Abstract
Background
Pain in infancy is poorly understood, and medical staff often have difficulty assessing whether an infant is in pain. Current pain assessment tools rely on behavioural and physiological measures, such as change in facial expression, which may not accurately reflect pain experience. Our ability to measure cortical pain responses in young infants gives us the first opportunity to evaluate pain assessment tools with respect to the sensory input and establish whether the resultant pain scores reflect cortical pain processing.
Methods and Findings
Cortical haemodynamic activity was measured in infants, aged 25–43 wk postmenstrual, using near-infrared spectroscopy following a clinically required heel lance and compared to the magnitude of the premature infant pain profile (PIPP) score in the same infant to the same stimulus (n = 12, 33 test occasions). Overall, there was good correlation between the PIPP score and the level of cortical activity (regression coefficient = 0.72, 95% confidence interval [CI] limits 0.32–1.11, p = 0.001; correlation coefficient = 0.57). Of the different PIPP components, facial expression correlated best with cortical activity (regression coefficient = 1.26, 95% CI limits 0.84–1.67, p < 0.0001; correlation coefficient = 0.74) (n = 12, 33 test occasions). Cortical pain responses were still recorded in some infants who did not display a change in facial expression.
Conclusions
While painful stimulation generally evokes parallel cortical and behavioural responses in infants, pain may be processed at the cortical level without producing detectable behavioural changes. As a result, an infant with a low pain score based on behavioural assessment tools alone may not be pain free.
Rebeccah Slater and colleagues show that although painful stimulation generally evokes parallel cortical and behavioral responses in infants, pain may produce cortical responses without detectable behavioral changes.
Editors' Summary
Background.
Pain is a sensory and emotional experience. It is normally triggered by messages transmitted from specialized receptors (nociceptors) in the body to integrative centers in the spinal cord and brainstem and on to the brain, where it undergoes higher sensory and cognitive analysis, allowing the body to respond appropriately to the stimuli. While the experience of pain may be considered to be unpleasant, it is a useful tool in communicating to us and to others that there is something wrong with our bodies. Ultimately, these responses help restrict further damage to the body and start the process of healing.
In a clinical setting, the ability to communicate about pain allows an individual to seek strategies to ease the pain, such as taking analgesics. Being unable to effectively communicate one's experience of pain leaves the individual vulnerable to prolonged suffering. One such vulnerable group is infants.
Ignored and untreated pain in infants has been shown to have immediate and long-term effects as a result of structural and physiological changes within the nervous system. For example, the body responds to untreated pain by increased release of stress hormones, which may be associated with increased morbidity and mortality in the short term. Long-term effects of pain may include altered pain perception, chronic pain syndromes, and somatic complaints such as sleep disturbances, feeding problems, and inability to self-regulate in response to internal and external stressors. It has been proposed that attention deficit disorders, learning disorders, and behavioral problems in later childhood may be linked to repetitive pain in the preterm infant.
Why Was This Study Done?
Until as recently as the 1990s, newborns in some clinical centres underwent surgery with minimal anesthesia. Also, newborns received little or no pain management postoperatively or for painful procedures such as lumbar punctures or circumcisions. Since then, there has been growing awareness amongst clinicians that pain may be experienced from the earliest stages of postnatal life and that inadequate analgesia may lead to the type of long-term consequences mentioned above. However, gauging how much pain infants and young children are experiencing remains a substantial challenge. The researchers in this study wanted to assess the association between cortical pain responses in young infants and currently used tools for the assessment of pain in these infants. These current tools are based on behavioral and physiological measures, such as change in facial expression, and it is possible that these tools do not give an adequate measure of pain especially in infants born preterm.
What Did the Researchers Do and Find?
Twelve clinically stable infants were studied on 33 occasions when they required a heel lance to obtain a blood sample for a clinical reason. The researchers examined the relationship between brain activity and a clinical pain score, calculated using the premature infant pain profile (PIPP) in response to a painful event. Activity in the somatosensory cortex was measured noninvasively by near-infrared spectroscopy, which measures brain regional changes in oxygenated and deoxygenated hemoglobin concentration. The PIPP is a well-established pain score that ascribes a value to infant behavior such as change in facial expression.
They found that changes in brain activity in response to a painful stimulus were related to the PIPP scores. These changes were more strongly linked to the behavioral components of the PIPP, e.g., facial expression, than physiological components, e.g., heart rate. They also found that a positive brain response could occur in the absence of any facial expression.
What Do These Findings Mean?
Behaviors to communicate pain require motor responses to sensory and emotional stimuli. The maturity of this complex system in infants is not clearly understood. The results of this study raise further awareness of the ability of infants to experience pain and highlight the possibility that pain assessment based on behavioral tools alone may underestimate the pain response in infants.
Additional Information.
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.0050129.
Important papers on pain in human neonates are discussed in the open access Paediatric Pain Letter with links to original articles
The Institute of Child Health in London has a Web site describing a three-year international project on improving the assessment of pain in hospitalized children, with many useful links
The International Association for the Study of Pain (IASP) provides accurate and up-to-date information and links about pain mechanisms and treatment
Introduction
One of the major barriers to providing adequate pain relief to infants and young children is gauging how much pain they are feeling and therefore how much analgesia is required. While adults can use visual analogue scales to report their pain, infants cannot report subjective pain intensity, and clinical pain scoring systems are based upon behavioural, physiological, and metabolic signs [1,2]. The well-established premature infant pain profile (PIPP), for example, ascribes a value to infant behaviours such as sleep state and change in facial expression to produce a composite pain score, which is weighted for age. Such scores have become the major outcome measure in recent analgesic trials of sucrose and morphine [3,4], despite the fact that they can arise from activation of subcortical somatic and autonomic motor pathways and may not be reliably linked to central sensory or emotional processing in the brain.
We have recently recorded the cortical haemodynamic activity produced by noxious stimulation in preterm infants, using near-infrared spectroscopy (NIRS). NIRS measures regional changes in oxygenated and deoxygenated haemoglobin concentration and, as with other techniques that use a haemodynamic approach to assess the functional activation of the brain, it is based on the assumption that increased tissue oxygenation represents an increase in regional cerebral blood flow, which is in turn associated with an increase in underlying neural activity [5]. Since noxious events and their intensity are encoded by frequency of firing and number of activated neurons [6], resultant changes in regional tissue oxygenation are thought to reflect pain intensity. Haemodynamic and electrophysiological analysis of cortical pain activity in adults have confirmed its central importance in pain perception and modulation [7]. Thus, NIRS recordings provide an objective and quantitative measure of infant cortical pain processing and may, in addition, provide further insight into pain awareness than behavioural and physiological responses alone. Our ability to record infant cortical activity in response to noxious stimulation has given us the first opportunity to look at the relationship between clinical pain assessment scores, on the basis of behavioural and physiological responses, with measurements of pain processing in the brain. Here, we present an analysis of the association between the cortical haemodynamic activity and the components of a clinical pain assessment tool (PIPP).
Methods
The hypothesis was that the clinical pain score, calculated using the PIPP, would correlate with the magnitude of evoked cortical haemodynamic activity recorded from the infant brain in response to a heel lance. The primary outcome measures were (i) cortical activity measured as the change in total haemoglobin (HbT) concentration using NIRS and (ii) a clinical pain score, calculated using the PIPP.
Infant Demographics
Infants aged between 25 and 43 wk postmenstrual age (PMA) were recruited from the Neonatal Unit at Elizabeth Garrett Anderson and Obstetric Hospital, University College London. Twelve infants were studied on 33 test occasions during a clinically required heel lance. Table 1 details the demographic characteristics of the infants. PMA was determined from antenatal ultrasound scans or from the maternal report of the last menstrual period. Medical charts were reviewed, and, at the time of the study, infants were assessed as clinically stable. Infants with congenital abnormalities or those receiving sedatives or analgesics were excluded from the study. One infant included in the study had a unilateral periventricular haemorrhage (PVH) as the aim was to correlate two measures across the whole neonatal population. Results relating to this infant have been identified in Figures 1 and 2.
Table 1.
Demographic Characterisation
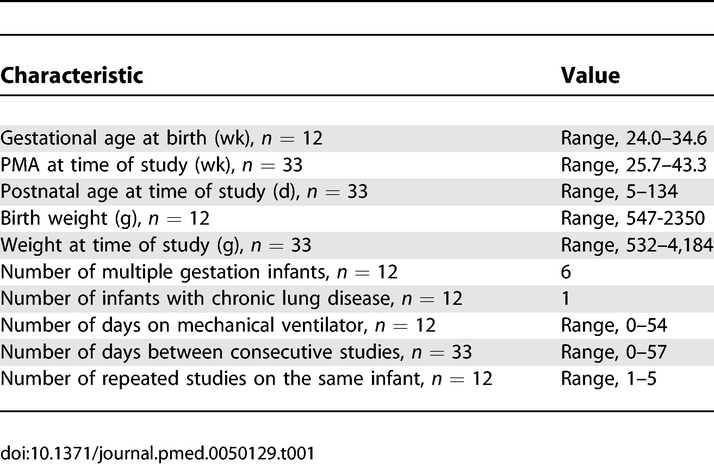
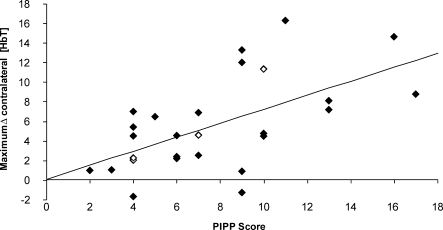
Figure 1. Relationship between the Haemodynamic Response and PIPP Score.
Relationship between maximum Δ[HbT] and the PIPP score following noxious stimulation. The open diamonds represent an infant with periventricular haemorrhage (PVH).
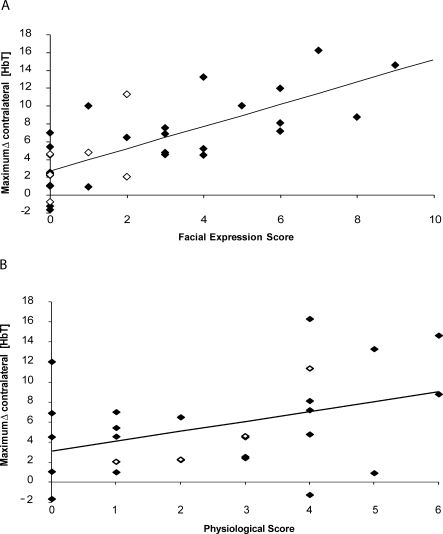
Figure 2. Relationship between the Haemodynamic Response and the Individual Components of the PIPP Score.
(A) Relationship between the haemodynamic response and facial expression score. Relationship between the maximum Δ[HbT] in the contralateral somatosensory cortex and the facial expression score.
(B) Relationship between the haemodynamic response and physiological score. Relationship between maximum Δ[HbT] in the contralateral somatosensory cortex and the physiological response. The open diamonds represent an infant with periventricular haemorrhage (PVH).
Study Plan
Heel lances were undertaken when blood samples were required for clinical diagnosis. No heel lances were performed solely for the purpose of the study. During the heel lance, cortical activity was assessed using NIRS, and the clinical pain score was calculated using the PIPP. This approach enabled simultaneous assessment of the cortical haemodynamic activity and the behavioural and physiological activity in response to a noxious event. Heel lances were performed when heart rate, oxygen saturation, and the haemodynamic cerebral activity were stable for a minimum of 20 s. Following the heel lance, the foot was not squeezed for a period of 30 s to ensure that the evoked response occurred only as a result of the initial stimulus. The time of the heel lance was marked on the video footage by an LED, which flashed when the spring-loaded blade was released from the heel lancet (Tenderfoot, Skafte Medlab AB), or by an auditory cue detected by a microphone. The study was approved by the University College London Hospital (UCLH) research ethics committee, and informed written parental consent was obtained for each infant.
Outcome Measures
NIRS.
A double channel near-infrared spectrophotometer (NIRO-200, Hamamatsu Photonics) was used to measure brain regional changes in oxygenated and deoxygenated haemoglobin concentration. This is a noninvasive technique that has been widely used in neonatal research to measure functional activation of the cortex [8–10]. The technique depends on the transparency of biological tissue to near-infrared light and uses the fact that the absorption of near-infrared light by oxygenated and deoxygenated haemoglobin depends on the oxygenation state [11,12]. The light emitters and detectors (optodes) were positioned symmetrically on either side of the infant's head over the somatosensory cortex, using the international 10–20 EEG placement system to identify key landmarks. Optodes were placed on the head at least 15 min prior to recording, and real-time changes in cerebral haemodynamic activity were monitored. Changes in oxyhaemoglobin [HbO2] and deoxyhaemoglobin [HHb] were measured. Change in total haemoglobin concentration [HbT] was calculated Δ[HbT] = Δ[HbO2] + Δ[HHb]. The baseline was determined 20 s prestimulus, and the cortical response to the stimulus was measured. The maximum change in [HbT] was defined as the maximum change from the mean prestimulus recording in the 20-s period post heel lance. These methods have been described in detail elsewhere [10].
PIPP.
For facial expression, a handheld camcorder was used to record facial actions for later analysis away from the clinical setting. The video recordings were used to calculate the behavioural state and facial expression components of the PIPP score. Behavioural state changes were assessed by observing the infants in the 15-s period prior to the heel lance and classifying the infant's behavioural state into one of four categories [13]. PMA was used to calculate the age weighting factor [13]. The facial expression component of the PIPP was analysed by a single observer watching the videos for the 30 s immediately following the heel lance. The presence of each facial expression indicator (nasolabial furrow, eye squeeze, and brow bulge) was assessed individually. The observer focused on one facial expression and used a stopwatch to calculate the duration over which the facial expression could be observed in the 30-s time window following the heel lance. This was repeated for each facial expression indicator. A score between 0 and 3 was assigned for each of the three facial expressions, and was dependent on the percentage of time that the facial expression was observed in the 30-s period after the heel lance.
For physiological data infants were monitored for changes in heart rate and oxygen saturation using a Nellcor 200 transcutaneous pulse oximeter. Heart rate and blood saturation values were downloaded to an external PC. If this was not possible, the values were manually read from the bedside monitors. Data analysis software was used to calculate the mean heart rate and oxygen saturation in the 15 s prior to the heel lance. The maximum heart rate and the minimum oxygen saturation in the 30-s period immediately following the heel lance were recorded. The maximum increase in heart rate and the percentage decrease in oxygen saturation from the 15-s prestimulus baseline period were calculated. A score between 0 and 3 was assigned for each of the physiological indicators.
Statistical Analysis
Intrarater and inter-rater reliability of assessment of behavioural state and facial expression were determined by a second assessment of the video recordings. For inter-rater reliability, the second coder was blinded to whether or not the infant was undergoing a heel lance. Comparisons between rating pairs were made using intraclass correlation. The intrarater reliability was 0.99, and the inter-rater reliability was 0.96. In 12 cases it was not possible to video record the infants. In these cases, the PIPP score was calculated in real time at the bedside. Real-time inter-rater reliability was 0.95.
The relationship between the PIPP score and the magnitude of the cortical haemodynamic response was assessed. The maximum change in [HbT], defined as the maximum change from the mean prestimulus recording in the 20-s period post stimulus, is referred to as the stimulus response and is used in all the statistical analyses. As the magnitude of the cortical haemodynamic response has been shown to be dependent on PMA and sleep state [10] the relationship between the unweighted PIPP score (i.e., the total PIPP score minus the score for gestational age and sleep state) and the cortical response was also established. In addition, the relationship between the facial expression score and the magnitude of the cortical activity was assessed. The sum of the three PIPP facial expression indicators (i.e., eye squeeze, brow bulge, and nasolabial furrow) is referred to throughout as the facial expression score. The percentage of infants who had a facial expression score of zero was calculated. The relationship between the physiological responses in the PIPP score and the magnitude of the cortical haemodynamic response was also assessed.
To examine the relationships between the individual components of the PIPP score and the haemodynamic response, a number of preliminary calculations were performed using restricted maximum likelihood mixed-model regression [14]. Mixed-model regression takes into consideration the inherent nonindependence among observations when more than one measurement is acquired from each individual. These analyses indicated a negligible level of correlation among the observations. Thus each analysis reduced to a simple regression problem involving independent observations. A number of calculations were performed to ensure that the regression results are robust, including model adequacy analysis, an assessment of the sensitivity to outliers, and a variety of regression diagnostics procedures for identifying potential overinfluential observations. The results were satisfactory. Each of the regression models was shown to be adequate, as judged by an assessment of various residual plots and Q-Q plot, and the absence of influential observations or marked outliers. Regression coefficients were calculated for each relationship, and 95% confidence interval [CI] limits and p-values are stated. Correlation coefficients for each relationship were also given. The mixed model regression analyses were performed using SAS PROC MIXED (SAS Version 9.1).
Results
Clinical Pain Scores (PIPP) and Cortical Haemodynamic Activity Are Well Correlated
An increase in HbT concentration in the contralateral somatosensory cortex was observed following a heel lance in 30 out of the 33 studies. The maximum change in HbT concentration in the contralateral and ipsilateral somatosensory cortex is shown for each test occasion (see Figure S1). A clear correlation between the PIPP score and the maximum change in [HbT] in the contralateral somatosensory cortex is shown in Figure 1 (regression coefficient = 0.72, 95% CI 0.32–1.11, p = 0.001; correlation coefficient = 0.566).
The Cortical Response Has a Stronger Correlation with Behavioural Components of the PIPP Compared with the Physiological Components
The PIPP score is made up of behavioural (facial expression) and physiological (heart rate and oxygenation) components. Figure 2 shows that the cortical activity was well correlated with the behavioural, facial expression component (regression coefficient = 1.26, 95% CI 0.84–1.67, p < 0.0001; correlation coefficient = 0.744) but only moderately correlated with the physiological component, calculated from the sum of the two physiological indicators (regression coefficient = 0.98, 95% CI 0.05–1.92, p = 0.04; correlation coefficient = 0.398).
The correlation between the PIPP score and the cortical response is therefore largely accounted for by the facial expression score (regression coefficient = 1.26, 95% CI 0.84–1.67, p < 0.0001; correlation coefficient = 0.744). Addition of the physiological score to the facial expression score does not improve the correlation (regression coefficient [excluding weighting factors] = 0.77, 95% CI 0.45–1.09, p < 0.0001; correlation coefficient = 0.699). The correlation between the measures is also less good when weighting factors for sleep state and age are added. This arises because the youngest sleeping infants tend to have the smallest cortical haemodynamic responses, and it is these infants whose scores are weighted most heavily.
Cortical Response to Painful Stimulation May Occur in the Absence of Facial Expression
In 13 of the 33 test occasions, no change in facial expression was observed. In 10 of these cases, infants still mounted a cortical haemodynamic response. The infants who did not display a change in facial expression were not different in terms of their clinical status (n = 8). In fact, seven out of the eight infants who did not display a change in facial expression on these test occasions did display a facial expression change when studied on different test occasions.
Discussion
The difficulty in accurate measurement of pain in infants is a major impediment in providing effective analgesia for infants undergoing neonatal intensive care. Here, for the first time, we have measured the cortical haemodynamic activity evoked by noxious stimulation in infants while simultaneously scoring their responses with a validated clinical behavioural and physiological assessment tool. Studies were undertaken when hospitalised infants underwent noxious procedures as part of their routine medical care. The results show that while observable behaviour and physiological changes are in general well correlated with cortical activation, it is possible to record significant cortical activity with no concomitant behavioural response.
Regression analysis demonstrated that following a noxious event, change in facial expression is the component of the PIPP that correlates best with the level of cortical activity in the contralateral somatosensory cortex. A weaker correlation was observed between the physiological response and the cortical haemodynamic response. This is consistent with previous studies which report facial expression is the most specific and consistent pain response [15,16] and poor correlation with behavioural and physiological indicators of pain [17]. This is supported by the attenuation of the behavioural but not the physiological responses to venepuncture by oral glucose intervention suggesting that the latter may be related to stress rather than pain [18]. The correlation of facial expression with cortical activity is likely to reflect underlying neural connections in the infant central nervous system and suggests that nociceptive pathways originating from the spinal cord and conveying information to the thalamus and somatosensory cortex more reliably activate the brainstem nuclei responsible for facial expression than they activate cardiovascular and respiratory control centres. Arbitrary weighting factors, which increase the pain score for the younger infants and those who are asleep, appear to distract from the true response to noxious stimulation.
Pain awareness consists of both sensory discriminative and emotional components, and pain behaviour can be expressions of either or both these components. Facial actions are thought to be an important emotional response to pain particularly where communication with others can help achieve relief or escape [19]. While careful scoring of facial expression has been a useful tool in studying infant pain [20], the facial actions themselves are a motor response—the actual subjective process of feeling emotions is generated by neural activity in the cortex and brainstem [21], and we know very little about this in infants. Here we have shown that somatosensory cortical activity can be recorded in response to a noxious stimulus in the absence of this facial motor response. How much this cortical response contributes to pain awareness or pain experience is not known, but our results do show that nociceptive information can generate significant sensory activity at higher levels of the central nervous system while failing to evoke a facial response. The absence of facial activity may simply be due to immature motor circuitry failing to produce synchronised and coordinated muscle contraction, or it may indicate a true absence of emotion. Since nociceptive activity is clearly transmitted to the brain on these occasions, we suggest that, either way, the infants may not actually be pain free. As a result, pain assessment based on behavioural tools alone should be interpreted with caution as they could under estimate the total pain response.
Supporting Information
Bar chart showing maximum Δ[HbT] in the contralateral and ipsilateral somatosensory cortex following heel lance on each test occasion. Test occasions ranked in terms of magnitude of the contralateral haemodynamic response.
(737 KB EPS)
Acknowledgments
We wish to thank Stewart Boyd for help with the analysis and interpretation of the data and Alan Worley for his technical support. We also thank Martin King for assistance with the mixed model regression analysis. We thank The Wellcome Trust, Medical Research Council, and SPARKS for funding this project.
Abbreviations
- CI
confidence interval
- HbT
total haemoglobin concentration
- NIRS
near-infrared spectroscopy
- PIPP
premature infant pain profile
- PMA
postmenstrual age
Footnotes
Author contributions. RS, JM, and MF conceived of and designed the study. RS, AC, and JM were involved in the acquisition of data. RS, AC, LF, JM, and MF analysed the data. RS, JM, and MF interpreted the data. RS, AC, LF, JM, MF revised the manuscript for critically important intellectual content. JM and MF obtained funding.
Funding: This study was funded by The Wellcome Trust, The Medical Research Council, and SPARKS. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing Interests: The authors have declared that no competing interests exist.
References
- Franck LS, Greenberg CS, Stevens B. Pain assessment in infants and children. Pediatr Clin North Am. 2000;47:487–512. doi: 10.1016/s0031-3955(05)70222-4. [DOI] [PubMed] [Google Scholar]
- McNair C, Ballantyne M, Dionne K, Stephens D, Stevens B. Postoperative pain assessment in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2004;89:F537–F541. doi: 10.1136/adc.2003.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajal R, Lenclen R, Jugie M, Paupe A, Barton BA, et al. Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics. 2005;115:1494–1500. doi: 10.1542/peds.2004-1425. [DOI] [PubMed] [Google Scholar]
- Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2004. p. CD001069. [DOI] [PubMed]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Price DD. Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol Interv. 2002;2:392–403. 339. doi: 10.1124/mi.2.6.392. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bartocci M, Winberg J, Papendieck G, Mustica T, Serra G, et al. Cerebral hemodynamic response to unpleasant odors in the preterm newborn measured by near-infrared spectroscopy. Pediatr Res. 2001;50:324–330. doi: 10.1203/00006450-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Meek JH, Firbank M, Elwell CE, Atkinson J, Braddick O, et al. Regional hemodynamic responses to visual stimulation in awake infants. Pediatr Res. 1998;43:840–843. doi: 10.1203/00006450-199806000-00019. [DOI] [PubMed] [Google Scholar]
- Slater R, Cantarella A, Gallella S, Worley A, Boyd S, et al. Cortical pain responses in human infants. J Neurosci. 2006;26:3662–3666. doi: 10.1523/JNEUROSCI.0348-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Reece H, Smith M, Elwell CE, Goldstone JC. Near infrared spectroscopy. Br J Anaesth. 1999;82:418–426. doi: 10.1093/bja/82.3.418. [DOI] [PubMed] [Google Scholar]
- Wyatt JS, Cope M, Delpy DT, Wray S, Reynolds EO. Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet. 1986;2:1063–1066. doi: 10.1016/s0140-6736(86)90467-8. [DOI] [PubMed] [Google Scholar]
- Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- Hand D, Crowder M. Practical longitudinal data analysis. London: Chapman and Hall; 1996. [Google Scholar]
- Grunau RV, Craig KD. Pain expression in neonates: facial action and cry. Pain. 1987;28:395–410. doi: 10.1016/0304-3959(87)90073-X. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Oberlander T, Holsti L, Whitfield MF. Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain. 1998;76:277–286. doi: 10.1016/S0304-3959(98)00046-3. [DOI] [PubMed] [Google Scholar]
- Johnston CC, Stevens BJ, Yang F, Horton L. Differential response to pain by very premature neonates. Pain. 1995;61:471–479. doi: 10.1016/0304-3959(94)00213-X. [DOI] [PubMed] [Google Scholar]
- Bauer K, Ketteler J, Hellwig M, Laurenz M, Versmold H. Oral glucose before venepuncture relieves neonates of pain, but stress is still evidenced by increase in oxygen consumption, energy expenditure, and heart rate. Pediatr Res. 2004;55:695–700. doi: 10.1203/01.PDR.0000113768.50419.CD. [DOI] [PubMed] [Google Scholar]
- Williams AC. Facial expression of pain: an evolutionary account. Behav Brain Sci. 2002;25:439–455. doi: 10.1017/s0140525x02000080. [DOI] [PubMed] [Google Scholar]
- Craig KD, Whitfield MF, Grunau RV, Linton J, Hadjistavropoulos HD. Pain in the preterm neonate: behavioural and physiological indices. Pain. 1993;52:287–299. doi: 10.1016/0304-3959(93)90162-I. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bar chart showing maximum Δ[HbT] in the contralateral and ipsilateral somatosensory cortex following heel lance on each test occasion. Test occasions ranked in terms of magnitude of the contralateral haemodynamic response.
(737 KB EPS)