Abstract
Introduction
Tumors of any histological origin can give rise to cutaneous and subcutaneous metastases during follow-up. This study aims to evaluate the costs and benefits of electrochemotherapy (ECT) with the Cliniporator™ vs other currently used methods in the control and treatment of cutaneous and subcutaneous advanced neoplasms.
Materials and methods
A cost-effectiveness analysis was carried out on ECT using the Cliniporator vs other techniques (radiotherapy, hyperthermia associated with radiotherapy and chemotherapy, interferon-alpha, and isolated limb perfusion) for the control and treatment of cutaneous and subcutaneous neoplasms. The direct health costs were attributed a value according to the Italian National Healthcare System. Resource consumption and clinical outcomes were derived from cost survey data collection and literature review.
Results
ECT is cost-effective with an incremental cost effectiveness ratio (ICER) of €1,571.53 to achieve a further additional response. Radiotherapy and interferon-alpha are the least effective strategies. A combination of hyperthermia, chemotherapy, radiotherapy, and interferon-alpha treatment are dominated by ECT (more costly and less effective). Isolated limb perfusion is the most effective treatment, but is very costly (€18,530.47) because of the use of antiblastic drugs (TNFα), with an ICER of €92,717.29.
Conclusions
After sensitivity analysis, the study results confirm the favorable cost-effectiveness ratio of ECT with the Cliniporator and justify its wider use.
Keywords: Cliniporator, cutaneous and subcutaneous tumors, electrochemotherapy, hyperthermia, chemotherapy, radiotherapy, interferon-alpha, isolated limb perfusion, cost-effectiveness
Introduction
Tumors of any histological origin can give rise to cutaneous and subcutaneous metastases during disease progression; in particular, around 8%–45% of the patients with malignant cutaneous melanoma develop metastases. Among these patients, 21.7% show satellite and in-transit metastases, 50.2% (regional) lymph node metastases, and 28.1% distant metastases (Meier et al 2002).
With the exception of distant metastases, surgery is the most widely used therapeutic option, followed by radiotherapy, isolated limb perfusion, hyperthermia, and, lastly, chemotherapy (Wolf et al 2003).
Some skin metastases are non-resectable because of their location (face, genitals). Complications such as bleeding and infections, as well as psychological issues, may require less invasive therapeutic options even in the 4th stage of the disease (Balch et al 2000).
Besides surgery, radiotherapy also proved to be effective in the treatment of metastatic melanomas, with fair percentages of complete response, which increase when radiotherapy is associated to hyperthermia (Kim et al 1978).
Hyperthermia can be applied to the whole body, to a body region, or locally. Among regional treatments, isolated limb perfusion with a variety of cytotoxic agents is often used in the treatment of melanoma metastases (Falk et al 2001).
Whereas the above-mentioned therapies have proven effective, results with chemotherapy are unsatisfactory and, after 20 years, decarbazine is still the most widely used cytotoxic agent (Wolf et al 2004).
Recently, electrochemotherapy (ECT) with the Cliniporator™ (IGEA Srl, Carpi [MO], Italy) has been added to clinical practice. ECT is an efficient local tumor ablation modality using electroporation, a physical method that enhances cell membrane permeability, and enables non-permeant or poorly permeant chemotherapeutic agents to enter cells, greatly enhancing their efficacy. Exposure of cells to electric pulses increases the cytotoxicity of suitable drugs (Sersa et al 1995; Mir et al 1998; Jaroszeski et al 2001).
Electrochemotherapy is an elective therapy for melanoma metastases; recent studies have proven that it is effective, easy to apply, and has a short application time, both for single and multiple lesions (Mir et al 2006).
This study aims to develop an economic evaluation of ECT and analyze, for the Italian National Healthcare System (SSN), the costs and the effectiveness of this innovative therapeutic tool in comparison with currently used treatments.
Materials and methods
A cost-effectiveness analysis was carried out on ECT using the Cliniporator vs other strategies (radiotherapy, hyperthermia in association with radiotherapy and chemotherapy, interferon-alpha, and isolated limb perfusion) for the control and treatment of cutaneous and subcutaneous neoplasms. The direct health costs were attributed a value from the point of view of the National Healthcare System. Resources consumption was derived from a literature review.
Cost analysis
No specific reimbursement rate for electroporation has been assigned; therefore in current clinical practice ECT is assimilated into “tumor ablation and electro-fulguration of tumors”. In order to compare more precisely the costs of ECT with the costs of the other methods, direct hospital costs, and amortization and maintenance costs for the Cliniporator were calculated. The costs associated to ECT were assessed according to the methods described in the European study “Standard operative procedures of the electrochemotherapy: instruction for the use of bleomycin or cisplatin administered either systemically or locally and electric pulse delivered by the Cliniporator by means of invasive or non invasive electrodes.” (Mir et al 2006).
Therefore, in order to determine direct hospital costs, six therapeutic courses were considered, depending on the kind of anesthesia and on the chemotherapy drug administered:
Treatment A (Blm): Local anesthesia, intratumoral administration of bleomycin.
Treatment A (CDDP): Local anesthesia, intratumoral administration of cisplatin.
Treatment B: Local anesthesia, intravenous administration of bleomycin.
Treatment C (Blm): General anesthesia, intratumoral administration of bleomycin.
Treatment C (CDDP): General anesthesia, intratumoral administration of cisplatin.
Treatment D: General anesthesia, intravenous administration of bleomycin.
The procedure was considered to last 28 minutes with local anesthesia, and 29.5 minutes with general anesthesia, with an average use of 1.3 electrodes per procedure (Mir et al 2006).
The costs relating to the staff involved (physicians and nurses) and to the required consumables, both for the treatment and the procedure, were attributed a value at the Azienda Ospedaliera (hospital) of Busto Arsizio (Varese) according to national indication of Agenzia Sanitaria Nazionale (ASSR 2007). The resulting costs ranged from a minimum of €1,229.00 (2nd procedure) to a maximum of €1,372.00 (6th procedure); the items that affected costs the most were the electrodes, with one electrode costing €900.00, including VAT (value added tax). In order to determine the amortization and maintenance costs, data given by the manufacturing company, IGEA Srl, were considered. The annual amortization cost, with a device purchase cost of €54,000.00, including VAT, was determined by considering an operational lifetime of 8 years and the use of the Cliniporator on at least 100 patients/year. Thus, the annual cost per patient (€67.50) for using the device was determined. Similar estimates were carried out for maintenance, based on a biannual cost of €6,000.00, VAT included, which resulted in an annual cost of around €30.00 per patient.
The manufacturer of the Cliniporator proposes either the purchase, or an annual lease of the device for €12,000.00 including VAT, which includes maintenance. With 100 patients a year potentially benefiting from the treatment with the Cliniporator, the unit cost would be €120.00 for the lease. In the final valuation of ECT, we considered resorting to amortization and maintenance in 50% of the cases, and to full service in the rest of the cases. The final value was determined to be just over €1,400.00 (Table 1).
Table 1.
Cost of electrochemotherapy
| Costs of all the categories with drugs and electrodes | |
|---|---|
| Treatment A Blm | €1,239.33 |
| Treatment A CDDP | €1,229.25 |
| Treatment B | €1,254.02 |
| Treatment C Blm | €1,357.45 |
| Treatment C CDDP | €1,347.37 |
| Treatment D | €1,372.14 |
| (A) Average cost value of the different therapeutic courses | €1,299.93 |
| Amortization cost of the equipment and manteinance | €97.50 |
| Annual full service | €120.00 |
| (B) Average cost value of amortizationand service cost | €108.75 |
| (A+B) Total cost | €1,408.68 |
Note: Treatment A (Blm): Local anesthesia, intratumoral administration of bleomycin.
Treatment A (CDDP): Local anesthesia, intratumoral administration of cisplatin.
Treatment B: Local anesthesia, intravenous administration of bleomycin.
Treatment C (Blm): General anesthesia, intratumoral administration of bleomycin.
Treatment C (CDDP): General anesthesia, intratumoral administration of cisplatin.
Treatment D: General anesthesia, intravenous administration of bleomycin.
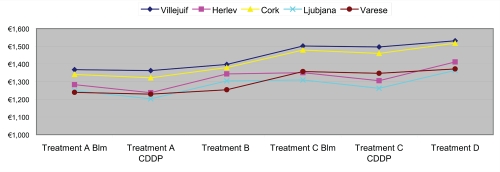
Moreover, our evaluation was in line with the costs estimated in the European study (Mir et al 2006), as shown in Figure 1.
Figure 1.
Electrochemotherapy: costs of the different therapeutic courses. Derived from Mir et al (2006).
Treatment A (Blm): Local anesthesia, intratumoral administration of bleomycin.
Treatment A (CDDP): Local anesthesia, intratumoral administration of cisplatin.
Treatment B: Local anesthesia, intravenous administration of bleomycin.
Treatment C (Blm): General anesthesia, intratumoral administration of bleomycin.
Treatment C (CDDP): General anesthesia, intratumoral administration of cisplatin.
Treatment D: General anesthesia, intravenous administration of bleomycin.
Cost of radiotherapy treatment (Table 2) was based on the “2006 National Tariff Nomenclator” (Health Ministry 2006) multiplied by numbers of cycles.
Table 2.
Cost of radiotherapy treatment
| Treatment | Code | Nr cycles | Nr admin.per cycle | Unit cost | Total cost per treatment |
|---|---|---|---|---|---|
| Electron teletherapy one or opposed fields | 92.25.01 | 5 | 5 | €38.42 | €960.50 |
| Simulation and individuation target | 92.29.01 | 1 | 1 | €54.23 | €54.23 |
| Dosimetric treatment plan | 92.29.04 | 1 | 1 | €23.86 | €23.86 |
| 2D treatment planning | 92.29.E | 1 | 1 | €50.00 | €50.00 |
| Start set-up, portal-films (each film) | 92.29.G | 1 | 1 | €35.64 | €35.64 |
| Total cost | €1,124.23 | ||||
Health Ministry 2006, National Tariff Nomenclator.
Interferon-alpha treatment costs proved to be very variable, depending on tumor localization and on the cytotoxic agent used. To assess cost of interferon-alpha treatment, units of consumed resources were multiplied by the prices reported in the official Italian price list (Informatore Farmaceutico 2007), taking into consideration the daily dosage and duration of the treatment (Table 3).
Table 3.
Cost of interferon-alpha treatment
| Drug | Dose | Dose die in U | Nr cycles | Nr admin. per cycle | Unit cost | Total cost |
|---|---|---|---|---|---|---|
| Interferon alfa 2b | 20,000,000 U/m2/die | 35200000 | 4 | 5 | €143.40 | €2,867.96 |
| 10,000,000 U/m2/die | 17600000 | 52 | 3 | €71.70 | €11,185.03 | |
| Total cost | €14,052.98 | |||||
Oncological hyperthermia is a therapy that can be used in association with radiotherapy and chemotherapy; by means of radiofrequency electromagnetic fields, the tumor tissue is heated up to a temperature nearing 43°C or above for about an hour; the heat heightens the radiotherapy and chemotherapy effects, with a significant improvement in the lesion control. The cost of hyperthermia is €65.80 per procedure, based on the Health Ministry 2006, National Tariff Nomenclator; we then added the costs of radiotherapy and chemotherapy (Table 4).
Table 4.
Cost of hyperthermia, chemotherapy and radiotherapy
| Treatment | Sessions or Cycles (mean) | Unit cost | Total cost | |||
|---|---|---|---|---|---|---|
| Hyperthermia | 6.13 | €65.80 | €403.57 | |||
| Chemotherapy* | 6.13 | €215.10 | €1,318.54 | |||
| Radiotherapy | 5.31 | €69.24 | €367.84 | |||
| Hyperthermia+Chemo+Radio | Total cost | €2,089.96 | ||||
Interferone alfa 2b.
Health Ministry 2006, National Tariff Nomenclator.
Isolated limb perfusion is a therapy used on some cancers located in the limbs, combining perfusion with antiblastic agents with hyperthermia (between 41°C and 43°C); it is a locoregional procedure which enables the release of high doses of a cytostatic drug in a limb with in-transit melanoma metastases. The technique used is sophisticated and requires accurate monitoring of heat dispersion and of limb temperature. Since there is no DRG (Diagnosis Related Groups) reimbursement in Italy to cover the costs of this procedure (Health Ministry, Hospital DRG tariffs, TUC, 2006), the isolated limb perfusion was attributed a value according the survey data on costs presented in Table 5. Table 6 summarizes costs of all treatment alternatives.
Table 5.
Cost of isolated limb perfusion
| Medical devices and others materials | €54.06 | |
| Physician’s time (*) | €84.59 | |
| Nurse’s time (*) | €92.59 | |
| Drugs (**) | ||
| TNF alpha | €12,734.24 | |
| Melfalan | €187.38 | |
| Heparin | €7.70 | |
| Emoderivates | €29.00 | |
| Total direct costs | €13,189.56 | |
| Hospitalization (nr. 8.5 die in general Surgery Dept.) | €2,252.50 | |
| Overhead costs | €3,088.41 | |
| Total cost per procedure | €18,530.47 | |
Table 6.
Summary of costs (all treatment alternatives)
| Treatment | Cost | Source |
|---|---|---|
| Radiotherapy | €1,124.23 | Health Ministry 2006, National Tariff Nomenclator |
| Electrochemotherapy (ECT) | €1,408.68 | Cost survey data collection |
| Interferon-alpha treatment | €14,052.98 | Informatore Farmaceutico 2007 |
| Hyperthermia+Chemo+Radio | €2,089.96 | Richting et al 2003; Informatore Farmaceutico, 2007, Health Ministry 2006, National Tariff Nomenclator |
| Isolated limb perfusion | €18,530.47 | Cost survey data collection |
Effectiveness analysis
The data on effectiveness were derived from a literature review. In particular, the effectiveness data on ECT were taken from Marty et al (2006). Effectiveness data are summarized in Table 7 and express the benefit as a function of the achieved tumor response.
Table 7.
Efficacy of the various strategies, based on tumor response
| Treatment | Effectiveness (OR*) | Range (min. - max.) | Source |
|---|---|---|---|
| Radiotherapy | 56.0% | (±10%) | Gaudy-Marqueste et al 2006 |
| ECT | 74.1% | 63.4%–84.8% | Marty et al 2006 |
| Hyperthermia+Chemo+Radio | 73.3% | (±10%) | Richtig et al 2003 |
| Interferon-alpha treatment | 43.6% | 196%–67.5% | Hahka-Kemppinen et al 1995; Mughal et al 1991; Sersa et al 2000 |
| Isolated limb perfusion | 92.6% | 87.5%–95.2% | Lienard et al 1999; Rossi et al 2004 |
Objective responses (the response relates to the treated patients).
The effectiveness data refer to cutaneous and subcutaneous metastases; according to the criteria of the WHO Handbook for Reporting Results of Cancer Treatment, success is evaluated based on the response, which can be: complete (CR), partial (PR), objective (OR = CR+PR), no change (NC), and progressive disease (PD). As shown in Table 7, the success is evaluated on the objective response.
Cost-effectiveness analysis
Cost-effectiveness analysis is a systematic method of comparing two or more alternative strategies. Their difference in cost (incremental cost) is compared with their difference in outcomes (incremental effectiveness) by dividing the former by the latter. This ratio is known as the incremental cost-effectiveness ratio (ICER). After determining the cost and effectiveness of each strategy, the programs are compared using the cost-effectiveness ranking algorithm, which consists of ranking the alternatives in the order of their costs, eliminating those that are dominated by simple dominance. In simple dominance, there is another alternative that is both more effective and less costly.
Results
Table 8 compares the cost, the effectiveness, the cost per response obtained, and the ICER between the different therapeutic options. The cost data in the first column (C) shows that radiotherapy proved to be the less costly therapeutic alternative in the treatment of cutaneous and subcutaneous metastases. Innovative ECT is the second most economical choice, followed by the combination of hyperthermia-chemotherapy-radiotherapy. Interferon-alpha treatment and isolated limb perfusion proved to be the costliest options.
Table 8.
Average cost, effectiveness, and cost-effectiveness ratio per achieved tumor response and incremental cost effectiveness ratio (ICER)
| C | E | C/E | ΔC | ΔE | ΔC/ΔE | |
|---|---|---|---|---|---|---|
| Treatment | Cost | Effectiveness | Average cost per achieved response | Delta cost | Delta effectiveness | ICER |
| Radiotherapy | €1,124.23 | 56.0% | €2,007.55 | - | - | - |
| ECT | €1,408.68 | 74.1% | €1,901.05 | €284.45 | 18.1% | €1,571.53 |
| Hyperthermia+Chemo+Radio | €2,089.96 | 73.3% | €2,851.24 | - | - | Dominated |
| Interferon-alpha treatment | €14,052.98 | 43.6% | €32,268.61 | - | - | Dominated |
| Isolated limb perfusion | €18,530.47 | 92.6% | €20,018.51 | €17,121.79 | 18.5% | €92,717.29 |
In terms of effectiveness (second column [E]), isolated limb perfusion is the first choice, with a very high percentage of tumor response, followed by ECT and the combination of hyperthermia-chemotherapy-radiotherapy (radiotherapy alone is much less effective). Interferon-alpha treatment proved to be the least effective choice.
The third column (C/E) expresses an average cost-effectiveness ratio indicating the average cost per objective tumor response to the treatment.
ECT, radiotherapy and the combination of hyperthemia-chemotherapy-radiotherapy have limited costs, particularly in comparison with isolated limb perfusion and Interferon-alpha treatment.
The sixth column (ICER = ΔC/ΔE) shows the ICER between the different treatment options.
The amount of €284.45 in the fourth column (ΔC) indicates the cost difference between ECT and radiotherapy. The 18.1% value in the fifth column (ΔE) represents the difference in effectiveness between the above-mentioned therapeutic alternatives; in order to achieve a homogeneous comparison, the considered effectiveness parameter is the objective response.
ECT proved therefore to be both more costly and more effective than radiotherapy; the value of €1,571.53 (ICER) represents the cost that the SSN would sustain by using ECT instead of radiotherapy to achieve a further additional objective response.
In these comparisons, ECT proved to be a dominant strategy with respect to the hyperthemia-chemotherapy-radiotherapy combination, since it is less costly and more effective.
Furthermore, ECT proved to be a dominant strategy with respect to interferon-alpha treatment, being less costly (€1,408.68 vs €14,052.98) and definitely more effective (74.1% vs 43.6%). If isolated limb perfusion is used instead of the innovative ECT, in order to achieve an additional objective response, the ICER value would significantly increase to €92,717.29.
Sensitivity analysis
A one-way sensitivity analysis was carried out on all the main cost parameters of ECT (±10%), and ECT’s effectiveness. The cost sensitivity analysis results do not alter the ranking of the various options already shown in the preceding tables. In particular, it is interesting to report the results of the sensitivity analysis of the electrode costs, since this value appeared to be the main cost driver in our analysis. If electrode cost declines by 10%, the total cost for ECT would be €1,291.68; if electrode cost increases by 10%, the total cost would be €1,525.68 (Tables 9-10). These new total cost values for ECT fall between the cost for radiotherapy and the cost for the combination of hyperthemia-chemotherapy-radiotherapy, which perfectly confirms the results of the base case (€1,408.68). In Tables 11-12 we consider a variation between mininum (63.4%) and maximum (84.8%) ECT effectiveness. Also in this case sensitivity analysis confirms the results of the base case scenario.
Table 9.
Sensibility analysis: −10% cost of electrodes per procedure
| C | E | C/E | ΔC | ΔE | ΔC/ΔE | |
|---|---|---|---|---|---|---|
| Treatment | Cost | Effectiveness | Average cost per achieved response | Delta cost | Delta effectiveness | ICER |
| Radiotherapy | €1,124.23 | 56.0% | €2,007.55 | - | - | - |
| ECT | €1,291.68 | 74.1% | €1,743.15 | €167.45 | 18.1% | €925.12 |
| Hyperthermia+Chemo+Radio | €2,089.96 | 73.3% | €2,851.24 | - | - | Dominated |
| Interferon-alpha treatment | €14,052.98 | 43.6% | €32,268.61 | - | - | Dominated |
| Isolated limb perfusion | €18,530.47 | 92.6% | €20,018.51 | €17,238.79 | 18.5% | €93,350.87 |
Table 10.
Sensibility analysis: +10% cost of electrodes per procedure
| C | E | C/E | ΔC | ΔE | ΔC/ΔE | |
|---|---|---|---|---|---|---|
| Treatment | Cost | Effectiveness | Average cost per achieved response | Delta cost | Delta effectiveness | ICER |
| Radiotherapy | €1,124.23 | 56.0% | €2,007.55 | - | - | - |
| ECT | €1,525.68 | 74.1% | €2,058.94 | €401.45 | 18.1% | €2,217.94 |
| Hyperthermia+Chemo+Radio | €2,089.96 | 73.3% | €2,851.24 | - | - | Dominated |
| Interferon-alpha treatment | €14,052.98 | 43.6% | €32,268.61 | - | - | Dominated |
| Isolated limb perfusion | €18,530.47 | 92.6% | €20,018.51 | €17,004.79 | 18.5% | €92,083.72 |
Table 11.
Sensibility analysis: 63.4% effectiveness of ECT (minimum)
| C | E | C/E | ΔC | ΔE | ΔC/ΔE | |
|---|---|---|---|---|---|---|
| Treatment | Cost | Effectiveness | Average cost per achieved response | Delta cost | Delta effectiveness | ICER |
| Radiotherapy | €1,124.23 | 56.0% | €2,007.55 | - | - | - |
| ECT | €1,408.68 | 63.4% | €2,221.89 | €284.45 | 7.4% | €3,843.87 |
| Hyperthermia+Chemo+Radio | €2,089.96 | 73.3% | €2,851.24 | €681.28 | 9.9% | €6,881.65 |
| Interferon-alpha treatment | €14,052.98 | 43.6% | €32,268.61 | - | - | Dominated |
| Isolated limb perfusion | €18,530.47 | 92.6% | €20,018.51 | €16,440.51 | 19.3% | €85,331.37 |
Table 12.
Sensibility analysis: 85.0% effectiveness of ECT (maximum)
| C | E | C/E | ΔC | ΔE | ΔC/ΔE | |
|---|---|---|---|---|---|---|
| Treatment | Cost | Effectiveness | Average cost per achieved response | Delta cost | Delta effectiveness | ICER |
| Radiotherapy | €1,124.23 | 56.0% | €2,007.55 | - | - | - |
| ECT | €1,408.68 | 84.8% | €1,661.18 | €284.45 | 28.8% | €987.66 |
| Hyperthermia+Chemo+Radio | €2,089.96 | 73.3% | €2,851.24 | - | - | Dominated |
| Interferon-alpha treatment | €14,052.98 | 43.6% | €32,268.61 | - | - | Dominated |
| Isolated limb perfusion | €18,530.47 | 92.6% | €20,018.51 | €17,121.79 | 7.8% | €220,452.27 |
Discussion
The cost-effectiveness results confirm the favorable cost-effectiveness ratio for ECT, its applicability, and its ease of use, with a cost of €1,901.05 per achieved response. The ICER analysis derived the additional cost for SSN in order to obtain a further tumor response. Such a value, if ECT is used instead of radiotherapy, would give an incremental cost of €1,571.53. On the other hand, the hyperthermia-chemotherapy-radiotherapy and interferon-alpha treatment options were dominated by ECT, whereas isolated limb perfusion has an extremely onerous cost-effectiveness ratio (€92,717.29) compared with ECT.
The analysis was carried out at standard costs and does not take into consideration the potential discretionary power of the physician in patient selection in clinical practice. Furthermore, our cost evaluation of ECT with the Cliniporator was in line with the values calculated in the European study (Mir et al 2006), as shown in Figure 1. The sensitivity analysis carried out on the main ECT cost driver, the electrode cost, gave results in line with the base-case scenario; the proposed data justify therefore a more widespread use of ECT with the use of the Cliniporator. The extremely high ICER value for isolated limb perfusion justifies its limited use. Lastly, interferon-alpha treatment proves to be a dominated strategy, being the least effective and most expensive of the considered therapies. Currently, ECT is applied mainly in the treatment of secondary cutaneous and subcutaneous tumors (metastases) of any histological origin, ie, not only skin cancers, but also solid tumors, carcinomas, sarcomas, and so on that give rise to cutaneous and subcutaneous metastases. Our analysis has some limitations: clinical outcomes, for example, were derived from literature review and the success was evaluated on the objective response in order to compare clinical effectiveness among alternatives. Further data on costs and outcomes for ECT need to be collected over a longer time frame. Conventional surgery, when applicable, appears in clinical practice to be the best choice (Wolf et al 2003); should surgery prove to be devastating for the patient, from the esthetic and functional points of view, ECT is an excellent alternative, one reason being the rapid application and the scarce side effects (a light muscle contraction on the treated site during the delivery of the electric pulses).
Use of ECT with the Cliniporator is preferable to isolated limb perfusion, which has a very high ICER value. Other advantages of ECT, not considered in this analysis, are the possibility of repeated applications on the same patient if new metastases develop, and the possibility of being used as an adjuvant to standard therapies. Often, for patients with many lesions, lesions in sensitive areas (face, head, neck), or large lesions, none of the alternatives to ECT can be applied. In these cases, ECT with the Cliniporator is not only the therapy of choice, but also the only feasible therapy.
Acknowledgments
The study was financially supported by IGEA Srl, Carpi (MO), Italy.
References
- ASSR. Agenzia per i Servizi Sanitari Regionali. 2007 [online] URL: URL: http://www.assr.it/spesasanitaria.htm#spesa_san.
- Balch CH, Buzaid A, et al. A new American Joint Committee on Cancer Staging System for Cutaneous Melanoma. Cancer. 2000;88:1484–91. doi: 10.1002/(sici)1097-0142(20000315)88:6<1484::aid-cncr29>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Falk MH, Issels LD. Hyperthermia in oncology. Int J Hyperthermia. 2001;17:11–18. doi: 10.1080/02656730150201552. [DOI] [PubMed] [Google Scholar]
- Gabriele P, Orecchia R, et al. Clinical hyperthermia, alone or with radiation therapy: results of a preliminary study on recurrences of cancers. Arch Geschwulstforsch. 1989;59:177–81. [PubMed] [Google Scholar]
- Gaudy-Marqueste C, Regis JM, et al. Gamma-Knife radiosurgery in the management of melanoma patients with brain metastases: a series of 106 patients without whole-brain radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65:809–16. doi: 10.1016/j.ijrobp.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Gehl J, Skovsgaard T, Mir LM. Enhancement of cytotoxicity by electropermeabilization: an improved method for screening drugs. Anticancer Drugs. 1998;9:319–25. doi: 10.1097/00001813-199804000-00005. [DOI] [PubMed] [Google Scholar]
- Gothelf A, Mir LM, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 2003;29:371–87. doi: 10.1016/s0305-7372(03)00073-2. [DOI] [PubMed] [Google Scholar]
- Hahka-Kemppinen M, Muhonen T, et al. Response of subcutaneous and cutaneous metastases of malignant melanoma to combined cytostatic plus interferon therapy. Br J Dermatol. 1995;132:973–7. doi: 10.1111/j.1365-2133.1995.tb16958.x. [DOI] [PubMed] [Google Scholar]
- Health Ministry. Nomenclatore delle Prestazioni di assistenza specialistica ambulatoriale, Ministero della Salute. 2006 National Tariff Nomenclator.2006. [Google Scholar]
- Health Ministry. Hospital DRG tariffs, TUC, Tariffa Unica Convenzionale, National DRG Tariff. 2006 [Google Scholar]
- Informatore Farmaceutico. Medicinali: Publisher Masson-Elsevier; 2007. [Google Scholar]
- Jaroszeski MJ, Coppola D, et al. Treatment of hepatocellular carcinoma in a rat model using f. Eur J Cancer. 2001;37:422–30. doi: 10.1016/s0959-8049(00)00412-3. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hahn EW, et al. Combination hyperthermia and radation therapy for cutaneous malignant melanoma. Cancer. 1978;41:2143–8. doi: 10.1002/1097-0142(197806)41:6<2143::aid-cncr2820410610>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lienard D, Eggermont AM, et al. Isolated limb perfusion with tumor nercrosis factor alpha and melphalan with or without interferon-gamma for the treatment of in transit melanoma metastases: a multicenter randomized phase II study. Melanoma Res. 1999;9:491–502. doi: 10.1097/00008390-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Feun Lynn G, Savaraj Niramol, et al. A clinical trial of intravenous vinorelbine tartrate plus tamoxifen in the treatment of patients with advanced malignant melanoma. Cancer. 2000;88:584–8. doi: 10.1002/(sici)1097-0142(20000201)88:3<584::aid-cncr14>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy - an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of the ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer. 2006;4(Suppl):3–13. [Google Scholar]
- Meier F, Will S, et al. Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Brit J Dermatol. 2002;147:62–70. doi: 10.1046/j.1365-2133.2002.04867.x. [DOI] [PubMed] [Google Scholar]
- Mir LM, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator™ by means of invasive or non-invasive electrodes. Eur J Cancer. 2006;4(Suppl):14–25. [Google Scholar]
- Mir LM, Glass LF, et al. Effective treatment of cutaneous and subcutaneous malignant tumours by electrochemotherapy. Br J Cancer. 1998;77:2336–42. doi: 10.1038/bjc.1998.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughal TI, Thomas MR, et al. Role of recombinant alpha-interferon in the treatment of advanced cutaneous malignant melanoma. Oncology. 1991;48:365–8. doi: 10.1159/000226960. [DOI] [PubMed] [Google Scholar]
- Popov P, Tukiainen E, et al. Soft-tissue sarcomas of the upper extremity: Surgical treatment and outcome. Plast Reconstr Surg. 2004;113:222–32. doi: 10.1097/01.PRS.0000095946.90511.1D. [DOI] [PubMed] [Google Scholar]
- Richtig E, Hoff M, et al. Efficacy of superficial and deep regional hyperthermia combined with systemic chemotherapy and radiotherapy in metastatic melanoma. J Dtsch Dermatol Ges. 2003;1:635–42. doi: 10.1046/j.1610-0387.2003.03719.x. [DOI] [PubMed] [Google Scholar]
- Rossi CR, Foletto M, et al. Hypertermic isolated limb perfusion with low-dose tumor necrosis factor- alpha and mephlan for bulky in-transit melanoma metastases. Ann Surg Oncol. 2004;11:173–7. doi: 10.1245/aso.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Sersa G, Borut S, et al. Electrochemotherapy with cisplatin: clinical experience in malignant melanoma patients. Clin Cancer Res. 2000;6:863–7. [PubMed] [Google Scholar]
- Sersa G, Cemazar M, et al. Antitumor effectiveness of electrochemotherapy with cis-diamminedichloroplatinum(II) in mice. Cancer Res. 1995;55:3450–5. [PubMed] [Google Scholar]
- Wolf IH, Richtig E, Kopera D, et al. Locoregional cutaneous metastases of malignant melanoma and their management. Dermatol Surg. 2004;30:244–7. doi: 10.1111/j.1524-4725.2004.30091.x. [DOI] [PubMed] [Google Scholar]
- Wolf IH, Smolle J, et al. Topical imiquimod in the treatment of metastatic melanoma to skin. Arch Dermatol. 2003;139:273–6. doi: 10.1001/archderm.139.3.273. [DOI] [PubMed] [Google Scholar]