Abstract
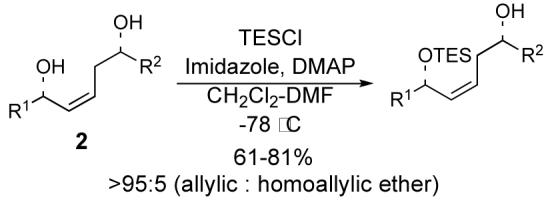 Treatment of unsaturated 1,5-diols 2 with TES-Cl (1.1 equiv), imidazole, and catalytic DMAP in 1 : 1 CH2Cl2-DMF at −78 °C effects selective silylation of the allylic alcohol with >95 : 5 chemoselectivity when the allylic and homoallylic alcohols are in similar steric environments.
Treatment of unsaturated 1,5-diols 2 with TES-Cl (1.1 equiv), imidazole, and catalytic DMAP in 1 : 1 CH2Cl2-DMF at −78 °C effects selective silylation of the allylic alcohol with >95 : 5 chemoselectivity when the allylic and homoallylic alcohols are in similar steric environments.
Our laboratory has reported a one-pot double allylboration reaction involving the sequential reaction of two aldehydes with the γ-boryl-substituted allylboranes 1 and 3 which provides (Z)-1,5-syn-diols 2 and (E)-1,5-anti-diols 4, respectively, with excellent diastereo- and enantioselectivity (Figure 1).1 In order to apply this method to the synthesis of structurally complex targets, including natural products, it is sometimes necessary to differentiate the two alcohols that result from the one-pot double allylboration reaction.2 We have previously reported that the allylic alcohol unit of 2 can be selectively protected with modest chemoselectivity as TBS ethers.3 We have examined this reaction in greater depth and report herein that optimal selectivity (≥95 : 5) is achieved by treating sterically unbiased diols 2 and 4 with triethylsilyl chloride (TES-Cl), imidazole, and catalytic DMAP in 1:1 CH2Cl2-DMF at −78 °C. This selective silylation of the allylic alcohol is attributed to subtle differences in the steric environment surrounding the two hydroxyl groups.
Figure 1.

Synthesis of (Z)-1,5-syn-diols and (E)-1,5-anti-diols.
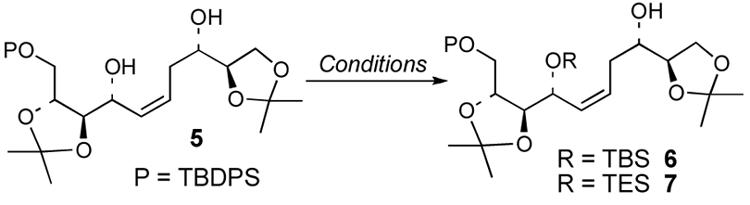
While examples are known of the selective silylation of a secondary allylic alcohol in the presence of a saturated secondary alcohol, the majority of cases involve sterically hindered or geometrically biased substrates in which the allylic alcohol undergoes preferential silylation.4,5 Relatively few examples of chemoselective silylation of allylic alcohols in the presence of saturated alcohols on substrates that lack a steric bias have been reported.3,6 With this background in mind, we examined the selective protection of diol 52a (Table 1). Examination of reaction conditions previously applied to sterically unbiased substrates (TBS-Cl, imidazole, DMAP, rt)3 provided a 39% yield of the mono-TBS ether consisting of an 86:14 mixture of allylic silyl ether 6 and the corresponding homoallylic silyl ether (entry 1). After exploring other conditions,7 we found the yield and chemoselectivity of silylation could be improved though the use of the more reactive silylating reagent, TES-Cl, which allowed these reactions to proceed at lower reaction temperatures. Optimal reaction conditions involved treatment of 5 with 1.1 equiv of TES-Cl, imidazole (1.15 equiv), and DMAP (0.05 equiv) in 1 : 1 CH2Cl2-DMF at −78 °C (entry 3).8
Table 1.
Optimization of Selective Silylation Conditions
 | |||||
|---|---|---|---|---|---|
| entry | conditionsa | yield | allylic: homoallylic |
bis-TES ether |
recovered 1,5-diol |
| 1 | TBSCl (1.05 equiv) Imidazole, DMAP CH2Cl2-DMF rt, 2 d |
39% | 86:14 | NA | 58% |
| 2 | TESCl (1.05 equiv) Imidazole, DMAP CH2Cl2-DMF 0 °C, 2 h |
65% | >95:5 | 7% | 8% |
| 3 | TESCl (1.1 equiv) Imidazole, DMAP CH2Cl2-DMF −78 °C, 2 h |
81% | >97:3 | 14% | NA |
1.15 equiv imidazole, 0.05 equiv DMAP
The optimal silylation conditions defined for 5 provided high levels of selecitivity for allylic alcohol silylation with other unsaturated (Z)-1,5-syn-diols (Table 2).9 Pseudosymmetrical 1,5-diols 81 and 106 which contain the same R1 and R2 substituents were silylated with excellent levels of chemoselectivity, providing nearly exclusive formation of the allylic silyl ether (entries 1 and 2). With substrates 10 and 12 (Entries 2 and 3) that have increased steric bulk around the 1,5-diol core, improved yields of the allylic monosilyl ethers 11 and 13 were obtained since formation bis-silyl ether product was suppressed (≤6% yield). The selective protection of dienylic alcohol 14, an intermediate in the synthesis of amphidinol 3,2b proceeds in good yield and chemoselectivity (76%, >97:3, entry 4).
Table 2.
Selective Protection of (Z)-1,5-syn-diols
| entry | substrate (R = H) |
producta (R = SiEt3) |
yieldb,c | allylic: homoallylic |
|---|---|---|---|---|
| 1 | 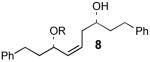 |
9 | 61% | >97:3 |
| 2 |  |
11 | 71% | >97:3 |
| 3 | 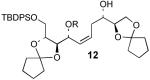 |
13 | 79% | >97:3 |
| 4 | 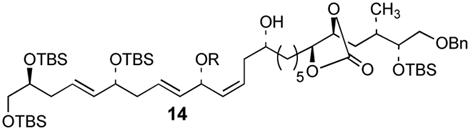 |
|||
| 15 | 76% | >97:3 | ||
| 5 | 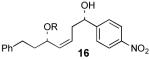 |
17 | 68% | 96:4 |
Reactions were performed by treating a solution of the 1,5-diol (1 equiv), imidazole (1.15 equiv), and DMAP (0.05 equiv) in CH2Cl2-DMF (1:1, 0.1 M) at −78 °C with TES-Cl (1.1 equiv)
Combined yield of mono-TES ethers (allylic and homoallylic)
bis-TES ethers were obtained as follows: entry 1, 17%; entry 2, 6%; entry 3, not isolated; entry 4, 12%; entry 5, 9% yield.
The scope of this selective protection reaction is not limited to (Z)-1,5-syn-diols, as (E)-1,5-anti-diols can also be monosilylated with good chemoselectivity (Table 3). Therefore, the selectivity for allylic alcohol silylation is not due to conformational effects specific to (Z)-1,5-syn-diols, e.g., deriving from A−1,3 interactions,10 or to an intramolecular hydrogen bonding network.11 Substrates 18 and 20 containing similar steric environments surrounding the allylic and homoallylic positions provided allylic silyl ethers 19 and 21 with ≥95:5 chemoselectivity. Increasing the size of the homoallylic subsutituent relative to the allylic subsutituent (22, entry 3) led to an improved yield of the allylic TES ether as less bis-silylation product was generated (2%). Increasing the size of the group neighboring the allylic alcohol, relative to the subsutituent adjacent to the homoallylic alcohol, as in 24, led to decreased selectivity for allylic alcohol protection, although interestingly, silylation of this position was still favored (84:16, entry 4). Modification the sterics and electronics of the system by introduction of an ester at the homoallylic position (26) led to a decrease selectivity to for silylation of the allylic alcohol (77:22, entry 5).
Table 3.
Selective Protection of (E)-1,5-anti-diols
| entry | substrate (R = H) |
producta (R = SiEt3) |
yieldb,c | allylic: homoallylic |
|---|---|---|---|---|
| 1 | 19 | 66% | 95:5 | |
| 2 | 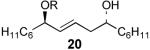 |
21 | 66% | >97:3 |
| 3 | 23 | 79% | >97:3 | |
| 4 | 25 | 44% | 84:16 | |
| 5 |  |
27 | 58% | 77:22 |
Reactions were performed by treating a solution of the 1,5-diol (1 equiv), imidazole (1.15 equiv), and DMAP (0.05 equiv) in CH2Cl2-DMF (1:1, 0.1 M) at −78 °C with TES-Cl (1.1 equiv)
Combined yield of mono-TES esters (allylic and homoallylic)
bis-TES ethers were obtained as follows: entry 1, 20%; entry 2, 2%, entry 3, 2%, entry 4, 16%, entry 5, 16%.
We considered two hypotheses to account for the selective allylic alcohol silylation presented in Tables 2 and 3. The first hypothesis focused on the differences in acidities between allylic and homoallylic alcohols. Generally, the pKa of alcohols that are adjacent to an alkene or alkyne are lower than the corresponding saturated alcohols due to the increased electronegativity of the unsaturated substituents.12 The increased acidity of the allylic alcohol could potentially facilitate hydrogen bonding to the base, thereby increasing the nucleophilicity of the allylic alcohol relative to the homoallylic alcohol. The second hypothesis focused on the subtle differences in the steric environment surrounding the allylic and homoallylic alcohols—namely the additional hydrogen atom flanking the homoallylic position. This hypothesis was initially less appealing because it did not appear to account for the allylic selective silylation of (E)-1,5-anti-diol 24 (Table 3), which contained a more sterically demanding cyclohexyl ring adjacent to the allylic alcohol.
We decided to investigate the first hypothesis by adjusting the acidities of the allylic and homoallylic alcohols. To probe the effect of changing the pKa of the two hydroxyl groups, a series of substrates were synthesized that contained similar steric environments but differing electronic environments (Table 4). Silylation of 1,5-diol 28 containing phenyl groups in both the R1 and R2 positions provided an 88:12 mixture of regioisomers at room temperature. The placement of an electron-withdrawing group on the aryl ring neighboring the homoallylic alcohol as in 30 and 32 (entries 2 and 3, Table 4) was predicted to decrease the amount of allylic alcohol silylation due to the increased acidity of the homoallylic alcohol.13 However, TES protection of 30 and 32 resulted in increased allylic alcohol protection, providing 31 and 33 with 95:5 and 94:6 selectivity, respectively (at room temperature). The installment of an electron-withdrawing group on the aryl ring neighboring the allylic alcohol as in entry 4 (Table 4, entry 4) resulted in a decrease in allylic selectivity, lowering the allylic:homoallylic silyl ether ratio to nearly 50:50. These results contradict the hypothesis that the greater acidity of the allylic alcohol results in preferential silylation. Rather, these results suggest instead that electron-withdrawing groups neighboring an alcohol affect selectivity by decreasing the nucleophilicity of that position (Table 4).14,15
Table 4.
Protection of Electronically Biased Substrates at Room Temperature
| entry | substrate (R = H) |
producta (R = SiEt3) |
yieldb | allylic: homoallylic |
|---|---|---|---|---|
| 1 |  |
29 | 55% | 88:12 |
| 2 | 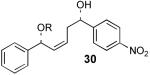 |
31 | 48% | 95:5 |
| 3 | 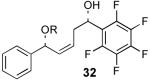 |
33 | 62% | 94:6 |
| 4 | 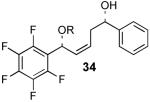 |
35 | 43% | 54:46 |
Reactions were performed by treating a solution of the 1,5-diol (1 equiv), imidazole (1.15 equiv), and DMAP (0.05 equiv) in CH2Cl2-DMF (1:1, 0.1 M) at rt with TES-Cl (1.1 equiv)
Combined yield of mono-TES ethers (allylic and homoallylic)
Data summarized in Table 5 for silylation of 8 with a series of trialkylsilyl chlorides are consistent with the second hypothesis that selective silylation of the allylic alcohol derives from the fact that the olefin adjacent to the allyic alcohol is less sterically demanding than the methylene group neighboring the homoallylic alcohol. The results summarized in Table 5 show the ratio of allylic to homoallylic silyl ether improves as the size and steric demands of the silyl chloride is increased.
Table 5.
Variation of Silyl Chloride
 | ||||
|---|---|---|---|---|
| silyl chloridea |
bis-silyl | mono-silyl 9a-e |
allylic: homoallylic |
recov. SM |
| TMS | 27% | 26% | 78:22 | 19% |
| TESCl | 16% | 59% | 89:11 | 14% |
| TBSCl | 8% | 55% | 89:11 | 33% |
| TBDPSCl | 12% | 53% | 92:8 | 33% |
| TIPS | 5% | 54% | 94:6 | 27% |
Reactions were performed by treating a solution of the 1,5-diol (1 equiv), imidazole (1.15 equiv), and DMAP (0.05 equiv) in CH2Cl2-DMF (1:1, 0.1 M) at room temperature with silyl chloride (1.1 equiv)
We also investigated the relationship between the stoichiometry of triethylsilyl chloride and the yield and chemoselectivity of the selective silylation reaction (Table 6). We observed that increasing the equivalents of TES-Cl in the reaction led to an increase in the ratio of allylic:homoallylic silyl ether products, from 94:6 when 0.1 equiv of TES-Cl is used (entry 1) to 98:2 when 1.1 equiv of TES-Cl is used (entry 6). These data imply that some of the initially formed homoallylic mono-TES ether undergoes a second silylation to give the 1,5-bis-protected ether 40 somewhat faster than the second silylation of homoallylic ether 9. It is quite clear from the data in Table 6 that homoallylic TES ether 9 is formed with excellent selectivity even at short conversion.
Table 6.
Effect of Increasing Equiv of TES-Cl on Ratio of Allylic:Homoallylic Ethers.
 | ||||
|---|---|---|---|---|
| equiv TESCl |
bis-TES 40 |
mono-TES 9 |
allylic: homoallylica |
recov. SM 8 |
| 0.10 | - | 6% | 94:6 | ND |
| 0.25 | - | 16% | 94:6 | ND |
| 0.50 | 2% | 35% | 95:5 | ND |
| 0.75 | 6% | 54% | 95:5 | 30% |
| 0.90 | 4% | 57% | 96:4 | 25% |
| 1.1 | 17% | 61% | 98:2 | 10% |
Allylic:homoallylic TES ether ratio determined by NMR spectroscopy (integration of homoallylic protons; S/N > 200:1, relaxation delay (d1) of ≥6 sec).
In conclusion, the selective protection of the allylic alcohol unit of unsaturated 1,5-diols 2 and 4 proceeds with high selectivity for substrates in which the allylic and homoallylic alcohols are in otherwise similar steric environments. These reactions are optimally executed at low temperatures using TES-Cl as the silylating agent, and provide the allylic TES ethers in good yields (typically 61-81%). The decreased steric environment surrounding the allylic alcohol relative to that surrounding the homoallylic alcohol is invoked to rationalize the selective allylic alcohol silylation described herein.
Supplementary Material
Acknowledgment
This work was supported by the National Institutes of Health (GM038436).
Footnotes
Supporting Information Available Experimental procedures and tabulated spectroscopic data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Flamme EM, Roush WR. J. Am. Chem. Soc. 2002;124:13644. doi: 10.1021/ja028055j. [DOI] [PubMed] [Google Scholar]
- 2.(a) Hicks JD, Flamme EM, Roush WR. Org. Lett. 2005;7:5509. doi: 10.1021/ol052322j. [DOI] [PubMed] [Google Scholar]; (b) Flamme EM, Roush WR. Org. Lett. 2005;7:1411. doi: 10.1021/ol050250q. [DOI] [PubMed] [Google Scholar]; (c) Lira R, Roush WR. Org. Lett. 2007;9:533. doi: 10.1021/ol0629869. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Owen RM, Roush WR. Org. Lett. 2005;7:3941. doi: 10.1021/ol0514303. [DOI] [PubMed] [Google Scholar]
- 3.Flamme EM, Roush WR. Beilstein J. Org. Chem. 2005;1:7. doi: 10.1186/1860-5397-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Chen S-H, Wei J-M, Vyas DM, Doyle TW, Farina V. Tetrahedron Lett. 1993;34:6845. [Google Scholar]; (b) Wyatt PG, Coomber BA, Evans DN, Jack TI, Fulton HE, Wonacott AJ, Colman P, Varghese J. Bioorg. Med. Chem. Lett. 2001;11:669. doi: 10.1016/s0960-894x(01)00019-1. [DOI] [PubMed] [Google Scholar]; (c) Kaneko M, Nakashima T, Uosaki Y, Hara M, Ikeda S, Kanda Y. Bioorg. Med. Chem. Lett. 2001;11:887. doi: 10.1016/s0960-894x(01)00094-4. [DOI] [PubMed] [Google Scholar]; (d) Horiguchi T, Oritani T, Kiyota H. Tetrahedron. 2003;59:1529. [Google Scholar]; (d) Nicolaou KC, Koftis TV, Vyskocil S, Petrovic G, Ling T, Yamada YMA, Tang W, Frederick MO. Angew. Chem. Int. Ed. 2004;43:4318. doi: 10.1002/anie.200460696. [DOI] [PubMed] [Google Scholar]; (f) Jaunzems J, Kashin D, Schonberger A, Kirschning A. Eur. J. Org. Chem. 2004:3435. [Google Scholar]; (g) Cho JH, Dernard DL, Sidwell RW, Kern ER, Chu CK. J. Med. Chem. 2006;49:1140. doi: 10.1021/jm0509750. [DOI] [PubMed] [Google Scholar]
- 5.(a) Ramsay MVJ, Roberts SM, Russell JC, Shingler AH, Slawin AMZ, Sutherland DR, Tiley EP, Williams DJ. Tetrahedron Lett. 1987;28:5353. [Google Scholar]; (b) Bliard C, Escribano FC, Lukacs G, Olesker A, Sarda P. J. Chem. Soc., Chem. Comm. 1987:368. [Google Scholar]; (c) Banks BJ, Bishop BF, Evans NA, Gibson SP, Goudie AC, Gration KAF, Pacey MS, Perry DA, Witty MJ. Bioorg. Med. Chem. 2000;8:2017. doi: 10.1016/s0968-0896(00)00120-6. [DOI] [PubMed] [Google Scholar]; (d) Konoki K, Sugiyama N, Murata M, Tachibana K, Hatanaka Y. Tetrahedron. 2000;56:9003. [Google Scholar]; (e) Crimmins MT, Katz JD, Washburn DG, Allwein SP, McAtee LF. J. Am. Chem. Soc. 2002;124:5661. doi: 10.1021/ja0262683. [DOI] [PubMed] [Google Scholar]; (f) Nagai K, Sunazuka T, Omura S. Tetrahedron Lett. 2004;45:2507. [Google Scholar]
- 6.(a) Karanewsky DS. Tetrahedron Lett. 1991;32:3911. [Google Scholar]; (b) Marco-Contelles J, de Opazo E. J. Org. Chem. 2000;65:5416. doi: 10.1021/jo000110o. [DOI] [PubMed] [Google Scholar]; (c) Smith AB, III, Adams CM, Barbosa SA, Degnan AP. J. Am. Chem. Soc. 2003;125:350. doi: 10.1021/ja0289649. [DOI] [PubMed] [Google Scholar]; (d) Nakata M, et al. Tetrahedron Lett. 2003;44:7747. [Google Scholar]
- 7.A table of other conditions examined during efforts to optimize the selectivity of silylation of diol 8 is provided in the Supporting Information.
- 8.The use of imidazole improved the selectivity for allylic alcohol silylation compared to other bases, while DMF and DMAP have little effect on chemoselectivity. See Supporting Information for details.
- 9.The identity of the major products as the allylic TES ethers was confirmed by acylation (Ac2O, DMAP) of the homoallylic hydroxyl groups to give the homoallylic acetate that was characterized by 1H NMR spectroscopy.
- 10.Hoffman RW. Chem. Rev. 1989;89:1841. [Google Scholar]
- 11.Haines AH. Tetrahedron Lett. 1969;15:1201. [Google Scholar]
- 12.The pKa of allyl alcohol is 15.5 while that of 1-propanol is 16.1. Serjeant EP, Dempsey B. Ionization Constants of Organic Acids in Aqueous Solution. Pergamon; New York, NY: 1979. (IUPAC Chemical Data Series, No. 23).
- 13.(a) Marin MAB, Nome F, Zanette D, Zucco C, Romsted LS. J. Phys. Chem. 1995;99:10879. [Google Scholar]; (b) Strong LE, Brummel CL, Lindower P. J. Solution Chem. 1987;16:105. [Google Scholar]
- 14.(a) Phan TB, Mayr H. Can. J. Chem. 2005;83:1554. [Google Scholar]; (b) Minegishi S, Mayr H. J. Am. Chem. Soc. 2003;125:286. doi: 10.1021/ja021010y. [DOI] [PubMed] [Google Scholar]
- 15.Example in 1,2-diol system: Sunazuka T, Hirose T, Harigaya Y, Takamatsu S, Hayashi M, Komiyama K, Omura S, Sprengeler PA, Smith AB., III J. Am. Chem. Soc. 1997;119:10247.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


