Abstract
The purpose of this review was to test contraceptive efficacy, cycle control, tolerability, and acceptability as found in the non-comparative studies with NuvaRing® by those found in the randomized trials comparing NuvaRing and combined oral contraceptives (COCs). All large non-comparative studies and all relevant randomized controlled trials (RCTs) between NuvaRing and a COC up to and including December 2006 were analyzed. Two large multi-center registration studies, 1 large daily clinical practice study, and 6 RCTs comparing NuvaRing and a COC were identified. The findings in the non-comparative studies were confirmed in the RCTs. Contraceptive efficacy was high showing no significant differences in comparison with the COC; cycle control was good and consistently better than that of the COC; compliance was high and comparable with that of the pill; the incidence of adverse events such as breast tenderness, headache, and nausea was low, but not lower than with the COC despite a halving of the systemic exposure to ethinyl estradiol (EE) with NuvaRing compared with a 30-μg EE-containing COC; the incidence of local and ring-related events was low but higher than with the COC, leading to higher discontinuation rates among NuvaRing users; acceptability was high and comparable between both contraceptives, resulting in a global improvement of sexual function with both methods. After study completion, women using NuvaRing were more likely to continue with their method than women using a COC. The good results with respect to contraceptive efficacy, cycle control, tolerability, and acceptability as achieved with NuvaRing in the large non-comparative registration studies were confirmed in the RCTs comparing NuvaRing with different COCs.
Keywords: acceptability, contraceptive efficacy, cycle control, NuvaRing, tolerability, vaginal ring
Introduction
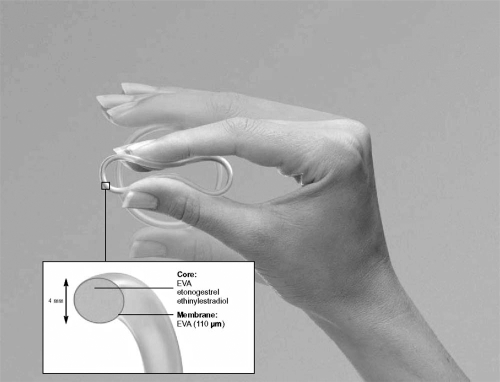
The combined contraceptive vaginal ring (CCVR, NuvaRing®, Organon Int., Oss, The Netherlands) is a new once-a-month method of hormonal contraception. It consists of a flexible, soft, transparent, ring measuring 54 mm in diameter and 4 mm thickness which can easily be inserted by the woman herself into the vagina (see Fig. 1). After 3 weeks of use the woman removes the ring, thereby introducing a ring-free week during which a withdrawal bleeding normally occurs.
Figure 1.
The combined contraceptive vaginal ring (NuvaRing®).
The ring is made of the copolymer evatane, in which the hormones ethinyl estradiol (EE, 2.7 mg) and etonogestrel (ENG, 11.7 mg) are equally dispersed. ENG is 3-ketodesogestrel, which is the active metabolite of the progestin desogestrel. Due to the composition of the ring, it steadily releases 15 μg EE and 120 μg ENG daily which are then continuously absorbed through the vaginal epithelium. Vaginal administration of contraceptive hormones with NuvaRing has the advantage of avoiding gastrointestinal absorption and hepatic first-pass metabolism, but does not produce elevated uterine concentrations of EE and ENG compared with a COC (Roumen and Dieben 2006). Compared with a COC containing 150 μg desogestrel and 30 μg EE, systemic exposure to ENG is similar for NuvaRing, whereas systemic exposure to EE with NuvaRing is approximately 50% of that for the COC (Timmer and Mulders 2000; van den Heuvel et al 2005). Ovulation suppression, as assessed by follicular diameter and serum hormone levels, is comparable (Mulders and Dieben 2001) or slightly lower (Duijkers et al 2004) between NuvaRing and COC use. Ovulations, however, do not occur (Duijkers et al 2004).
Contraceptive efficacy, cycle control, tolerability, and acceptability of NuvaRing have been established in several large non-comparative multi-center registration studies (Roumen et al 2006; Dieben et al 2002), and in daily clinical practice (Roumen et al 2006). It is interesting to find out whether the results of these studies could be confirmed in randomized controlled trials (RCTs) comparing NuvaRing with COCs, as the working mechanism (ovulation inhibition) and the contraindications for both methods are very similar. Up to and including December 2006, the results of 6 such trials have been published in 10 peer-reviewed articles. The results of the non-comparative studies will be compared with those of these RCTs, and reviewed here.
Contraceptive efficacy
Contraceptive efficacy with NuvaRing was examined in two 1-year, open-label, non-comparative studies conducted in 52 Western European centers and in 48 centers in the US and Canada, involving nearly 1200 women each (Roumen et al 2001; Dieben et al 2002). Throughout the study period, less unwanted pregnancies occurred in Western Europe (n = 6) than in North America (n = 15). The Pearl indices for the intent-to-treat (ITT) populations were 0.65 in the European study, and 1.75 in the American study (Table 1). The method-related Pearl indices were lower: 0.40 (95% CI 0.08–1.16), and 1.27 (95% CI 0.51–2.62), respectively. Pooling the pregnancies of both studies together resulted in a Pearl index of 1.18 (95% CI 0.73–1.80). The overall cumulative pregnancy rate of in-treatment pregnancy, derived from life table analysis, was 1.18% (95% CI 0.68–1.69), and comparable with the Pearl index. The pooled method-related Pearl index was 0.77 (95% CI 0.37–1.40).
Table 1.
Contraceptive efficacy with NuvaRing in 2 non-comparative studies, and in 2 randomized controlled trials comparing NuvaRing with 2 different COCs: 150LNG/30EE and DRSP/30EE (ITT population)
| Study | Method (number of participants) | Pearl index | 95% CI |
|---|---|---|---|
| Roumen et al 2001 | NuvaRing (n = 1145) | 0.65 | 0.24–1.41 |
| Dieben et al 2002 | NuvaRing (n = 1177) | 1.75 | 0.98–2.89 |
| Oddsson et al 2005a | NuvaRing (n = 512) | 1.23 | 0.40–2.86 |
| 150LNG/30EE COC (n = 518) | 1.19 | 0.39–2.79 | |
| Ahrendt et al 2006 | NuvaRing (n = 499) | 0.25 | 0.01–1.36 |
| DRSP/30EE COC (n = 484) | 0.99 | 0.27–2.53 |
Abbreviations: CI, confidence interval; COCs, combined oral contraceptives; DRSP, drospirenone; EE, ethinyl estradiol; ITT, intent-to-treat; LNG, levonorgestrel.
Occurrence of pregnancy was also determined in a Phase III, open-label, group-comparative, multi-center trial conducted in 9 European and 2 South American countries (Oddsson et al 2005a). In this study, 1030 women were randomized to 13 cycles of treatment with NuvaRing (n = 512) or a 150 levonorgestrel (LNG)/30EE COC (n = 518) and comprised the ITT population. The per protocol (PP) population comprised 899 subjects (NuvaRing = 440; COC = 459). A total of 10 pregnancies occurred during treatment in the ITT population (NuvaRing = 5; COC = 5). Of these, 3 subjects in the NuvaRing group and 2 subjects in the COC group had no protocol violations or only minor protocol violations that occurred after the estimated date of conception, reducing the number of PP in-treatment pregnancies to 5 (NuvaRing = 3; COC = 2), with no significant difference between PP treatment groups. The Pearl indices for the ITT populations were 1.23 and 1.19 per 100 women-years for the ring and COC groups, respectively (Table 1). No significant difference was found between the two treatment groups. The estimated cumulative probabilities of in-treatment ITT pregnancy after cycle 13 were 1.20% (95% CI 0.14–2.26%) for the ring group and 1.07% (95% CI 0.13–2.00%) for the COC group. For the PP population, the estimated probabilities were 0.71% (95% CI 0.00–1.52%) and 0.43% (95% CI 0.00-1.01%) for the ring and COC groups, respectively. The results show therefore that contraceptive efficacy was comparable for NuvaRing and this COC.
These results were confirmed in another open-label, group-comparative, multi-center trial conducted in 10 countries in Europe (Ahrendt et al 2006). In this study, 983 women were randomly treated with NuvaRing (n = 499) or a COC containing 3 mg drospirenone and 30 μg EE (DRSP/30EE) (n = 484) for 13 cycles (ITT population). There were 5 pregnancies during the study, 1 in the NuvaRing group and 4 in the COC group. Two pregnancies in the COC group were associated with protocol violations. Pearl indices for the ITT population were 0.25 and 0.99 per 100 women-years for the NuvaRing and COC groups, respectively (Table 1). These results were not significantly different (p = 0.37).
Cycle control
Bleeding patterns were evaluated in the two 1-year, open-label, non-comparative studies conducted in 52 Western European centers and in 48 centers in the US and Canada (Roumen et al 2001; Dieben et al 2002). Combining the data of the PP populations of both studies resulted in a withdrawal bleeding in 98.5% of women (95% CI 97.7%–99.0%); early withdrawal bleeding (starting before the ring-free week) in 6.1% of women (95% CI 5.1%–8.4%); and late withdrawal bleeding (persisting after the ring-free week) in 23.9% of women (95% CI 20.5%–26.5%). In most women, early or late withdrawal bleeding was restricted to spotting only. The incidence of irregular bleeding was very low, with an average of 5.5% per cycle over cycles 1–13, and slightly declining during the study. Irregular bleeding was less frequent in the European study (4.4% of women per cycle) than in the North American study (7.0% of women per cycle). In the majority of cycles, the irregular bleeding was restricted to spotting only. Cycles with an “intended bleeding pattern”, defined as a cycle with a withdrawal bleeding in the ring-free week, no early withdrawal bleeding or continued withdrawal bleeding, and no irregular bleeding, can be calculated to be 63.0% in the combined data.
Bleeding patterns were also examined in an observational study among 854 women in The Netherlands who started with NuvaRing (Roumen et al 2006). In the subgroup of participants who had previously used a COC (86.8%), the percentage of women who reported irregular blood loss during the first three NuvaRing cycles decreased from 32% to 16% (p < 0.0001) (spotting from 20% to 9%, and bleeding from 12% to 7%). In total, after 3 cycles, 42% of the ring users reported a decrease in blood loss during the ring-free week compared with the pill-free week following the end of a cycle of COC use, whereas 12% of the ring users reported an increase. In 39% of the cases, after 3 cycles of ring use the blood loss was reported to be of shorter duration.
Data on cycle control with NuvaRing versus the 150LNG/30EE COC were obtained in the randomized, comparative, multicenter, Phase III study conducted in 11 countries (Oddsson et al 2005a, b). In the ITT analysis, the incidence of breakthrough bleeding and spotting over cycles 2–13, the primary efficacy parameter, was lower with NuvaRing (range 2.0%–6.4%) than the COC (range 3.5%–12.6%), and for cycles 2 and 9 the lower incidence with NuvaRing was confirmed as statistically significant (p < 0.003). As shown in Table 2, the incidence of intended bleeding pattern was significantly higher over all cycles with NuvaRing (58.8%–72.8%) than with the COC (43.4%–57.9%) (p < 0.005). This was mainly due to a lower incidence of continued withdrawal bleeding for the NuvaRing group (21.7%–27.3%) than the COC group (33.8%–39.0%) in cycles 1–13 and this was statistically significant for all cycles (p < 0.02). Continued withdrawal bleeding with spotting days occurred less frequently in the NuvaRing group (16.2%–23.2%) in cycles 1–13 and was statistically significant for cycles 1, 3–7, 9, 10, and 12 (p < 0.05) compared with the COC group (25.6%–30.7%). There was no significant difference in the incidence of early withdrawal bleeding or spotting between both treatment groups.
Table 2.
The incidence of intended bleeding pattern - defined as cycles with a withdrawal bleeding, no early or continued withdrawal bleeding, and no irregular bleeding - during NuvaRing and COC use in 1 large non-comparative study and 2 large randomized controlled trials
| Study | Method (number of participants) | Cycles with an intended bleeding pattern (%) | p value |
|---|---|---|---|
| Dieben et al 2002 | NuvaRing (n = 2322) | 63.0 | |
| Oddsson et al 2005b (13 cycles) | NuvaRing (n = 512) | 58.8–72.8 | <0.005 |
| 150LNG/30EE COC (n = 518) | 43.4–57.9 | for all cycles | |
| Milsom et al 2006 (13 cycles) | NuvaRing (n = 499) | 55.2–68.5 | <0.01 |
| DRSP/30EE COC (n = 484) | 35.6–56.6 | for all cycles |
Abbreviations: COCs, combined oral contraceptives; DRSP, drospirenone; EE, ethinyl estradiol.
Cycle control was also studied in the women participating in the European open-label, group-comparative, multi-center trial of Ahrendt et al (2006). A significantly higher incidence of intended bleeding in the NuvaRing group (55.2%–68.5%) compared with the DRSP/30EE COC group (35.6%–56.6%) was found for each of the ITT cycles 1–12 (p < 0.01). Breakthrough bleeding or spotting during cycles 2–13 ranged from 3.6% to 6.2% in the NuvaRing group and from 4.7% to 10.4% in the COC group. The incidence of breakthrough bleeding/spotting was lower with NuvaRing than with the COC for all cycles except cycles 11 and 12, but this did not achieve statistical significance. However, significantly fewer breakthrough spotting episodes were observed in the NuvaRing group during cycles 1, 3, 4, 6, and 10 (p < 0.05), whereas significantly more early withdrawal bleedings were observed in the NuvaRing group compared with the COC group during cycles 1, 5, 8, and 12 (p < 0.05). Between-group differences for continued withdrawal bleeding were statistically significant in each of cycles 1–12 in favor of NuvaRing (p < 0.0001). In both groups, early and continued withdrawal bleeding consisted mainly of spotting days during the ring/pill period. The incidence of cycles (1–13) without withdrawal bleeding ranged from 0.2% to 3.2% for NuvaRing users and from 0.5% to 1.7% for COC users, with no statistically significant differences between the groups in any cycle.
Bleeding patterns were also studied using the Quick Start approach, which means that the contraceptive method is initiated immediately, at the time of the clinic visit, regardless of menstrual cycle day (Westhoff et al 2005). In an open-label, one-center, controlled trial, 201 women were randomly assigned to NuvaRing (ITT = 78) or a triphasic COC containing norgestimate (0.18 mg during the first week; 0.215 mg during the second week; 0.25 mg during the third week) and 25 μg EE (NOR/25EE) (ITT = 78). Using the standardized definitions of the clinically important WHO menstrual indices, which exclude any menstrual events in progress at the beginning and end of the reference period, the NuvaRing users experienced significantly fewer bleeding-spotting days (14.5 versus 19.2 days, respectively, p < 0.001) during the 84-day reference period (three 28-day cycles). Ethnicity, weight, body mass index, and smoking were not associated with the number of bleeding-spotting days. In the COC group, however, there was a weak association between nulliparity and bleeding-spotting days, particularly for older nulliparous participants. No significant differences in bleeding patterns were found based on analysis of cycle week at study enrolment. The difference in bleeding-spotting days was largely attributable to a difference in bleeding, with NuvaRing users experiencing fewer bleeding-only days (9.1 versus 11.9 days, respectively). The number of bleeding-spotting episodes was also significantly lower (2.4 versus 3.0, respectively) in NuvaRing users, and their bleeding-spotting-free intervals were significantly longer (21.2 versus 19.0 days, respectively). Fifteen percent of NuvaRing users experienced prolonged bleeding (at least 1 bleeding-spotting episode lasting 10 or more days), compared with 31% of pill users (p = 0.04). At the end of the study, the women answered exit questions about their perceptions of bleeding during the 84-day reference period compared with their bleeding at times when they were not using hormonal contraception. A significantly greater proportion of women using NuvaRing perceived a decrease in duration of bleeding than those on the COC (p < 0.01). Similarly, NuvaRing users were less likely to report an increase in flow, although this difference did not reach statistical significance. NuvaRing users were more likely to report no change or a “good” change in their bleeding, whereas COC users were more likely to report a “bad” change (p < 0.01). Hence, alongside conventional method initiation, the Quick Start approach also gave women using NuvaRing better bleeding patterns than those using the COC.
An open-label, single-center, cross-over study was carried out in which women were randomly assigned to either NuvaRing (ITT = 33) or a COC containing 100 μg levonorgestrel and 20 μg EE (100LNG/20EE) (ITT = 31) for 3 consecutive 28-day cycles, directly followed by three cycles of the alternative study drug (Veres et al 2004). During NuvaRing use, there were fewer total bleeding days, and the number of inter-menstrual bleeding days averaged 0.2 days compared with 1.4 days with COC use (p < 0.001).
A prospective study during 1 year was carried out in 280 women who were randomized to either NuvaRing (n = 94), or a COC containing 100 μg levonorgestrel and 20 μg EE (100LNG/20EE) (n = 94), or a COC containing 60 μg gestodene and 15 μg EE (60 gestodene [GES]/15EE) (n = 92) (Sabatini and Cagiano 2006). The incidence of irregular bleeding was significantly lower in the NuvaRing group as compared with both COC groups in all cycles (p < 0.05).
Bleeding patterns in extended ring regimens were examined in a 1-year open-label, comparative study in 10 European centers and 10 centers in the US (Miller et al 2005). The women were randomly assigned to 1 of 4 regimens: monthly (28-day cycle), every other month (49-day cycle), every third month (91-day cycle), or continuous (264-day cycle). The mean percentages of bleeding and/or spotting days were 17.6% (28-day), 15.5% (49-day), 20.9% (91-day), and 24.4% (364-day). Discontinuation rates for unacceptable bleeding were higher for the 91-day and 364-day cycles compared with the 28-day cycle.
Tolerability
Compliance
In the non-comparative studies, compliance with the prescribed NuvaRing regimen was higher in Europe (90.8%) than in North America (79.9%) (Roumen et al 2001; Dieben et al 2002). The first experience in daily clinical practice in The Netherlands revealed a compliance of 88% (Roumen et al 2006). In this study, 3% of women inserted the ring too early or too late, and 3.4% removed it too early or too late. Another 3.2% had intentionally not complied with the prescribed regimen in order to regulate their cycle, whereas 0.9% reported loss of the ring. In the first cycle, 2.1% of women left the ring outside the vagina for longer than the advised 3 h, and 1.8% of women did this during the second and third cycles.
In most randomized studies, compliance with the prescribed regimen was high and comparable between the NuvaRing groups and the COC groups (87.4% versus 86.6% (Oddsson et al 2005a), and 89.2% versus 85.5% (Ahrendt et al 2006), of ITT cycles, respectively).
Blood pressure
In the non-comparative studies, no clinically relevant changes from baseline were observed in blood pressure (Roumen et al 2001; Dieben et al 2002). In most randomized studies, blood pressure did not change significantly from baseline in either NuvaRing or COC users (Veres et al 2004; O’ Connell et al 2005; Oddsson et al 2005a; Ahrendt et al 2006; Roumen and Dieben 2006; Sabatini and Cagiano 2006). In 1 study, 4 subjects in the NuvaRing group (0.8%) and 8 in the COC group (1.5%) experienced hypertension (Oddsson et al 2005a).
Body weight
In the non-comparative European study, mean body weight increased by 0.43 ± 3.35 kg over the 13 cycles of treatment (Roumen et al 2001). A decrease in body weight from screening to the last visit of ≥7% was reported for 8% of the women while an increase in body weight of ≥7% was reported for 10% of women. The combined data of the non-comparative European and American studies showed a mean body weight increase by 0.84 ± 3.81 kg over the 13 cycles of treatment (Dieben et al 2002). In most randomized studies no marked changes in body weight were seen between comparator groups (O’Connell et al 2005; Roumen and Dieben 2006; Sabatini and Cagiano 2006).
In the study of Oddsson et al (2005a), fewer NuvaRing users had an increase of ≥7% in body weight from baseline than COC (150LNG/30EE) users (8.4% versus 9.8%, respectively), and more women had a decrease of ≤7% (6.8% versus 5.0%, respectively). However, no significant differences between groups in body weight were found at study end.
In the study of Milsom et al (2006) which compared NuvaRing with DRSP/30EE, effects on body weight and body composition were relatively small and similar with both contraceptives over 1 year. For the NuvaRing ITT group, the estimated mean body weight change from baseline to the last assessment was 0.37 kg (two-sided 95% CI 0.10–0.64). For the COC ITT group, the estimated mean bodyweight change from baseline was −0.03 kg (2-sided 95% CI −0.29 to 0.23). In both cases, the upper limit of the 2-sided 95% CI was below the pre-specified 1.5 kg, and therefore it was concluded that weight neutrality of both NuvaRing and the COC was demonstrated. Individual data were not given.
In the Quick Start trial, the women were weighed upon enrolment and at exit after three cycles (NuvaRing = 82; COC = 79) (O’Connell et al 2005; Westhoff et al 2005). Participants gained an average of 2.8 lb over 3 months (95% CI 1.9–3.6 lb; p < 0.001). Weight change ranged from an 11-lb weight loss to a 20-lb weight gain (SD = 5.5). Subjects underwent an average increase in their BMI of 0.6 kg/m2, ranging from a decrease of 1.8 kg/m2 to an increase of 3.2 kg/m2 (SD = 0.9). These gains were similar between NuvaRing and COC groups (NuvaRing = 2.5 lb, COC = 3.1 lb (p = 0.49); NuvaRing = 0.53 kg/m2, COC = 0.58 kg/m2 (p = 0.76)), regardless of weight class, baseline weight, baseline BMI or the season of study enrolment or exit. Therefore, a small, but clinically unimportant weight gain was demonstrated with both contraceptives. Again, individual data were not given.
Premenstrual syndrome and dysmenorrhea
The first experience in daily clinical practice in The Netherlands showed that during the first three months of NuvaRing use, in comparison with the preceding contraceptive method, the percentage of women with complaints of dysmenorrhoea decreased from 42% to 26% (p < 0.0001), and of those with complaints of PMS from 45% to 29% (Roumen et al 2006). After 1 year of treatment, moderate or severe PMS or dysmenorrhea decreased in both the NuvaRing and COC groups (DRSP/30EE) with no apparent differences between the treatments (Milsom et al 2006). The proportion of subjects reporting moderate or severe PMS symptoms decreased from 12.6% to 4.5% in the NuvaRing group and from 14.7% to 4.7% in the COC group (screening versus cycle 13). The proportion of subjects reporting moderate or severe dysmenorrhea also decreased at study end compared with screening (decreasing from 17.4% to 5.9% in the NuvaRing group and from 19% to 6.4% in the COC group).
Negative mood impact (irritability and depression), however, was experienced less by NuvaRing users (4.2%) than by COC (100LNG/20EE and 60GES/15EE) users (8.5% and 8.6%, respectively) (p < 0.05) (Sabatini and Cagiano 2006).
Adverse events
In the combined data of the non-comparative European and American studies, 65.5% of women reported at least one adverse event, of which 37.5% were considered by the investigator as at least possibly related to NuvaRing use (Dieben et al 2002). The most frequently reported adverse events were headache, vaginitis, and leukorrhea. The incidence of breast tenderness and nausea was low. Vaginal discomfort was reported by 2.4% of women, and device-related events by 4.4%. As shown in Table 3, there were no major differences in the incidence of these events between the European and North American studies (Roumen et al 2001; Dieben et al 2002).
Table 3.
Percentage of women reporting adverse events with NuvaRing and a COC in two non-comparative studies and three randomized controlled trials
| Adverse event | Method | NuvaRing (n = 2322) (Europe3 -America4) | NuvaRing (n = 512) vs 150LNG/30EE COC (n = 518)5 | NuvaRing (n = 499) vs DRSP/30 EE COC (n = 484)6 | NuvaRing (n = 94) vs 100LNG/20EE COC (n = 94)7 | NuvaRing (n = 94) vs 60GES/15EE COC (n = 92) 7 |
|---|---|---|---|---|---|---|
| Breast tenderness | NuvaRing | 2.6 (1.9–3.3) | 3.1 | 3.2 | 4.2 | 4.2 |
| COC | 1.3 | 4.7 | 6.3 | 6.5 | ||
| Headache | NuvaRing | 5.8 (6.6–5.0) | 7.2 | 6.8 | 6.3 | 6.3 |
| COC | 5.8 | 7.6 | 9.5 | 9.7 | ||
| Nausea | NuvaRing | 3.2 (2.8–3.6) | 2.7 | 0.8 | 2.1 | 2.1 |
| COC | 4.0 | 3.7 | 7.4 | 5.4 | ||
| Leukorrhea | NuvaRing | 4.8 (5.3–4.2) | 3.5 | 3.2 | - | - |
| COC | 0.2 | 1.0 | - | - | ||
| Vaginal discomfort | NuvaRing | 2.4 (2.2–2.6) | - | 1.4 | - | - |
| COC | - | 0.0 | - | - | ||
| Vaginitis | NuvaRing | 5.6 (5.0–6.2) | 3.9 | 4.6 | - | - |
| COC | 1.0 | 2.1 | - | - | ||
| Ring-related events1 | NuvaRing | 4.4 (3.8–5.0) | 4.7 | 6.6 | - | - |
| COC | 0.0 | 0.42 | - | - |
Ring-related adverse events comprise foreign body sensation, coital problems, and expulsions.
dyspareunia.
Abbreviations: COCs, combined oral contraceptives; DRSP, drospirenone; EE, ethinyl estradiol; GES, gestodene; LNG, levonorgestrel.
The proportion of subjects reporting adverse events was comparable in the NuvaRing group and the COC (150LNG/30EE) group (57.6% and 54.3%, respectively) (Oddsson et al 2005a). Similar percentages of adverse events considered by the investigator to be at least possibly related to study treatment were also observed when the ring was compared with 150LNG/30EE or DRSP/30EE (NuvaRing: 28.9% (Oddsson et al 2005a), and 29.1% (Ahrendt et al 2006) versus COC: 22.1% (Oddsson et al 2005a), and 23.5% (Ahrendt et al 2006). The incidence of estrogen-related adverse events such as breast tenderness, headache, and nausea were comparable between treatment groups (Table 3) (Oddsson et al 2005a; Ahrendt et al 2006; Sabatini and Cagiano 2006). The main differences in at least “possibly” treatment-related adverse events between both groups were the higher incidences of local events such as leukorrhea, vaginal discomfort, vaginitis, and ring-related events (comprising foreign body sensation, coital problems and expulsions) in the NuvaRing groups than in the COC groups.
In the combined data of the non-comparative European and American studies, 15.1% of women discontinued because of adverse events (Roumen et al 2001; Dieben et al 2002). The most frequently reported adverse events that resulted in withdrawal were device-related problems (2.5%), headache (1.3%), emotional lability (1.2%), weight increase (1.0%), bleeding irregularities (0.8%), vaginitis (0.7%), and leukorrhea (0.6%). Ring-related problems were also the most important reason for premature discontinuation (3.9% of women) in the Netherlands’ study (Roumen et al 2006).
In most randomized studies, discontinuation due to adverse events was higher in the NuvaRing groups than in the COC groups (NuvaRing: 11.3% (Oddsson et al 2005a), and 12.2% (Ahrendt et al 2006), versus COC: 8.7% (Oddsson et al 2005a), and 9.9% (Ahrendt et al 2006), respectively), due to the additional ring-related events. In another study, however, the discontinuation rate was lower in NuvaRing users (11.7%) than in COC (100LNG/20EE and 60GES/15EE) users (22.3% and 30.4%, respectively) (Sabatini and Cagiano 2006).
In the “genital symptoms” study, Veres et al reported that the ring never slipped (ring descent with bearing down) in 46 (71.9%) NuvaRing users, while 6 (9.4%) users reported ring slippage at least once a week or more (Veres et al 2004). Ring slippage was not associated with an increase in vaginal wetness scores or any examination or laboratory finding. In this study, 72% of men never or rarely felt the ring during coitus, 92% reported no change in sensation during intercourse, and 87% did not feel the ring move during intercourse. Of the 24 men who reported 10 or more coital events with ring use, 4 (16%) reported that the ring would sometimes come out during coitus and 2 of these men thought that it interrupted sexual activity.
In the study by Guida et al (2005), 89% of women and 68% of partners never felt NuvaRing during sexual intercourse (10% of women and 24% of partners felt it occasionally, and 1% of women and 8% of partners always felt it).
The open-label, randomized, cross-over study of Veres et al (2004) was specifically designed to investigate genital symptoms, signs, examination, and laboratory findings with NuvaRing use compared with 100LNG/20EE. At baseline, 15% of women had yeast on culture; during NuvaRing use, 18.8% of women were positive for yeast by culture compared with 22.5% of women during COC use (p = 0.12). At baseline, 83.8% of women were positive for any Lactobacillus by culture, and at subsequent visits this percentage was similar and not different by method (p = 0.28). However, the concentration of Lactobacillus colony-forming units positive for hydrogen peroxide (H2O2) production increased during NuvaRing use (2.67-fold difference, 95% CI 1.49–4.78; p < 0.001) and increased over baseline values, possibly due to some sort of preferential delivery of EE to the vaginal tissue and suggesting improvement of the vaginal flora. All other examination and laboratory findings were not significantly different, including Nugent Gram stain score, vaginal white blood cell count, vaginal pH values and discharge weight. Most subjects reported few genital symptoms with either method, but 63% of subjects reported vaginal wetness during NuvaRing use compared with 43% during COC use. Most vaginal symptoms were scored as mild, and a severe score was very rare and sporadic. Vaginal wetness had the highest symptom score among NuvaRing users, but the scores were still quite low considering they represent 3-cycle averages of 28-day totals. There was a non-significant trend over the entire study of decreased reporting of symptoms, with on average 20% fewer total symptoms reported after cross-over (p = 0.28), perhaps reflecting improved method tolerance over time for both methods. However, both the ring-first and pill-first subjects reported on average more vaginal wetness during NuvaRing use than during COC use (2.74-fold difference, 95% CI 1.80–4.18; p < 0.001). There was also a carry-over effect for total symptoms, with ring-first subjects reporting more symptoms with subsequent pill use than pill-first-subjects (p = 0.045). Women who reported more vaginal wetness did not differ on laboratory findings from women who did not report this symptom, except for increased cervical ectopy at baseline, to predict the occurrence of this symptom and the finding of leukorrhea on examination (p = 0.03).
These findings were confirmed in another study (Sabatini and Cagiano 2006), in which after three cycles vaginal dryness was reported by less NuvaRing users (2.1%) than by COC (100LNG/20EE and 60GES/15EE) users (12.7% and 30.4%, respectively) (p < 0.005).
Serious adverse events
In most studies, no serious adverse events were reported (Roumen et al 2001; Dieben et al 2002; Veres et al 2004; O’Connell et al 2005; Roumen and Dieben 2006). One case of a cerebral venous sinus thrombosis was reported in the Netherlands’ study (Roumen et al 2006), and 3 cases (0,2%) of deep vein thrombosis, possibly related to study medication, were reported in the NuvaRing groups (Miller et al 2005; Oddsson et al 2005a; Ahrendt et al 2006).
Acceptability
In the combined data of the non-comparative European and North-American studies, the proportion of women who reported at least occasionally feeling the ring during intercourse was 18% and higher in the discontinuers (23%) than the completers (15%) (Roumen et al 2001; Dieben et al 2002). The percentage of partners feeling the ring during intercourse was 32% (discontinuers 37%, completers 29%). Most partners, however, in both the completers (83%) and the discontinuers (83%) groups did not object to women using the ring. From all participating women, 85% were satisfied or very satisfied with the ring at last assessment, and 90% of respondents (97% in completers and 75% in discontinuers) indicated that they would recommend the ring to others. There were no major differences between Europe and North-America.
Compared to the preceding method of contraception, the percentage of (very) satisfied users increased from 34% to 72% during NuvaRing use in The Netherlands, whereas the percentage of (very) dissatisfied users decreased from 44% to 16% (Roumen et al 2006). Of the study population, 82% of women and 67% of their partners never or rarely felt the ring during intercourse. For the women who completed all 3 cycles, these percentages were 85% and 68%, respectively, and for the group of women who discontinued, these percentages were 65% and 62%, respectively. In couples who felt the ring during intercourse, 56% of women and 38% of their partners found it unpleasant. From the 82.6% of women who completed the 3-month study period, 80% continued with NuvaRing use. The most frequently reported reasons for satisfaction (more than one answer possible) were once-a month administration (54%), low hormonal dose (31%), and ease of use (27%). The most frequently reported reasons for dissatisfaction were general adverse events (16%), local adverse events like expulsion (8%), and/or inconvenience during intercourse (7%).
In the comparison with DRSP/30EE, both NuvaRing and COC were found to be highly acceptable (Ahrendt et al 2006). The majority of women were satisfied with NuvaRing (59% very satisfied, 25% satisfied) and the COC (54% very satisfied, 33% satisfied) and would recommend the method to others (87% NuvaRing, 92% COC).
In the comparison with 100LNG/20EE, average levels of satisfaction reported at exit visit were 4.3 ±0.9 for NuvaRing use and 3.6 ±1.0 for COC use (p < 0.001), based on a scale of 1 to 5, where 1 = dissatisfied and 5 = best method (Veres et al 2004). At study completion, 50% of all women planned to continue using the ring, compared with 28% of women who planned to continue using the COC (6% chose another method and 16% chose no contraception). Ninety-three percent of partners said that they would definitely or possibly recommend NuvaRing to other couples.
User satisfaction and method continuation were also assessed in the previously described group of women with a Quick Start approach (O’Connell et al 2005; Westhoff et al 2006; Schafer et al 2006). At 3 months, 174 subjects (87%) had follow-up interviews (Schafer et al 2006). Significantly more NuvaRing users than COC users were very satisfied with their method (61% versus 34%; p = 0.003), and chose to continue their method (79% versus 59%; p < 0.001). Both the higher user satisfaction and the continuation of NuvaRing for birth control were not associated with prior use of vaginal contraceptives or products, masturbation, discomfort with intercourse or other behaviors that involve genital touching such as waxing and shaving pubic hair or having tattoos and/or body piercings. Neither demographic characteristics nor vaginal experiences identified successful ring users.
In an open-label, randomized, single center study, the influence of intravaginal and oral hormonal contraception on the sexual life of women and their partners was evaluated (Guida et al 2005). Healthy women with a permanent partner and an active sexual life were randomly assigned to NuvaRing (ITT = 26) or a COC containing 150 μg desogestrel and 20 μg EE (ITT = 25) for 6 consecutive 28-day cycles. A control group was also included, consisting of 25 women not using hormonal contraception. Sexual activity of the women and their partners was assessed by the Interviewer Ratings of Sexual Function (IRSF) questionnaire, at the start of the study and after cycles 3 and 6. Compared with women not using hormonal contraception, women in both the NuvaRing and the COC groups reported a global improvement of sexual function after 3 months, which was sustained until the 6-month assessment. Compared with the group not using hormonal contraception, in both contraceptive groups, significant improvements were observed for anxiousness (p < 0.001), sexual pleasure (p < 0.001), frequency and intensity of orgasm (p < 0.001), satisfaction (p < 0.001), sexual interest (p < 0.01), and complicity (p < 0.01). Only women using NuvaRing reported a significant increase in sexual fantasy compared with the women using the COC or no hormonal contraception (p < 0.001). A significant increase in the frequency of sexual intercourse was seen in both contraceptive groups at cycle 3 (p < 0.001) and at cycle 6 (p < 0.001) in comparison with baseline, and with women not using hormonal contraception at cycle 3 (p < 0.001) and at cycle 6 (p < 0.001). After 3 cycles, a significant reduction in anxiety and an increase in pleasure and satisfaction, frequency and intensity of orgasm were reported for partners of women in both the NuvaRing and the COC groups. A significant increase in sexual interest, complicity, and sexual fantasy was observed only in partners of women using NuvaRing. As for the women, the improvement of sexual function in the male partners of both contraceptive groups showed substantial consistency after 6 months.
The improved sexuality among NuvaRing users was confirmed in another study, in which after 12 cycles an increase of sexual desire and sexual satisfaction was reported by more NuvaRing users (75.5%, and 77.6%, respectively) than by COC (100LNG/20EE) users (26.5%, and 46.8%, respectively) (p < 0.005), and COC (60GES/15EE) users (30.4%, and 22.8%, respectively) (p < 0.005) (Sabatini and Cagiano 2006).
Discussion
In this review, contraceptive efficacy, cycle control, tolerability, and acceptability of NuvaRing as established in several large non-comparative multi-center registration studies (Roumen et al 2001; Dieben et al 2002), and in daily clinical practice (Roumen et al 2006), were compared with those of RCTs comparing NuvaRing with COCs (Veres et al 2004; Guida et al 2005; O’Connell et al 2005; Oddsson et al 2005a, b; Westhoff et al 2005; Ahrendt et al 2006; Milsom et al 2006; Sabatini and Cagiani 2006; Schafer et al 2006). The reason for this testing was that results of registration studies may be biased by the willingness of the participants and the fact, that these studies were funded by, undertaken by, analyzed by and the results written up by staff of the company which manufactures the CCVR. Although the latter is also true for most of the RCTs, these studies represent the highest possible level of evidence.
The good contraceptive efficacy during the first year of NuvaRing use in the non-comparative studies (Roumen et al 2001; Dieben et al 2002) was confirmed in the RCTs, in which contraceptive efficacy was comparable between NuvaRing and the COC (Oddsson et al 2005a; Ahrendt et al 2006). This finding is not surprising, as ovarian suppression was shown to be adequate with both methods (Mulders and Dieben 2001; Duijkers et al 2004). This result should be interpreted with caution, of course, as it is not predictive of the long-term contraceptive results.
The incidence of estrogen-related adverse events such as breast tenderness, headache, and nausea was low in the non-comparative registration studies (Roumen et al 2001; Dieben et al 2002). Although this finding could be confirmed in the RCTs, the incidence of estrogen-related adverse events was not significantly lower with NuvaRing compared with the COC (Oddsson et al 2005a; Ahrendt et al 2006; Sabatini and Cagiano 2006). This is somewhat disappointing, as the systemic exposure to EE with NuvaRing (releasing 15 μg EE per day) compared with that for a COC containing 30 μg EE per pill, is approximately 50% (Timmer and Mulders 2000; van den Heuvel et al 2005). In the non-comparative studies, no clinically relevant changes from baseline were observed in blood pressure and body weight (Roumen et al 2001; Dieben et al 2002). Also no apparent differences between NuvaRing and the COC were found in blood pressure changes (Veres et al 2004; O’Connell et al 2005; Oddsson et al 2005a; Ahrendt et al 2006; Roumen and Dieben 2006; Sabatini and Cagiano 2006), body weight changes (Oddsson et al 2005a; Westhoff et al 2005; Roumen and Dieben 2006; Sabatini and Cagiano 2006; Schafer et al 2006), or decreasing rates of PMS and dysmenorrhea complaints (Milsom et al 2006).
An important finding in the non-comparative studies was the good cycle control with NuvaRing (Roumen et al 2001; Dieben et al 2002; Roumen et al 2006). This was confirmed in the RCTs, in which a better cycle control with NuvaRing than with the COC was a consistent finding (Sabatini and Cagiano 2006). Cycle control is a key factor that affects contraceptive acceptability, compliance, and convenience, especially for the vaginal route of administration. The incidence of an intended bleeding pattern, defined as a cycle with only a withdrawal bleeding in the hormone-free week, was high in the combined data of the large international registration studies (Roumen et al 2001; Dieben et al 2002). This high incidence of an intended bleeding pattern with NuvaRing was confirmed in the RCTs, and shown to be significantly higher in most cycles with NuvaRing than with the COC (Oddsson et al 2005b; Milsom et al 2006). This was mainly due to a significantly lower incidence of withdrawal bleeding persisting after the hormone-free week in the NuvaRing groups compared with the COC. This continued withdrawal bleeding consisted mainly of spotting days in both groups. A better bleeding pattern with NuvaRing than with the COC was also found when the same women used both methods in a cross-over study (Veres et al 2004). Even after initiation of the contraceptive method regardless of menstrual cycle day, the bleeding patterns with NuvaRing were better than with the COC (Westhoff et al 2005). The superior cycle control with NuvaRing is remarkable, as the total daily dose of EE is only half of that of a 30 μg EE COC. So, other mechanisms must be responsible for this. A local effect is unlikely, as no elevated concentrations of EE and ENG were found in the endometrial and myometrial tissues with NuvaRing compared with a 20 EE μg COC (Roumen and Dieben 2006). It is most obvious, therefore, that the considerably lower variation in daily EE serum levels with NuvaRing compared with the COC is an important causative factor (van den Heuvel et al 2005).
It is not surprising, that the vaginal route of hormone administration was associated with higher incidences of local adverse events such as leukorrhea, vaginal discomfort, vaginitis, and ring-related events comprising foreign body sensation, coital problems and expulsions, than the oral route in both the non-comparative studies and the RCTs (Roumen et al 2001; Dieben et al 2002; Oddsson et al 2005a; Ahrendt et al 2006). In one study, increased vaginal wetness during NuvaRing use was accompanied by an improvement of the bacterial flora in the vagina (Veres et al 2004). In another study, less vaginal dryness was reported by NuvaRing users (Sabatini and Cagiano 2006). As could be expected, discontinuation rates due to local and ring-related adverse events were also higher in the NuvaRing groups than in the COC groups (Oddsson et al 2005a; Ahrendt et al 2006). The incidences of serious adverse events were low and comparable in both groups. However, the number of participants in the studies was too small and the duration of the studies too short to provide any reliable information on the incidence of infrequent but serious adverse events like thromboembolism. Unfortunately, the influence of the lower EE exposure with NuvaRing on coagulation factors and lipid metabolism remains unknown, as no RCTs between NuvaRing and the COC on these important metabolic parameters have been published yet. In two different open-label, non-randomized comparative studies, both NuvaRing and a 150LNG/30EE COC were associated with minimal effects on hemostatic variables and on lipid profile (Magnusdóttir et al 2004; Tuppurainen et al 2004).
In the non-comparative studies and the RCTs, both NuvaRing and the COC were found to be highly acceptable methods of contraception (Roumen et al 2001; Dieben et al 2002; Ahrendt et al 2006; Roumen et al 2006). Compared with women not using hormonal contraception, both women using NuvaRing and the COC reported a global improvement of sexual function (Guida et al). The higher satisfaction and stronger preference for method continuation of NuvaRing users is remarkable, and its explanation did not emerge from the studies (Veres et al 2004; Schafer et al 2006). The most important reason is possibly, that the once-a-month use of NuvaRing is easy and more convenient for many women (Roumen et al 2006), although NuvaRing compliance was not different from the daily pill regimen (Dieben et al 2002; Oddsson et al 2005a; Ahrendt et al 2006). Also, the superior cycle control is a possible attractive reason for many women. A third argument could be, that many women feel that the lower daily EE dose and the less fluctuating hormone levels with the CCVR are less harmful for their general health (Roumen et al 2006). Lastly, the significant increase in sexual desire, satisfaction and fantasy of women and partners of women using the CCVR is a possible contributing factor (Guida et al 2005; Sabatini and Cagiano 2006).
Footnotes
Disclosures The author has no conflicts of interest to disclose.
References
- Ahrendt HJ, Nisand I, Bastianelli C, et al. Efficacy, acceptability and tolerability of the combined contraceptive ring, NuvaRing, compared with an oral contraceptive containing 30 μg ethinyl estradiol and 3 mg of drospirenone. Contraception. 2006;74:451–7. doi: 10.1016/j.contraception.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Dieben TOM, Roumen FJME, Apter D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol. 2002;100:585–93. doi: 10.1016/s0029-7844(02)02124-5. [DOI] [PubMed] [Google Scholar]
- Duijkers IJM, Klipping C, Verhoeven CHJ, Dieben TOM. Ovarian function with the contraceptive vaginal ring or an oral contraceptive: a randomized study. Hum Reprod. 2004;19:2668–73. doi: 10.1093/humrep/deh493. [DOI] [PubMed] [Google Scholar]
- Guida M, Di Spiezio Sardo A, Bramante S, et al. Effects of two types of hormonal contraception – oral versus intravaginal – on the sexual life of women and their partners. Hum Reprod. 2005;20:1100–6. doi: 10.1093/humrep/deh686. [DOI] [PubMed] [Google Scholar]
- Magnusdóttir EM, Bjarnadóttir RI, Önundarson PT, et al. The contraceptive vaginal ring (NuvaRing®) and hemostasis: a comparative study. Contraception. 2004;69:461–7. doi: 10.1016/j.contraception.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Miller L, Verhoeven CHJ, ’t Hout J. Extended regimens of the contraceptive vaginal ring. Obstet Gynecol. 2005;106:473–82. doi: 10.1097/01.AOG.0000175144.08035.74. [DOI] [PubMed] [Google Scholar]
- Milsom I, Lete I, Bjertnaes A, et al. Effects on cycle control and bodyweight of the combined contraceptive ring, NuvaRing, versus an oral contraceptive containing 30 microg ethinyl estradiol and 3 mg drospirenone. Hum Reprod. 2006;21:2304–11. doi: 10.1093/humrep/del162. [DOI] [PubMed] [Google Scholar]
- Mulders TMT, Dieben TOM. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril. 2001;75:865–70. doi: 10.1016/s0015-0282(01)01689-2. [DOI] [PubMed] [Google Scholar]
- O’Connell KJ, Osborne LM, Westhoff C. Measured and reported weight change for women using a vaginal contraceptive ring vs a low-dose oral contraceptive. Contraception. 2005;72:323–7. doi: 10.1016/j.contraception.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Oddsson K, Leifels-Fischer B, de Melo NR, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1-year randomized trial. Contraception. 2005a;71:176–82. doi: 10.1016/j.contraception.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Oddsson K, Leifels-Fischer B, Wiel-Masson D, et al. Superior cycle control with a contraceptive vaginal ring compared with an oral contraceptive containing 30 μg ethinylestradiol and 150 μg levonorgestrel: a randomized trial. Hum Reprod. 2005b;20:557–62. doi: 10.1093/humrep/deh604. [DOI] [PubMed] [Google Scholar]
- Roumen FJME, Apter D, Mulders TMT, et al. Efficacy, tolerability and acceptability of a novel contraceptive vaginal ring releasing etonogestrel and ethinyl oestradiol. Hum Reprod. 2001;16:469–75. doi: 10.1093/humrep/16.3.469. [DOI] [PubMed] [Google Scholar]
- Roumen FJME, Dieben TOM. Comparison of uterine concentrations of ethinylestradiol and etonogestrel – after use of a contraceptive vaginal ring and an oral contraceptive. Fertil Steril. 2006;85:57–62. doi: 10.1016/j.fertnstert.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Roumen FJME, op ten Berg MMT, Hoomans EHM. The combined contraceptive vaginal ring (NuvaRing®): first experience in daily clinical practice in the Netherlands. Eur J Contracept Reprod Health Care. 2006;11:14–22. doi: 10.1080/13625180500389547. [DOI] [PubMed] [Google Scholar]
- Sabatini R, Cagiano R. Comparison profiles of cycle control, side effects and sexual satisfaction of three hormonal contraceptives. Contraception. 2006;74:220–3. doi: 10.1016/j.contraception.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Schafer JE, Osborne LM, Davis AR, et al. Acceptability and satisfaction using Quick Start with the contraceptive vaginal ring versus an oral contraceptive. Contraception. 2006;73:488–92. doi: 10.1016/j.contraception.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Timmer CJ, Mulders TMT. Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinet. 2000;39:233–42. doi: 10.2165/00003088-200039030-00005. [DOI] [PubMed] [Google Scholar]
- Tuppurainen M, Klimscheffskij R, Venhola M, et al. The combined contraceptive vaginal ring (NuvaRing®) and lipid metabolism: a comparative study. Contraception. 2004;69:389–94. doi: 10.1016/j.contraception.2004.01.004. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MW, van Bragt AJM, Alnabawy AKM, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168–74. doi: 10.1016/j.contraception.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Veres S, Miller L, Burington B. A comparison between the vaginal ring and oral contraceptives. Obstet Gynecol. 2004;104:555–63. doi: 10.1097/01.AOG.0000136082.59644.13. [DOI] [PubMed] [Google Scholar]
- Westhoff C, Osborne LM, Schafer J, et al. Bleeding patterns after immediate initiation of an oral compared with a vaginal hormonal contraceptive. Obstet Gynecol. 2005;106:89–96. doi: 10.1097/01.AOG.0000164483.13326.59. [DOI] [PubMed] [Google Scholar]