Abstract
The physiological adaptations of the neonatal rat to hypoxia from birth include changes in gastrointestinal function and intermediary metabolism. We hypothesized that the hypoxic lactating dam would exhibit alterations in mammary gland function leading to changes in the concentration of milk peptides that are important in neonatal gastrointestinal development. The present study assessed the effects of chronic hypoxia on peptides produced by the mammary glands and present in milk. Chronic hypoxia decreased the concentration of epidermal growth factor (EGF) in expressed milk and pup stomach contents and decreased maternal mammary gland Egf mRNA. The concentration of parathyroid hormone-related protein (PTHrp) was unchanged in milk and decreased in pup stomach contents; however, mammary Pthlh mRNA was increased by hypoxia. There was a significant increase in adiponectin concentrations in milk from hypoxic dams. Chronic hypoxia decreased maternal body weight, and pair feeding normoxic dams an amount of food equivalent to hypoxic dam food intake decreased body weight to an equivalent degree. Decreased food intake did not affect the expression of Egf, Pthlh, or Lep mRNA in mammary tissue. The results indicated that chronic hypoxia modulated mammary function independently of hypoxia-induced decreases in maternal food intake. Decreased EGF and increased adiponectin concentrations in milk from hypoxic dams likely affect the development of neonatal intestinal function.
Keywords: lactation, leptin, adiponectin, stomach contents
Introduction
The energy demands placed on lactating mammals are increased compared to nonlactating animals [1-3]. Alterations in maternal food intake may have profound effects on the development of the neonate via changes in milk composition and/or production [4-6]. Systemic hypoxia modulates a number of physiological systems and has significant effects on food intake [7-14]. In addition, chronic hypoxia during lactation may affect the composition of expressed milk via alterations in maternal metabolism and mammary gland physiology [15-17].
We and others have previously shown that chronic hypoxia from birth attenuates body weight gain in neonatal rats [18-20]. We hypothesized that the lactating dam exhibits significant changes in mammary gland function and milk composition in response to chronic hypoxia, which would be very important in the maternal neonatal adaptation to altitude. This theory may also provide an approach to exploring possible modifications to the food composition given to hypoxic neonates. That is, a maternal adaptation in mammary gland physiology and milk composition may reveal physiological changes that could be exploited in clinical neonatology.
We have also previously demonstrated changes in the lipid content and profile of the stomach contents (used as a surrogate for milk composition) of hypoxic nursing pups [15, 16, 21]. Comprehensive lipid profiling revealed large increases in diacylglyceride concentrations in the stomach contents of hypoxic pups, along with increases in specific saturated (e.g., palmitate) and polyunsaturated (e.g., alpha-linolenate) fatty acid concentrations [15]. Changes in milk lipid composition may have profound effects on the neonatal adaptation to hypoxia, including alterations in hepatic function [8, 10, 18].
Milk also contains a variety of bioactive proteins that influence neonatal function [5, 22, 23]. We have previously demonstrated increases in lactase and maltase activity in the small intestine of PD7 rat pups exposed to hypoxia from birth [24]. Intestinal disaccharidase activity may serve as an index of mucosal functional development in the neonatal rat [25]. We have also demonstrated changes in intermediary metabolites and insulin sensitivity in neonates exposed to hypoxia from birth [18, 20, 26]. As a result, we were particularly interested in four peptides contained in milk that may alter gastrointestinal development and intermediary metabolism in the nursing neonatal rat pup. Milk-derived epidermal growth factor (EGF) induces the maturation of small intestinal enzyme activity and promotes differentiation of intestinal epithelial cells [5, 25]. Milk-derived parathyroid hormone-related peptide (PTHrp) plays a role in small intestinal growth and differentiation, in addition to influencing neonatal calcium homeostasis [27, 28]. The adipose tissue-derived hormones leptin and adiponectin, also present in milk, affect a wide variety of physiological systems in the neonate, including modulation of hypothalamic-pituitary-adrenal (HPA) axis function, insulin sensitivity, short-term feeding behavior, and metabolic rate [29-34].
The present study investigated the effects of chronic hypoxia from birth to postnatal day (PD) 7. Real-time PCR analysis of mammary gene expression was performed, with a special focus on the expression of the genes encoding for EGF (Egf), PTHrp (Pthlh), leptin (Lep), and adiponectin (Adipoq). The concentrations of these peptides were assessed in maternal milk and/or stomach contents of nursing pups. We hypothesized that exposure to chronic hypoxia causes significant changes in milk-derived peptide composition that are reflected in changes in gene expression. This adaptation may modulate neonatal small intestinal enzyme activity, which we have demonstrated previously [35]. Because we have also shown that hypoxia lowers maternal food intake [7], we studied normoxic dams that were fed ad libitum and normoxic dams that were pair fed to account for the effects of decreased caloric intake per se on milk composition. Maternal body weight and mammary gene expression were measured as part of this initial investigation.
Methods
Animal treatment
The Institutional Animal Care and Use Committee of Aurora Health Care approved the animal protocols. Timed-pregnant Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN; n = 36) were obtained at 14 days gestation and maintained on a standard diet and water ad libitum in a controlled environment (0600-1800 lights on). As soon as a litter was completely delivered, the dam and her pups were immediately moved to an environment chamber and exposed to 21% (normoxia group; n = 14) or 12% (hypoxia group; n = 13) O2 as described previously [12, 13, 16, 20, 36]. Litter size was normalized to 12 pups per litter (mixed sexes). Maternal food intake was measured in normoxic and hypoxic dams as described previously [7]. The amount of food equivalent to the average daily intake of the hypoxic dams was then given to a separate set of normoxic dams (pair-fed group; n = 9).
Collection of pup stomach contents, dam’s milk, and mammary tissue
All experimentation was performed on the morning of PD7. Pups were removed from their dams and quickly decapitated. Recently ingested stomach contents were harvested from the forestomach through a midline abdominal incision, weighed, and frozen in liquid N2. Only full stomachs were analyzed, and separate samples were taken from 2-3 pups per litter (n = 12 per treatment; normoxia and hypoxia groups only). Rat milk was obtained from lactating dams as described previously [16, 37]. Briefly, after the removal of the entire litter, dams were kept in the environment chambers for one hour. Each dam was lightly anesthetized with methohexital sodium (Brevital; ∼0.3 mg/kg i.p.) and weighed, followed by administration of oxytocin (15 IU i.p.; Sigma Chemical, St. Louis, MO). Five minutes after the oxytocin injection, nipples were warmed (water-soaked cotton), and expressed milk was collected by gentle aspiration and frozen for further analysis (n = 7-8 per treatment; normoxia and hypoxia groups only). Mammary tissue was collected from nipples not used for milk collection, with one sample from each dam. Tissue was weighed and frozen in liquid N 2 for RNA extraction and real-time PCR analyses (n = 7-9 per treatment; normoxia, hypoxia, and normoxia pair-fed groups).
RNA isolation and real-time PCR analysis
RNA samples were prepared by tissue extraction with guanidine thiocyanate, purified by CsCl2 centrifugation, and treated with DNase before assay to destroy any contaminating genomic DNA. Each RNA sample was assayed in triplicate; a fourth tube per sample containing no reverse transcriptase was included as a control for contaminating genomic DNA. Messenger RNA levels were quantitated using the Taqman RT-PCR protocol (Applied Biosystems, Inc. [ABI], Foster City, CA). Emitted fluorescence was detected with the ABI Prism 7700 Sequence Detection System (SDS) instrument. Data analysis was performed with the SDS software. The manufacturer’s protocol was followed, with the exception that the concentrations of certain reagents were adjusted as follows: each reaction tube contained 0.25 μgtotal RNA, 2.5 μl TaqMan buffer A (10×), 2 μl dNTP mix (2.5 mmol/l dATP, 2.5 mmol/l dCTP, 2.5 mmol/l dGTP and 5 mmol/l dUTP), 0.375 μl each of forward and reverse primers (20 μmol/l), 4 μl MgCl2 (25 mmol/l), 0.025 μl probe (100 μmol/l), 0.25 μl MuLV reverse transcriptase (50 units/μl), and 0.25 μl Amplitaq Gold (5 units/μl) in a total volume of 25 μl. Values in parentheses indicate stock, not final, concentrations. One tube per assay contained water in place of RNA as a control for RNA or DNA contamination of the solutions. The temperature profile was 30 minutes at 42°C, 10 minutes at 95°C, 15 seconds at 95°C/1 minute at 60°C for 40 cycles, and 5 minutes at 23°C. Primer (Integrated DNA Technologies, Inc., Coralville, IA) and probe (ABI) sequences are presented in Table 1. The number of cycles to reach a given threshold value (Ct) is inversely related to the initial abundance of a specific mRNA at the start of the reaction.
Table 1.
Nucleotide sequences of primers and probes used for real-time PCR
| Gene | Gene symbol | Forward primer | Reverse primer | Probe |
|---|---|---|---|---|
| epidermal growth factor | Egf | ATG GTG GCG TGT GCA TGT AT | ATC GTT CTC CAA TAT AGC CAA TGA C | CAG TTG CAC ACG TAG CGG TCC ACG |
| parathyroid hormone-like peptide | Pthlh | ACA AGG GCA AGT CCA TCC AA | CTC CGC AAT CAG ATG GTG G | ACT TGC GCC GCC GTT TCT TCC |
| leptin | Lep | GGT CAC CGG TTT GGA CTT CAT | GGT CTG GTC CAT CTT GGA CAA | CCC GGG CTT CAC CCC ATT CTG |
| adiponectin | Adipoq | ACC CCT GGC AGG AAA GGA | CCC TAC GCT GAA TGC TGA GTG | AGC CCG GAG AAG CCG CTT ACA TGT |
| nerve growth factor | Ngf | CAT AGC GTA ATG TCC ATG TTG TTC T | TCT CCC TCT GGG ACA TTG CT | CGG TTC TGC CTG TAC GCC GAT CA |
| solute carrier family 2 (facilitated glucose transporter), member 4 | Slc2a4 | TCC ATC CCA CAA GGC ACC | AAT CAT GCC ACC CAC AGA GAA | CAC TAC CCT TTG GGC TCT CTC CGT GG |
| solute carrier family 16 (monocarboxylic acid transporter), member 8 | Slc16a8 | GTG TAT TTC GCC AGC GCA C | GTA ACC AAG GGC AGC AGC A | ACC GTA TCT GGG CCT TTG GGA TCG |
| thyroid hormone receptor alpha | Thra | TCA TCA CCC CGG CCA TC | CGG AGA ACA TGG GCA GTT TT | CCG CGT GGT GGA CTT TGC CA |
| thyroid hormone receptor beta | Thrb | GAC ACA CTT TTG GCC CAA ACT C | AAG CGG CTG GCG TGG | CGG ACC TGC GGA TGA TTG GAG C |
| solute carrier family 5 (sodium iodide symporter), member 5 | Slc5a5 | CTT GCT CGA CAG ACA GCG TCT | TCC CTT CAC CAG GCT CTC CT | CCC AAA GGA AGA CAC TGC CAC CCT G |
| hydroxysteroid 11-beta dehydrogenase 1 | Hsd11b1 | TTG AGT CAA GCT GCT CCC AA | TTG CGC AGA ACT GTG CCT | CAG GAA TGC GCC CTG GAG ATC A |
| hydroxysteroid 11-beta dehydrogenase 2 | Hsd11b2 | TTG GCA AGG AGA CAG CTA AGA A | TCC AAC ACA GTG GCC AGC | TGG ATG CCA TGG GCT TCA CG |
| ribosomal protein L19 | Rpl19 | GCT GAT CAA AGA TGG CCT GAT | CGG GCC AAG GTG TTC TTC | TGA CTG TCC ATT CCC GGG CTC G |
EGF assay
EGF concentrations in milk and stomach contents were measured by a modification of a radioimmunoassay published previously [38]. Anti-rat EGF antibody, purified rat EGF, and goat anti-rabbit IgG were purchased from Biomedical Technologies (Stoughton, MA). [125I]EGF (specific activity 186 μCi/μ g) was prepared by a standard chloramine-T reaction and fractionated by HPLC (Peptide Radioiodination Service Center, University of Mississippi, University, MS). The standard curve ranged from 0.025 to 2.5 ng/tube. Milk was diluted 1:50 and 1:100 in assay buffer. Stomach contents were homogenized in assay buffer (1:1 w/v) and then diluted 1:50 in the assay buffer. The linearity of dilution and recovery of EGF in the samples were verified.
PTHrp, adiponectin, and leptin assays
The linearity of dilution and recovery of the analytes from milk and stomach contents were verified for each assay. PTHrp was measured by two-site immunoradiometric assay (Diagnostic Systems Laboratories, Inc., Webster, TX) specific for PTHrp(1-86) as described previously for the analysis of rat milk [28]. Milk and homogenized stomach contents were diluted 1:500 and 1:1000 in assay buffer. Adiponectin was measured by ELISA (B-Bridge International, Inc., Sunnyvale, CA) after a 1:500 dilution of milk in assay buffer. Leptin was measured by ELISA (Crystal Chem, Inc., Downers Grove, IL) after 1:5 and 1:10 dilutions of milk in assay buffer.
Statistical analyses
Data for milk, stomach contents, and body weight measurements were analyzed by one-way analysis of variance (ANOVA), with a value of p<0.05 considered significant. The Ct (cycles to threshold) values obtained from real-time PCR were also analyzed by one-way ANOVA, with a value of p<0.05 considered significant. All post hoc analyses were performed by the Student-Newman-Keuls method for multiple comparisons (SigmaStat 2.03).
Results
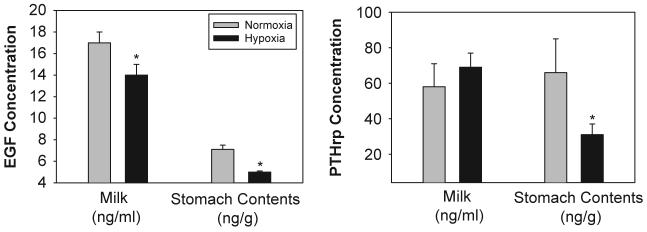
Hypoxia from birth to PD7 significantly decreased EGF concentrations in both milk (p<0.001) and stomach contents (p<0.04; Fig. 1). The magnitude of the decreases between the two sample sources was similar (20-30%). The concentration of PTHrp in expressed milk was not affected by hypoxia (p>0.05); however, PTHrp concentrations in the stomach contents of hypoxic pups were decreased by more than 50% (p<0.04; Fig. 1). Leptin concentrations in milk were not affected by hypoxia (p>0.05). Adiponectin concentrations in milk were significantly increased by hypoxia (779±186 ng/ml) compared to normoxic control (470±42ng/ml; p<0.03).
Fig. 1.
Effects of chronic hypoxia on the concentration of EGF and PTHrp in maternal milk and pup stomach contents. Dams and their newborn litters were placed in an environmental chamber at 21%O2 (normoxia group) or 12 % O 2 (hypoxia group) from birth to PD7.Milk was expressed(n=7-8per treatment) and neonatal stomach contents (n=12 per treatment) were harvested after one week of continuous hypoxia (on PD7). Data are presented as mean±SEM.*Indicates a significant difference from thenormoxiagroup(p<0.05).
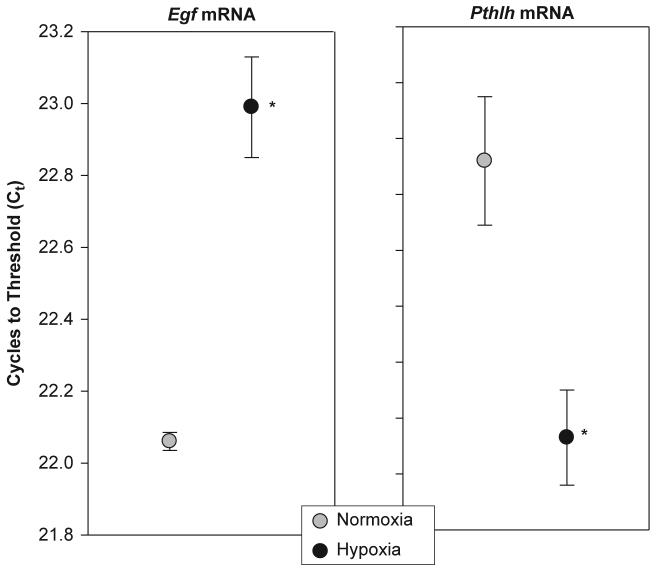
Fig. 2 illustrates the effects of hypoxia on mammary tissue Egf and Pthlh mRNA expression. As outlined in the Materials and Methods section, the Ct value (y-axis) is inversely related to the amount of mRNA present in the sample; therefore, the data are plotted from high to low Ct. In Fig. 2, the panel on the left illustrates a significant hypoxia-induced decrease in mammary Egf mRNA expression (p<0.005). The right-hand panel shows that Pthlh mRNA expression in mammary tissue was significantly increased by hypoxia (p<0.003). Table 2 lists mRNA expression data for other genes chosen for their possible significance in the mammary response to hypoxia. The only other gene to be significantly influenced by hypoxia encodes a sodiumiodide symporter (Slc5a5; p<0.001). Note that the expression of Rpl19, which served as an endogenous control to account for sample and assay variability, was not significantly altered by hypoxia.
Fig. 2.
Effects of chronic hypoxiaon the expression of Egf and Pthlh mRNA in the maternal mammary gland. Dams and their newborn litters were placed in an environmental chamber at 21 %O2 (normoxia group) or 12 % O2 (hypoxia group) from birth to PD7. The units of the y-axis (C t)reflect the number of real-time PCR cycles needed to reach the signal intensity of a predetermined threshold value. Note that a decrease in the C t value indicates an increase in mRNA expression. Data are presented as mean±SEM.*Indicates asignificant difference from the normoxia group(p<0.05)(n=7-8samples per treatment).
Table 2.
Effects of hypoxia on gene expression in mammary glands of nursing rats
| Gene | Gene symbol | Normoxia | Hypoxia | p-value |
|---|---|---|---|---|
| nerve growth factor | Ngf | 27.48 ± 0.24 | 27.94 ± 0.20 | p> 0.05 |
| solute carrier family 2 (facilitated glucose transporter), member 4 | Slc2a4 | 26.23 ± 0.15 | 26.37 ± 0.24 | p> 0.05 |
| leptin | Lep | 27.50 ± 0.29 | 28.08 ± 0.16 | p> 0.05 |
| solute carrier family 16 (monocarboxylic acid transporter), member 8 | Slc16a8 | 22.89 ± 0.30 | 22.86 ± 0.08 | p> 0.05 |
| thyroid hormone receptor alpha | Thra | 21.53 ± 0.13 | 21.45 ± 0.10 | p> 0.05 |
| thyroid hormone receptor beta | Thrb | 22.83 ± 0.34 | 22.64 ± 0.12 | p> 0.05 |
| solute carrier family 5 (sodium iodide symporter), member 5 | Slc5a5 | 25.30 ± 0.10 | 24.44 ± 0.13 | p< 0.001 |
| hydroxysteroid 11-beta dehydrogenase 1 | Hsd11b1 | 25.38 ± 0.34 | 25.13 ± 0.21 | p> 0.05 |
| hydroxysteroid 11-beta dehydrogenase 2 | Hsd11b2 | 27.55 ± 0.19 | 27.47 ± 0.22 | p> 0.05 |
| adiponectin | Adipoq | 22.82 ± 0.43 | 22.17 ± 0.28 | p> 0.05 |
| ribosomal protein L19 | Rpl19 | 18.03 ± 0.29 | 17.93 ± 0.16 | p> 0.05 |
The data reported indicate the PCR cycle at which a threshold was reached (Ct). The SDS software automatically set the threshold line in the exponential phase of the amplifi cation. A lower Ct value indicates an increase in mRNA expression due to hypoxia. Data are presented as mean ± SEM (n = 8 samples per treatment; each sample run in triplicate in PCR assay)
Fig. 3 shows the effects of hypoxia and pair feeding on maternal body weight, as compared to the normoxic control. Hypoxia from parturition to PD7 significantly decreased maternal body weight (from 281 ±4g to 247±5g; p<0.001). The hypoxia-induced decrease in maternal body weight coincided with the decrease in food intake that we previously reported [7]. Based on these observations, normoxic dams (with their litters) were pair fed according to hypoxic dam intake. As shown in Fig. 3, pair-fed normoxic dams also exhibited a significant decrease in body weight (p<0.001), equal to the magnitude of the decrease in hypoxic dams.
Fig. 3.
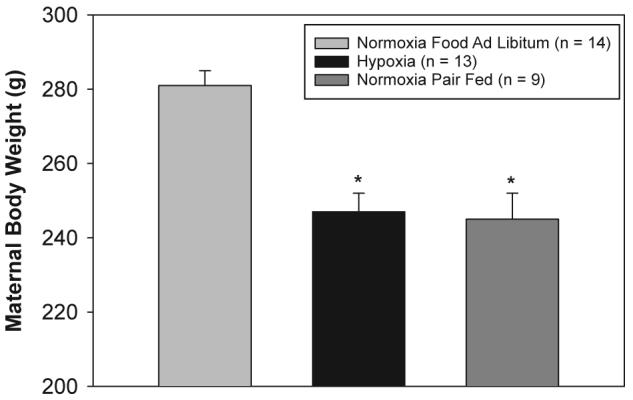
Effects of chronic hypoxia and pair feeding on maternal body weight. Dams and their newborn litters were placed in an environmental chamber at 21 %O2 (normoxiagroup)or 12 % O2 (hypoxia group) from birth to PD7. Maternal food intake was measured daily. A separate set of normoxic dams was then fed an amount of food equivalent to the daily intake of the hypoxic dams (normoxia pair-fed group). Maternal body weight was measured daily. The data represent maternal body weight at PD7 and are presented as mean ± SEM.*Indicates asignificant difference from the normoxia group (p<0.05).
As a screening tool to examine the effects of pair feeding on lactation, real-time PCR analyses were performed on control and pair-fed maternal mammary tissue (Table 3). Interestingly, the mRNA expression of Egf, Pthlh, and Slc5a5 was not affected by decreased food intake (p> 0.05), although it was affected by hypoxia per se. Pair feeding significantly increased the expression of the following genes in maternal mammary tissue: Thra, Thrb, Hsd11b1, and Slc2a4. As with hypoxia, there was no effect of pair feeding on Rpl19 expression.
Table 3.
Effects of pair feeding on gene expression in mammary glands of nursing rats
| Gene | Gene symbol | Normoxia food ad libitum | Normoxia pair fed | p-value |
|---|---|---|---|---|
| epidermal growth factor | Egf | 21.56 ± 0.17 | 21.76 ± 0.06 | p> 0.05 |
| solute carrier family 2 (facilitated glucose transporter), member 4 | Slc2a4 | 25.61 ± 0.14 | 25.29 ± 0.05 | p< 0.04 |
| leptin | Lep | 27.09 ± 0.27 | 27.36 ± 0.40 | p> 0.05 |
| solute carrier family 16 (monocarboxylic acid transporter), member 8 | Slc16a8 | 21.81 ± 0.12 | 21.96 ± 0.06 | p> 0.05 |
| thyroid hormone receptor alpha | Thra | 22.32 ± 0.06 | 21.77 ± 0.08 | p< 0.001 |
| thyroid hormone receptor beta | Thrb | 21.81 ± 0.07 | 21.18 ± 0.08 | p< 0.001 |
| solute carrier family 5 (sodium iodide symporter), member 5 | Slc5a5 | 24.97 ± 0.13 | 25.11 ± 0.12 | p> 0.05 |
| hydroxysteroid 11-beta dehydrogenase 1 | Hsd11b1 | 25.50 ± 0.06 | 24.82 ± 0.08 | p< 0.001 |
| hydroxysteroid 11-beta dehydrogenase 2 | Hsd11b2 | 27.88 ± 0.08 | 27.51 ± 0.09 | p< 0.02 |
| parathyroid hormone-like peptide | Pthlh | 23.67 ± 0.45 | 24.49 ± 0.28 | p> 0.05 |
| ribosomal protein L19 | Rpl19 | 16.32 ± 0.32 | 16.09 ± 0.32 | p> 0.05 |
The data reported indicate the PCR cycle at which a threshold was reached (Ct). The SDS software automatically set the threshold line in the exponential phase of the amplification. A lower Ct value indicates an increase in mRNA expression due to pair feeding. Data are presented as mean ± SFM (n = 8-9 samples per treatment; each sample run intriplicate in PCR assay)
Discussion
The present study demonstrated that chronic hypoxia induces changes in the concentrations of bioactive peptides in maternal milk. A majority of these changes correlate with changes in mRNA expression. Hypoxia from birth to PD7 decreased the concentrations of EGF in expressed milk and pup stomach contents, while also decreasing the expression of the Egf gene in mammary tissue. PTHrp concentrations were unaltered in milk but were decreased in the stomach contents of hypoxic pups. Interestingly, Pthlh mRNA expression was significantly increased in mammary tissue from hypoxic dams. Adiponectin concentrations were increased in milk from hypoxic dams, but there were no significant changes in Adipoq mRNA expression in mammary tissue from these rats. There was no change in milk leptin concentrations or Lep mRNA expression as a result of hypoxia.
We have previously shown that chronic hypoxia in the neonatal rat elicits a multitude of physiological adaptations, including changes in intermediary metabolites and HPA axis function [7,13,15,16,18,20,26,36]. As part of a systematic approach to investigating these adaptations, we have also shown that the lipid composition of pup stomach contents (used as a surrogate for milk composition) was increased considerably during hypoxia [15, 16]. Stomach contents of hypoxic pups had increased concentrations of diacylglycerides and saturated (14:0 and 16:0) and polyunsaturated (18:3n-3 and 20:5n-3) fatty acids [15]. Chronic hypoxia also elicited changes in neonatal digestive function, including increased small intestinal lactase and maltase activity and decreased pancreatic lipase activity [35,39].
Based on these observations, it seems plausible that our model of chronic hypoxia could offer unique insight into maternal-neonatal interactions at altitude. In addition, maternal adaptations to hypoxia also may provide valuable information that could be applied to hypoxic neonates in a clinical setting (e.g., suggesting possible dietary manipulations). Because we have not yet determined the role that changes in milk hormone concentrations have on neonatal function, this discussion will speculate mainly about the potential functional significance of the changes observed.
Effects of hypoxia on epidermal growth factor
Breast milk contains a wide variety of bioactive substances, including peptides that modulate many aspects of growth and development [5,22,23]. We chose to measure the concentrations of EGF, adiponectin, leptin, and PTHrp. EGF has been shown to modulate numerous aspects of gastrointestinal (GI) development in rats, including small intestinal brush border disaccharidase activity and functional development of enterocytes [23, 25, 38]. In the developing rat stomach, EGF has trophic effects on oxyntic mucosal growth without affecting functional development [40,41].
In the present study, chronic hypoxia decreased the concentration of EGF in maternal milk and pup stomach contents and also decreased the expression of Egf mRNA in maternal mammary tissue. Decreased delivery of milk-derived EGF to hypoxic pups would, at first glance, seem to be maladaptive in nature. For example, necrotizing enterocolitis (NEC) induced by repeated asphyxia in neonatal rats could be reversed upon administration of exogenous EGF [42]. Interestingly, supplementation of the neonatal diet with polyunsaturated fatty acid also reversed NEC using a similar model [43]. Conversely, decreased milk-derived EGF may be a beneficial adaptation for pup survival. Chronic moderate hypoxia (i.e., 12 %O2) from birth may not trigger the development of NEC. Likewise, accelerated maturation of GI function may not be suitable for the processing of dietary con-stituents presented to hypoxic pups, such as increased milk lipid content [15, 16]. Changes in dietary lipid composition have been shown to directly modulate small intestinal function [44].
Corticosterone and insulin also have been shown to influence the maturation and function of the small intestine [45,46].Wehave reported that hypoxia from birth to seven days of age increased plasma corticosterone and insulin in suckling pups [16,20,36]. Glucocorticoids and insulin shift the maturation of enzyme functional capacity towards that of the adult, including increased sucrase and decreased lactase activity [46]. We have previously shown that lactase and maltase activity was increased in the small intestine of hypoxic pups, while there were no changes in sucrase activity [35]. Therefore, the enzyme profiles did not correlate with changes in circulating hormone concentrations. This discrepancy may be explained by the interplay of other factors, such as decreased milk-derived EGF, changes in milk macronutrient composition, and / or direct effects of hypoxia on intestinal function. We propose that maintenance of a relatively immature GI phenotype may better match nutrient intake, its assimilation into growing tissues, and its use as metabolic fuel with the physiological and biochemical demands of chronic neonatal hypoxia.
Effects of hypoxia on PTHrp, adiponectin, and leptin
Parathyroid hormone-related protein (PTHrp) is found in a variety of tissues in both fetuses and adults, including epithelia, mesenchymal tissues, endocrine glands, and the central nervous system [27]. PTHrp normally serves as a paracrine regulator of calcium metabolism and cellular growth and development, and it also plays a critical role in the control of digestive function [27,28,47].In addition, Pthlh mRNA has been detected in the rat stomach [47]. The present results indicated a significant increase in Pthlh expression in mammary tissue of hypoxic dams; however, there was a significant decrease in PTHrp concentration in the stomach contents of hypoxic pups. The discordance between PTHrp concentrations in milk and stomach contents may be due to increased plasma corticosterone in hypoxic pups [7].Previous studies found that stress-induced increases in plasma corticosterone inhibited Pthlh expression in the rat stomach and that this could partly explain the gastric hypercontractility associated with stress [47]. We speculate that the hypoxia-induced increase in maternal mammary Pthlh expression may serve to maintain gastric function in the hypoxic neonate. Conversely, it is also possible that intragastric PTHrp metabolism is increased, as hypoxia has been shown to modulate gastric function [48,49]. Future studies should measure Pthlh expression in the neonatal stomach to resolve this issue.
The adipocyte-derived peptides adiponectin and leptin modulate a broad spectrum of physiological functions but are most widely known for their involvement in the control of intermediary metabolism and food intake [30-33].Neonatal plasma adiponectin concentrations are much higher than those in adults, correlate with birth weight, and may be responsible for increased insulin sensitivity in newborns [30,50]. We have previously shown that chronic hypoxia in the newborn rat increased plasma adiponectin concentrations [13]. The present findings indicated increased concentrations of adiponectin in milk from hypoxic dams. To our knowledge, it is unknown whether adiponectin can be taken up intact from milk and transferred to the plasma compartment in the neonate. These changes may serve to main tain some degree of insulin sensitivity in the hypoxic neonate. We have shown that hypoxia induces mild insulin resistance in the neonate, which could be partly due to increased adrenal glucocorticoid production [7,20,36].Alternatively,lowoxygenmay directly inhibit insulin signaling, as highlighted in a recent publication by Kang and colleagues [24]. Whether increased plasma adiponectin concentrations in hypoxic pups was due to dietary influences or to changes in endogenous production remains to be elucidated.
We have previously measured decreased plasma leptin concentrations in newborn rats exposed to hypoxia from birth [20].Leptin increases metabolic rate, modulates the HPA axis, and inhibits food intake [14,32,34]. Others have shown that the neonatal stomach absorbs milk-derived leptin and that this may control short-term feeding behavior [33]. We did not observe an effect of chronic hypoxia on milk leptin concentrations or mammary Lep expression in the present study. It is likely then that low oxygen decreased endogenous leptin production in the neonate, possibly via decreased adipose tissue mass or through a direct effect on Lep gene transcription [51]. The decrease in plasma leptin in the hypoxic newborn may serve to maintain adequate food intake and override the anorectic effects of hypoxia per se.
Real-time PCR analyses revealed only one other significant effect of chronic hypoxia on mammary gene expression. Hypoxia significantly increased the expression of Slc5a5 (Na+ /I - symporter), a gene whose product is responsible for the active transport of iodide into thyroid follicular cells and other tissues [52].Active iodide transport in the mammary gland, and the transfer of iodide to milk, provides the necessary substrate needed for neonatal thyroid hormone synthesis [52]. We have previously shown that neonatal pups exposed to chronic hypoxia can maintain normal thyroid function [36]. Increased mammary Slc5a5 expression may have served to provide increased iodide to the neonate, thus maintaining adequate thyroid function in a situation that requires coordinated metabolic adaptations, such as hypoxia [53]. Further studies will be necessary to fully evaluate the significance of this finding.
Effects of pair feeding on mammary gene expression
The inhibitory effects of hypoxia on food intake have been well described, and we have shown that chronic hypoxia inhibits maternal food intake [7,14,19].The present study assessed the possible confounding effects of decreased caloric intake per se on mammary function and secretory activity during hypoxia. A separate set of normoxic dams was pair fed the amount of food that was consumed daily by hypoxic dams [7]. Hypoxic and pair-feeding normoxic dams showed a decrease in body weight, with the decreases being nearly equal in magnitude. We used real-time PCR analyses of mammary gene expression as an initial screen to evaluate mammary function. Pair feeding did not affect Egf, Pthlh, Lep, or Slc5a5 mRNA expression. These results support the hypothesis that hypoxia per se, rather than hypoxia-induced maternal anorexia, modulated the expression of these genes and the concentration of their products in milk and stomach contents.
Interestingly, pair feeding affected a different set of genes that were not affected by chronic hypoxia. Decreased maternal food intake per se caused a significant increase in the expression of Thra and Thrb (thyroid hormone receptors alpha and beta) in mammary tissue. Previous studies have shown that decreased maternal food intake decreases mammary milk production, while others have shown that thyroid hormones were essential for growth hormone- and prolactin-stimulated milk production [4,6,53].Upregulation of mammary thyroid hormone receptor expression may have served to maintain adequate milk production in the face of decreased food intake.
Maternal glucocorticoids modulate mammary gland function during lactation by stimulating milk protein synthesis and inhibiting mammary gland apoptosis [54]. At the cellular level, the concentration of glucocorticoid available for binding to gluco-corticoid and mineralocorticoid receptors is determined by the activity of the 11 β-hydroxysteroid dehydrogenases (11 βHSD1 and 11 βHSD2)[55]. In the present study, we detected expression of the genes encoding these enzymes (Hsd11b1 and Hsd11b2)in mammary tissue and showed that decreased maternal food intake (i.e., pair feeding) increased the expression of both genes. Because the enzyme activities of 11 β HSD1 and 11 βHSD2oppose one another, it is difficult to assign a functional correlate based on changes in mammary gene expression. Additional measurements, including maternal plasma and milk corticosterone and 11-dehydrocorticosterone concentrations, will be necessary for further assessment of the effects of decreased food intake on mammary Hsd11b1 and Hsd11b2 expression.
Active glucose uptake and transport into mammary epithelial cells, via the glucose transporters (GLUTs), are required for milk synthesis [56, 57]. Internalized glucose is utilized as a substrate in the synthesis of lactose, the major milk carbohydrate, and in some species glucose may be converted into lipids or amino acids [57]. Pair feeding normoxic dams the equivalent of hypoxic dam food intake elicited a small but significant increase in maternal mammary Slc2a4 mRNA expression. This gene encodes the GLUT4 protein, most widely known as the insulin-sensitive glucose transporter associated with muscle and adipose tissue [58]. Previous studies indicated that expression of Slc2a4 was confined to mammary adipocytes, and it has not been detected in the milk-synthesizing cells of the mammary epithelium [56]. Taken together with the present results, these findings indicate that decreased maternal food intake per se may have altered insulin-sensitive glucose metabolism in mammary adipocytes. Systemic hypoxia is frequently encountered in the neonatal intensive care unit and is associated with significant morbidity and mortality [9,59,60].The physiological adaptations to neonatal hypoxia have been well described, yet optimal treatment strategies aimed at decreasing morbidity and mortality remain to be identified. Our experimental model allowed the examination of maternal influences on neonatal adaptations to hypoxia, as both dams and their litters were exposed to hypoxia. The present results indicated that decreased EGF and increased adiponectin concentrations in milk from hypoxic dams might confer an adaptive advantage in gastrointestinal and metabolic function to hypoxic neonates. It has become clear that dietary composition significantly affects normal development. Therefore, it is logical to suggest that dietary formulations for hypoxic infants will have a serious impact on their health [3,21]. This fact takes on extra significance because most sick infants are not breast fed. While preliminary in nature, the present study outlines the possibility that the evaluation of changes in expressed milk and mammary gland gene expression might lead to changes in formula composition for hypoxic neonates.
Acknowledgments
The authors would like to thank Barbara M. Jankowski and Peter J. Homar for their expert technical assistance. This study was supported in part by NIH grant DK54685 to HR, by Aurora St. Luke’s Medical Center, and by the Medical College of Wisconsin Summer Program for Undergraduate Research to JVH.
References
- 1.Butte NF, King JC. Energy requirements during pregnancy and lactation. Public Health Nutr. 2005;8:1010–1027. doi: 10.1079/phn2005793. [DOI] [PubMed] [Google Scholar]
- 2.Dewey KG. Energy and protein requirements during lactation. Annu Rev Nutr. 1997;17:19–36. doi: 10.1146/annurev.nutr.17.1.19. [DOI] [PubMed] [Google Scholar]
- 3.Picciano MF. Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J Nutr. 2003;133:1997–2002. doi: 10.1093/jn/133.6.1997S. [DOI] [PubMed] [Google Scholar]
- 4.Flint DJ, Vernon RG. Effects of food restriction on the responses of the mammary gland and adipose tissue to prolactin and growth hormone in the lactating rat. J Endocrinol. 1998;156:299–305. doi: 10.1677/joe.0.1560299. [DOI] [PubMed] [Google Scholar]
- 5.Grosvenor CE, Picciano MF. Baumrucker CR.Hormones and growth factors in milk. Endocr Rev. 1993;14:710–728. doi: 10.1210/edrv-14-6-710. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen KM. Effects of under- and overnutrition on lactation in laboratory rats. J Nutr. 1998;128:390–393. doi: 10.1093/jn/128.2.390S. [DOI] [PubMed] [Google Scholar]
- 7.Bruder ED, Lee JJ, Widmaier EP, Raff H. Microarray and real-time PCR analysis of adrenal gland gene expression in the 7-day-old rat: effects of hypoxia from birth. Physiol Genomics. 2007;29:193–200. doi: 10.1152/physiolgenomics.00245.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiodi H, Whitmore S. Lipid metabolism in suckling rats with fatty liver induced by hypoxia. Experientia. 1974;30:463–465. doi: 10.1007/BF01926294. [DOI] [PubMed] [Google Scholar]
- 9.Harris RJ. Plasma nonesterified fatty acid and blood glucose levels in healthy and hypoxemic new born infants. J Pediatr. 1974;84:578–584. doi: 10.1016/s0022-3476(74)80685-2. [DOI] [PubMed] [Google Scholar]
- 10.Lee PC, Jelinek B, Struve M, Bruder ED, Raff H. Effect of neonatal hypoxia on the development of hepatic lipase in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:1341–1347. doi: 10.1152/ajpregu.2000.279.4.R1341. [DOI] [PubMed] [Google Scholar]
- 11.White MM, MacCullough RE, Dyckes R, Robertson AD, Moore LG. Effects of pregnancy and chronic hypoxia on contractile responsiveness to alpha1-adrenergic stimulation. J Appl Physiol. 1998;85:2322–2329. doi: 10.1152/jappl.1998.85.6.2322. [DOI] [PubMed] [Google Scholar]
- 12.Raff H, Sandri RB, Segerson TP. Renin, ACTH, and adrenocortical function during hypoxia and hemorrhage in conscious rats. Am J Physiol. 1986;250:240–244. doi: 10.1152/ajpregu.1986.250.2.R240. [DOI] [PubMed] [Google Scholar]
- 13.Raff H, Bruder ED. Adiponectin and resistin in the neonatal rat: effects of dexamethasone and hypoxia. Endocrine. 2006;29:341–344. doi: 10.1385/ENDO:29:2:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westerterp-Plantenga MS, Westerterp KR, Rubbens M, Verwegen CR, Richelet JP, Gardette B. Appetite at “high altitude” [Operation Everest III (Comex-’97)]: a simulated ascent of Mount Everest. J Appl Physiol. 1999;87:391–399. doi: 10.1152/jappl.1999.87.1.391. [DOI] [PubMed] [Google Scholar]
- 15.Bruder ED, Lee PC, Raff H. Lipid and fatty acid profiles in the brain, liver, and stomach contents of neonatal rats: effects of hypoxia. Am J Physiol Endocrinol Metab. 2005;288:314–320. doi: 10.1152/ajpendo.00362.2004. [DOI] [PubMed] [Google Scholar]
- 16.Raff H, Bruder ED, Jankowski BM, Goodfriend TL. Neonatal hypoxic hyperlipidemia in the rat: effects on aldosterone and corticosterone synthesis in vitro. Am J Physiol Regul Integr Comp Physiol. 2000;278:663–668. doi: 10.1152/ajpregu.2000.278.3.R663. [DOI] [PubMed] [Google Scholar]
- 17.Seagroves TN, Hadsell D, MacManaman J, Palmer C, Liao D, MacNulty W, Welm B, Wagner KU, Neville M, Johnson RS. HIF1alpha is a critical regulator of secretory differentiation and activation, but not vascular expansion, in the mouse mammary gland. Development. 2003;130:1713–1724. doi: 10.1242/dev.00403. [DOI] [PubMed] [Google Scholar]
- 18.Bruder ED, Lee PC, Raff H. Metabolic consequences of hypoxia from birth and dexamethasone treatment in the neonatal rat: comprehensive hepatic lipid and fatty acid profiling. Endocrinology. 2004;145:5364–5372. doi: 10.1210/en.2004-0582. [DOI] [PubMed] [Google Scholar]
- 19.Moromisato DY, Moromisato MY, Brasel JA, Cooper DM. Effect of growth hormone therapy in mitigating hypoxia-induced and food restriction-induced growth retardation in the newborn rat. Crit Care Med. 1999;27:2234–2238. doi: 10.1097/00003246-199910000-00028. [DOI] [PubMed] [Google Scholar]
- 20.Raff H, Bruder ED, Jankowski BM, Colman RJ. Effect of neonatal hypoxia on leptin, insulin, growth hormone and body composition in the rat. Horm Metab Res. 2001;33:151–155. doi: 10.1055/s-2001-14929. [DOI] [PubMed] [Google Scholar]
- 21.Bowen RA, Clandinin MT. Dietary low linolenic acid compared with docosahexaenoic acid alter synaptic plasma membrane phospholipid fatty acid composition and sodium-potassium ATPase kinetics in developing rats. J Neurochem. 2002;83:764–774. doi: 10.1046/j.1471-4159.2002.01156.x. [DOI] [PubMed] [Google Scholar]
- 22.Murphy KG, Dhillo WS, Bloom SR. Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev. 2006;27:719–727. doi: 10.1210/er.2006-0028. [DOI] [PubMed] [Google Scholar]
- 23.Read LC, Francis GL, Wallace JC, Ballard FJ. Growth factor concentrations and growth-promoting activity in human milk following premature birth. J Dev Physiol. 1985;7:135–145. [PubMed] [Google Scholar]
- 24.Kang SG, Brown AL, Chung JH. Oxygen tension regulates the stability of insulin receptor substrate-1 (IRS-1) through caspase-mediated cleavage. J Biol Chem. 2007;282:6090–6097. doi: 10.1074/jbc.M610659200. [DOI] [PubMed] [Google Scholar]
- 25.Foltzer-Jourdainne C, Garaud JC, Nsi-Emvo E, Raul F. Epidermal growth factor and the maturation of intestinal sucrase in suckling rats. Am J Physiol. 1993;265:459–466. doi: 10.1152/ajpgi.1993.265.3.G459. [DOI] [PubMed] [Google Scholar]
- 26.Bruder ED, Lee PC, Raff H. Dexamethasone treatment in the newborn rat: fatty acid profiling of lung, brain, and serum lipids. J Appl Physiol. 2005;98:981–990. doi: 10.1152/japplphysiol.01029.2004. [DOI] [PubMed] [Google Scholar]
- 27.Strewler GJ. The physiology of parathyroid hormone-related protein. N Engl J Med. 2000;342:177–185. doi: 10.1056/NEJM200001203420306. [DOI] [PubMed] [Google Scholar]
- 28.VanHouten J, Dann P, MacGeoch G, Brown EM, Krapcho K, Neville M, Wysolmerski JJ. The calcium-sensing receptor regulates mammary gland parathyroid hormone-related protein production and calcium transport. J Clin Invest. 2004;113:598–608. doi: 10.1172/JCI18776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunger D, Ong K. Abundance of adiponectin in the newborn. Clin Endocrinol. 2004;61:416–417. doi: 10.1111/j.1365-2265.2004.02128.x. [DOI] [PubMed] [Google Scholar]
- 30.Kotani Y, Yokota I, Kitamura S, Matsuda J, Naito E, Kuroda Y. Plasma adiponectin levels in newborns are higher than those in adults and positively correlated with birth weight. Clin Endocrinol. 2004;61:418–423. doi: 10.1111/j.1365-2265.2004.02041.x. [DOI] [PubMed] [Google Scholar]
- 31.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 32.Mistry AM, Swick A, Romsos DR. Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am J Physiol. 1999;277:742–747. doi: 10.1152/ajpregu.1999.277.3.R742. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez J, Oliver P, Miralles O, Ceresi E, Pico C, Palou A. Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology. 2005;146:2575–2582. doi: 10.1210/en.2005-0112. [DOI] [PubMed] [Google Scholar]
- 34.Walker CD, Salzmann C, Long H, Otis M, Roberge C, Gallo-Payet N. Direct inhibitory effects of leptin on the neonatal adrenal and potential consequences for brain glucocorticoid feedback. Endocr Res. 2004;30:837–844. doi: 10.1081/erc-200044096. [DOI] [PubMed] [Google Scholar]
- 35.Lee PC, Struve M, Raff H. Effects of hypoxia on the development of intestinal enzymes in neonatal and juvenile rats. Exp Biol Med. 2003;228:717–723. doi: 10.1177/153537020322800611. [DOI] [PubMed] [Google Scholar]
- 36.Raff H, Jacobson L, Cullinan WE. Elevated corticosterone and inhibition of ACTH responses to CRH and ether in the neonatal rat: effect of hypoxia from birth. Am J Physiol Regul Integr Comp Physiol. 2003;285:1224–1230. doi: 10.1152/ajpregu.00259.2003. [DOI] [PubMed] [Google Scholar]
- 37.Nagasawa H. A device for milk collection from mice. Lab Anim Sci. 1979;29:633–635. [PubMed] [Google Scholar]
- 38.Schaudies RP, Grimes J, Wray HL, Koldovsky O. Identification and partial characterization of multiple forms of biologically active EGF in rat milk. Am J Physiol. 1990;259:1056–1061. doi: 10.1152/ajpgi.1990.259.6.G1056. [DOI] [PubMed] [Google Scholar]
- 39.Lee PC, Struve M, Lewis SM, Raff H. Neonatal hypoxia in the rat: effects on exocrine pancreatic development. J Pediatr Gastroenterol Nutr. 2002;34:542–547. doi: 10.1097/00005176-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Dembinski AB, Johnson LR. Effect of epidermal growth factor on the development of rat gastric mucosa. Endocrinology. 1985;116:90–94. doi: 10.1210/endo-116-1-90. [DOI] [PubMed] [Google Scholar]
- 41.Puccio F, Lehy T. Oral administration of epidermal growth factor in suckling rats stimulates cell DNA synthesis in fundic and antral gastric mucosae as well as in intestinal mucosa and pancreas. Regul Pept. 1988;20:53–64. doi: 10.1016/0167-0115(88)90057-2. [DOI] [PubMed] [Google Scholar]
- 42.Feng J, Besner GE. Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2007;42:214–220. doi: 10.1016/j.jpedsurg.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 43.Caplan MS, Russell T, Xiao Y, Amer M, Kaup S, Jilling T. Effect of poly-unsaturated fatty acid (PUFA) supplementation on intestinal inflammation and necrotizing enterocolitis (NEC) in a neonatal rat model. Pediatr Res. 2001;49:647–652. doi: 10.1203/00006450-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Pacha J. Development of intestinal transport function in mammals. Physiol Rev. 2000;80:1633–1667. doi: 10.1152/physrev.2000.80.4.1633. [DOI] [PubMed] [Google Scholar]
- 45.Menard D, Arsenault P, Gallo-Payet N. Epidermal growth factor does not act as a primary cue for inducing developmental changes in suck-ling mouse jejunum. J Pediatr Gastroenterol Nutr. 1986;5:949–955. doi: 10.1097/00005176-198611000-00023. [DOI] [PubMed] [Google Scholar]
- 46.Morisset J. Regulation of growth and development of the gastrointestinal tract. J Dairy Sci. 1993;76:2080–2093. doi: 10.3168/jds.S0022-0302(93)77543-8. [DOI] [PubMed] [Google Scholar]
- 47.Blackshaw LA. Parathyroid hormone-related peptide: A jack of all trades that masters gastric responses to stress. J Gastroenterol Hepatol. 2003;18:1–3. doi: 10.1046/j.1440-1746.2003.02939.x. [DOI] [PubMed] [Google Scholar]
- 48.Yamaji R, Sakamoto M, Miyatake K, Nakano Y. Hypoxia inhibits gastric emptying and gastric acid secretion in conscious rats. J Nutr. 1996;126:673–680. doi: 10.1093/jn/126.3.673. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimoto M, Sasaki M, Naraki N, Mohri M, Miki K. Regulation of gastric motility at simulated high altitude in conscious rats. J Appl Physiol. 2004;97:599–604. doi: 10.1152/japplphysiol.01061.2003. [DOI] [PubMed] [Google Scholar]
- 50.Weyermann M, Beermann C, Brenner H, Rothenbacher D. Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clin Chem. 2006;52:2095–2102. doi: 10.1373/clinchem.2006.071019. [DOI] [PubMed] [Google Scholar]
- 51.Ambrosini G, Nath AK, Sierra-Honigmann MR, Flores-Riveros J. Transcriptional activation of the human leptin gene in response to hypoxia. Involvement of hypoxia-inducible factor 1. J Biol Chem. 2002;277:34601–34609. doi: 10.1074/jbc.M205172200. [DOI] [PubMed] [Google Scholar]
- 52.Tazebay UH, Wapnir IL, Levy O, Dohan O, Zuckier LS, Zhao QH, Deng HF, Amenta PS, Fineberg S, Pestell RG, Carrasco N. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med. 2000;6:871–878. doi: 10.1038/78630. [DOI] [PubMed] [Google Scholar]
- 53.Capuco AV, Kahl S, Jack LJ, Bishop JO, Wallace H. Prolactin and growth hormone stimulation of lactation in mice requires thyroid hormones. Proc Soc Exp Biol Med. 1999;221:345–351. doi: 10.1046/j.1525-1373.1999.d01-91.x. [DOI] [PubMed] [Google Scholar]
- 54.Berg MN, Dharmarajan AM, Waddell BJ. Glucocorticoids and progesterone prevent apoptosis in the lactating rat mammary gland. Endocrinology. 2002;143:222–227. doi: 10.1210/endo.143.1.8584. [DOI] [PubMed] [Google Scholar]
- 55.Draper N, Stewart PM. 11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J Endocrinol. 2005;186:251–271. doi: 10.1677/joe.1.06019. [DOI] [PubMed] [Google Scholar]
- 56.Burnol AF, Leturque A, Loizeau M, Postic C, Girard J. Glucose transporter expression in rat mammary gland. Biochem J. 1990;270:277–279. doi: 10.1042/bj2700277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shennan DB, Peaker M. Transport of milk constituents by the mammary gland. Physiol Rev. 2000;80:925–951. doi: 10.1152/physrev.2000.80.3.925. [DOI] [PubMed] [Google Scholar]
- 58.Ishiki M, Klip A. Mini-review: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology. 2005;146:5071–5078. doi: 10.1210/en.2005-0850. [DOI] [PubMed] [Google Scholar]
- 59.Frankel L, Stevenson DK. Metabolic emergencies of the newborn: hypoxemia and hypoglycemia. Compr Ther. 1987;13:14–19. [PubMed] [Google Scholar]
- 60.Friedman AH, Fahey JT. The transition from fetal to neonatal circulation: normal responses and implications for infants with heart disease. Semin Perinatol. 1993;17:106–121. [PubMed] [Google Scholar]