one preliminary study that demonstrated feasibility [44]. That study used genetically engineered Herpes Simplex Virus (HSV) that was radiolabeled using 111Indium-oxine, and injected intravenously into the animals after focused ultrasound BBB disruption on one side of the brain. The brains were harvested 24 hours after injection of the virus, and subjected to serial sectioning. Autoradiography and histology were performed on the sections and there was a clear Right-Left difference in the brains at the sites of the sonications, indicating focal viral delivery. Serial autoradiographs demonstrated the viral distribution in each of the locations. ROI analysis showed a maximal R/L ratio of 3.56 for a 2.3 MPa location, and 1.74 for a 1 MPa location at the frequency of 1.63 MHz with 100ms bursts repeated at the rate of 1 Hz for 20s.
4. Ultrasound aided delivery of molecules to other neural tissues
Other neural tissues such as spinal cords are also protected by endothelial cells with TJs similar to the brain. Although the spinal cord is protected by a more variable bony structure than the brain it is likely that the same methods could be used to disrupt the BBB in this structure. We are currently performing a preliminary study investigating large molecular agent delivery into the spinal cord. The initial experiments demonstrate feasibility, but much more work needs to be done for exposure optimization.
5. Future potential of ultrasound in agent delivery to the brain
The development of a method that can induce focal and transient disruption of the BBB without permanent tissue damage may make image-guided targeted molecular therapeutic and imaging agent delivery feasible in the brain. For example, advances in tumor cell biology have led to the availability of new types of anti-cancer chemotherapeutic agents that are superior to the conventional agents in that they can precisely target the signal-transduction system unique to malignant tumor cells, thereby lowering their toxic effects on normal cells. Herceptin® (Trastuzumab) is a humanized monoclonal antibody (mAb) that targets human epidermal growth-factor receptor 2 (HER2/c-erbB2) expressed in the breast cancer cells of some patients. Herceptin® has been used to treat breast cancer patients and succeeded remarkably in controlling local and distal breast cancer lesions [53]. Although breast cancer often metastasizes to the brain [61], Herceptin® could only be used to treat extracranial lesions because there is currently no efficient method to deliver it to the CNS. The increased use of Herceptin® to treat breast cancer patients has resulted in a higher incidence of brain metastasis from primary lesions[62,63]. When Herceptin® was used as a first-line therapy in breast cancer patients, metastatic extracranial lesions responded to the agent in 71% of the patients who continued to develop metastatic lesions in the brain [62]. If this method can be implemented clinically it could have significant application in treating patients with metastatic brain disease. There are also other effective anti-cancer agents that do not penetrate the BBB. For example, Rituxan® (Rituximab), an anti-CD20 mAb, has also been shown to be effective in patients with lymphoma [54]. The ability to aid the delivery of these new agents through the BBB could have a large impact in health care due to the large number of patients that may benefit from these new treatments but who eventually develop brain metastases. This is just one example of the potential of image-guided, focused, ultrasound-induced BBB disruption, and we believe that there are many other potential uses for this technology after it has been clinically tested for the tumor treatments.
6. Conclusions
A number of animal studies have demonstrated that local BBBD is possible with burst focused ultrasound exposures and intravascular micro-bubbles. The required acoustic power values are over 100 times smaller than those required to produce thermal damage in tissue and are thus safe to deliver through intact skull. Two major mechanisms have been identified for the disruption including opening of the TJs and active transport by vacuoles. BBBD is associated with minimal or no damage to the vasculature or the surrounding brain tissue. Survival studies have verified the lack of adverse events in test animals. Focal delivery of antibodies and chemotherapy agents has been shown. However, the quantity of brain uptake and the therapeutic effectiveness of drug delivery for a specific intervention has to be demonstrated in appropriate models before the clinical potential can be determined.
Acknowledgments
Sources of Support: NIH (grant numbers EB00705 and EB003268), Terry Fox Foundation, and CRCP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Mol Med Today. 1996;2:106–113. doi: 10.1016/1357-4310(96)88720-x. [DOI] [PubMed] [Google Scholar]
- 2.Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42:1083–1099. doi: 10.1097/00006123-199805000-00082. [DOI] [PubMed] [Google Scholar]
- 3.Johansson BB. The physiology of the blood-brain barrier. Adv Exp Med Biol. 1990;274:25–39. doi: 10.1007/978-1-4684-5799-5_2. [DOI] [PubMed] [Google Scholar]
- 4.Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- 5.Nag S. Morphology and molecular properties of cellular components of normal cerebral vessels. Methods Mol Med. 2003;89:3–36. doi: 10.1385/1-59259-419-0:3. [DOI] [PubMed] [Google Scholar]
- 6.Doolittle ND, Miner ME, Hall WA, Siegal T, Jerome E, Osztie E, McAllister LD, Bubalo JS, Kraemer DF, Fortin D, Nixon R, Muldoon LL, Neuwelt EA. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 2000 Feb. 1;88(3):637–47. doi: 10.1002/(sici)1097-0142(20000201)88:3<637::aid-cncr22>3.0.co;2-y. 88 637–647. [DOI] [PubMed] [Google Scholar]
- 7.Bakay L, Hueter TF, Ballantine HT, Sosa D. Ultrasonically produced changes in the blood-brain barrier. Arch Neurol. 1956;76:457–467. doi: 10.1001/archneurpsyc.1956.02330290001001. [DOI] [PubMed] [Google Scholar]
- 8.Bakay L, Ballantine HT, Bell E. P32 uptake by normal and ultrasonically irradiated brain tissue from cerebrospinal fluid. Arch Neurol. 1959;1:59–67. doi: 10.1001/archneur.1959.03840010061007. [DOI] [PubMed] [Google Scholar]
- 9.BALLANTINE HT, Jr, Bell E, MANLAPAZ J. Progress and problems in the neurological applications of focused ultrasound. J Neurosurg. 1960;17:858–876. doi: 10.3171/jns.1960.17.5.0858. [DOI] [PubMed] [Google Scholar]
- 10.Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron. 1960;ME-7:166–181. doi: 10.1109/iret-me.1960.5008041. [DOI] [PubMed] [Google Scholar]
- 11.Heimburger RF. Ultrasound augmentation of central nervous system tumor therapy. Indiana Medicine. 1985;78:469–476. [PubMed] [Google Scholar]
- 12.Guthkelch AN, Carter LP, Cassady JR, Hynynen K, Iacono RP, Johnson PC, Obbens EAMT, Roemer RB, Seeger JF, Shimm DS, Stea B. Treatment of malignant braintumors with focussed ultrasound hyperthermia and radiation:Results of a phase I trial. J Neuro-Oncology. 1991;10:271–284. doi: 10.1007/BF00177540. [DOI] [PubMed] [Google Scholar]
- 13.Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through intact skull. Ultrasound Med Biol. 1998;24:275–283. doi: 10.1016/s0301-5629(97)00269-x. [DOI] [PubMed] [Google Scholar]
- 14.Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol. 2002;47:1219–1236. doi: 10.1088/0031-9155/47/8/301. [DOI] [PubMed] [Google Scholar]
- 15.Aubry JF, Tanter M, Pernot M, Thomas JL, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am. 2003;113:84–93. doi: 10.1121/1.1529663. [DOI] [PubMed] [Google Scholar]
- 16.Hynynen K, Clement GT, McDannold N, Vykhodtseva N, King R, White PJ, Vitek S, Jolesz FA. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: A preliminary rabbit study with ex vivo human skulls. Magn Reson Med. 2004;52:100–107. doi: 10.1002/mrm.20118. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340:1085–1090. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 18.Pernot M, Aubry JF, Tanter M, Boch AL, Marquet F, Kujas M, Seilhean D, Fink M. In vivo transcranial brain surgery with an ultrasonic time reversal mirror. J Neurosurg. 2007;106:1061–1066. doi: 10.3171/jns.2007.106.6.1061. [DOI] [PubMed] [Google Scholar]
- 19.Hynynen K, Clement G. Clinical applications of focused ultrasound-the brain. Int J Hyperthermia. 2007;23:193–202. doi: 10.1080/02656730701200094. [DOI] [PubMed] [Google Scholar]
- 20.Yin X, Hynynen K. A numerical study of transcranial focused ultrasound beam propagation at low frequency. Phys Med Biol. 2005;50:1821–1836. doi: 10.1088/0031-9155/50/8/013. [DOI] [PubMed] [Google Scholar]
- 21.Clement GT, White PJ, Hynynen K. Enhanced ultrasound transmission through the human skull using shear mode conversion. J Acoust Soc Am. 2004;115:1356–1364. doi: 10.1121/1.1645610. [DOI] [PubMed] [Google Scholar]
- 22.Patrick JT, Nolting MN, Goss SA, Dines KA, Clendenon JL, Rea MA, Heimburger RF. Ultrasound and the blood-brain barrier. Adv Exp Med Biol. 1990;267:369–381. doi: 10.1007/978-1-4684-5766-7_36. [DOI] [PubMed] [Google Scholar]
- 23.McDannold N, Vykhodtseva N, Jolesz FA, Hynynen K. MRI investigation of the threshold for thermally induced blood-brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med. 2004;51:913–923. doi: 10.1002/mrm.20060. [DOI] [PubMed] [Google Scholar]
- 24.Ng KY, Cho CW, Henthorn TK, Tanguay RL. Effect of heat preconditioning on the uptake and permeability of R123 in brain microvessel endothelial cells during mild heat treatment. J Pharm Sci. 2004;93:896–907. doi: 10.1002/jps.20015. [DOI] [PubMed] [Google Scholar]
- 25.Vykhodtseva NI, Hynynen K, Damianou C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med Biol. 1995;21:969–979. doi: 10.1016/0301-5629(95)00038-s. [DOI] [PubMed] [Google Scholar]
- 26.Mesiwala AH, Farrell L, Wenzel HJ, Silbergeld DL, Crum LA, Winn HR, Mourad PD. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med Biol. 2002;28:389–400. doi: 10.1016/s0301-5629(01)00521-x. [DOI] [PubMed] [Google Scholar]
- 27.Reinhard M, Hetzel A, Kruger S, Kretzer S, Talazko J, Ziyeh S, Weber J, Els T. Blood-brain barrier disruption by low-frequency ultrasound. Stroke. 2006;37:1546–1548. doi: 10.1161/01.STR.0000221813.27519.0b. [DOI] [PubMed] [Google Scholar]
- 28.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 29.Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105:445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 31.Dayton P, Klibanov A, Brandenburger G, Ferrara K. Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles. Ultrasound Med Biol. 1999;25:1195–1201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 32.Apfel RE. Acoustic cavitation: A possible consequence of biomedical use of ultrasound. Br J Cancer. 1995;V(Suppl):140–146. [PMC free article] [PubMed] [Google Scholar]
- 33.Crum LA, Fowlkes JB. Acoustic cavitation generated by microsecond pulses of ultrasound. Nature. 1986;319:52–54. [Google Scholar]
- 34.Margulis MA. Sonochemistry of cavitation. Gordon and Breach Publishers; Luxembourg: 1995. [Google Scholar]
- 35.McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006;51:793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- 36.McDannold N, Vykhodtseva N, Hynynen K. Use of ultrasound pulses combined with definity for targeted blood-brain barrier disruption: a feasibility study. Ultrasound Med Biol. 2007;33:584–590. doi: 10.1016/j.ultrasmedbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang FY, Fu WM, Yang RS, Liou HC, Kang KH, Lin WL. Quantitative evaluation of focused ultrasound with a contrast agent on blood-brain barrieer disruption. Ultrasound Med Biol. 2007 doi: 10.1016/j.ultrasmedbio.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Mychaskiw G, Badr AE, Tibbs R, Clower BR, Zhang JH. Optison (FS069) disrupts the blood-brain barrier in rats. Anesth Analg. 2000;91:798–803. doi: 10.1097/00000539-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Raymond SB, Skoch J, Hynynen K, Bacskai BJ. Multiphoton imaging of ultrasound/Optison mediated cerebrovascular effects in vivo. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600336. [DOI] [PubMed] [Google Scholar]
- 40.Lo EH, Pan Y, Matsumoto K, Kowall NW. Blood-brain barrier disruption in experimental focal ischemia: comparison between in vivo MRI and immunocytochemistry. Magn Reson Imaging. 1994;12:403–411. doi: 10.1016/0730-725x(94)92533-x. [DOI] [PubMed] [Google Scholar]
- 41.Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol. 2004;30:979–989. doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. NeuroImage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 43.Sheikov N, McDannold N, Sharma S, Hynnen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound in Med Biol. 2007 doi: 10.1016/j.ultrasmedbio.2007.12.015. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheikov N, McDannold N, Jolesz F, Zhang YZ, Tam K, Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med Biol. 2006;32:1399–1409. doi: 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 45.McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med Biol. 2005;31:1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 46.McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med Biol. 2005;31:1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 47.McDannold N, Vykhodtseva NI, Hynynen K. Blood-brain barrier disruption induced by focused ultrasound appears to be characterized by the mechanical index. Ultrasound in Med Biol. 2007 doi: 10.1016/j.ultrasmedbio.2007.10.016. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDannold NJ, Vykhodtseva N, Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption. Ultrasound in Med Biol. 2007 doi: 10.1016/j.ultrasmedbio.2007.11.009. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi JJ, Pernot M, Small SA, Konofagou EE. Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med Biol. 2007;33:95–104. doi: 10.1016/j.ultrasmedbio.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 50.Hynynen K, McDannold N, Martin H, Jolesz FA, Vykhodtseva N. The threshold for brain damage in rabbits induced by bursts of ultrasound in the presence of an ultrasound contrast agent (Optison) Ultrasound Med Biol. 2003;29:473–481. doi: 10.1016/s0301-5629(02)00741-x. [DOI] [PubMed] [Google Scholar]
- 51.Schlachetzki F, Holscher T, Koch HJ, Draganski B, May A, Schuierer G, Bogdahn U. Observation on the integrity of the blood-brain barrier after microbubble destruction by diagnostic transcranial color-coded sonography. J Ultrasound Med. 2002;21:419–429. doi: 10.7863/jum.2002.21.4.419. [DOI] [PubMed] [Google Scholar]
- 52.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121:901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 53.Baselga J. Clinical trials of single-agent trastuzumab (Herceptin) Semin Oncol. 2000;27:20–26. [PubMed] [Google Scholar]
- 54.Victorov IV, Prass K, Dirnagl U. Improved selective, simple, and contrast staining of acidophilic neurons with vanadium acid fuchsin. Brain Res Brain Res Protoc. 2000;5:135–139. doi: 10.1016/s1385-299x(00)00004-0. [DOI] [PubMed] [Google Scholar]
- 55.Hall WA, Doolittle ND, Daman M, Bruns PK, Muldoon L, Fortin D, Neuwelt EA. Osmotic blood-brain barrier disruption chemotherapy for diffuse pontine gliomas. J Neurooncol. 2006;77:279–284. doi: 10.1007/s11060-005-9038-4. [DOI] [PubMed] [Google Scholar]
- 56.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340:1085–1090. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 57.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Treat LH, McDannold N, Hynynen K. Transcranial MRI-Guided Focused Ultrasound-Induced Blood-Brain Barrier Opening in Rats. 2004. pp. 998–1000. [Google Scholar]
- 59.Cummings J, McArdle CS. Studies on the in vivo disposition of adriamycin in human tumours which exhibit different responses to the drug. Br J Cancer. 1986;53:835–838. doi: 10.1038/bjc.1986.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fulci G, Chiocca EA. The status of gene therapy for brain tumors. Expert Opin Biol Ther. 2007;7:197–208. doi: 10.1517/14712598.7.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Markesbery WR, Brooks WH, Gupta GD, Young AB. Treatment for patients with cerebral metastases. Arch Neurol. 1978;35:754–756. doi: 10.1001/archneur.1978.00500350058012. [DOI] [PubMed] [Google Scholar]
- 62.Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, Bunnell C, Rue M, Gelman R, Winer E. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 63.Clayton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, Wilkinson PM, Welch RS, Magee B, Wilson G, Howell A, Wardley AM. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91:639–643. doi: 10.1038/sj.bjc.6601970. [DOI] [PMC free article] [PubMed] [Google Scholar]


