Abstract
Psoriasis is a chronic inflammatory, immune-mediated skin disorder that affects 1.5–1.8 million people in Italy. The most common form of the disease is chronic plaque psoriasis, affecting about 90% of psoriasis patients, with about 20%–30% of them suffering from a moderate or severe condition. Little information is available about the economic impact of psoriasis in European countries. The primary objective of this study was to perform a cost-of-illness analysis of patients with moderate and severe plaque psoriasis in Italy. Therefore, direct, indirect costs, and intangible costs (quality of life – QoL) were assessed. In this national, multicenter, prospective, 3-month cost-of-illness study of moderate and severe plaque psoriasis, direct and indirect costs were assessed from the patient, third-party payer (National Health Service, NHS), and societal perspectives. From November 2003 to October 2004 consecutive patients were enrolled over a 1-year period, in order to minimize seasonal fluctuations in disease severity. 150 patients enrolled in 6 investigational sites in Italy, completed the study, and were eligible to be analyzed according to the study protocol. Intangible costs (QoL) were measured using SF36 and DLQI questionnaires. The mean total cost for psoriasis (average Psoriasis Area Severity Index [PASI] score 21.4), including direct and indirect items, was €8,371.61 per patient per year. The mean cost for patients with moderate disease (PASI ≤ 20) was €5,226.04, while the mean cost for patients with more severe disease (PASI > 20) was €11,434.40 per year. Disease heavily affected QoL measured using SF36, and the impairment was greater in patients affected by a more severe form of disease. Moderate and severe plaque psoriasis is associated with extremely high costs, which are related to disease severity. Data from this study show that the more severe plaque psoriasis, the higher the direct and indirect costs for its management. Direct costs are higher than indirect costs; hospitalization represents the most significant item, accounting for 30% of the total expenses. QoL in moderate and severe plaque psoriasis is low compared with the population at large, confirming the high impact of plaque psoriasis on QoL. The relatively high average annual costs per patient point to the need for a more efficient and long-term control of psoriasis.
Keywords: cost of illness, psoriasis, quality of life
Introduction
Psoriasis is a chronic inflammatory skin disorder that affects approximately 1–3% of the world population and almost 2% of the European population, about 1.5–1.8 million people in Italy (Plunkett et al 1998; Sterry et al 2004; Naldi et al 2004). The most common form of the disease is chronic plaque psoriasis, an immune-mediated disorder that affects about 90% of psoriasis patients, with about 20%–30% of them having a moderate or severe condition. Its high prevalence and chronic nature have profound effects on the patients’ quality of life.
Psoriasis severity is defined by the extent of body surface involved, the lesion characteristics, or the impact of the disease on quality of life (QoL) (Finlay et al 2005). In general, patients with a body-surface involvement higher than 10%, or a Psoriasis Area Severity Index (PASI) >10, or a Dermatology Life Quality Index (DLQI) >10 are considered appropriate candidates for systemic therapy (Kirby et al 2000; Shikiar et al 2003). However, even patients with less extensive disease, but localized to certain body areas (hands, face, genitalia), may need systemic therapy or phototherapy instead of topical therapy (Rapp et al 1999). Psoriasis can be associated with other diseases (Del Giglio et al 2007). The most common co-morbidities include psoriatic arthritis and anxiety/depression disorders (Gisondi et al 2005; Kimball et al 2005). Psoriatic arthritis affects approximately 10%–15% of psoriasis patients (Parisier et al 2007). More recently, an association has also been reported between psoriasis and metabolic disorders, including abdominal obesity, atherogenic dyslipidemia, hypertension, and diabetes, which produces an unfavorable cardiovascular risk profile (Gelfand et al 2006; Neimann et al 2006; Malerba et al 2006; Shapiro et al 2006; Boehncke et al 2007; Gisondi et al 2007). Major factors that may contribute to this increased cardiovascular risk also include cigarette smoking, physical inactivity, hyperhomocysteinemia, and psychological stress, all of which have an higher prevalence among psoriasis patients (Malerba et al 2006). The cardiovascular risk factors involved in the metabolic syndrome are more strongly associated with severe psoriasis than with mild psoriasis.
A higher risk for arterial and venous thrombosis (Mallbris et al 2004), and a 3-fold increased risk of myocardial infarction have been reported especially in young patients with severe psoriasis (Gelfand et al 2006). The presence of these co-morbidities should be carefully evaluated in patients with psoriasis, when selecting the most appropriate therapy. As a matter of fact, many traditional systemic therapies such as CsA and MTX may negatively affect cardiovascular risk factors, especially in the long term (Gisondi et al 2007). In addition, patients with cardiovascular diseases are often under treatment with several drugs, and drug interactions must also be taken into account when selecting an antipsoriatic treatment. Because the disease is usually persistent and progressive, patients with a diagnosis of psoriasis usually need lifelong care, which also means a lifetime of expenses. From an economic perspective, there are many influencing factors on the costs of psoriasis to be taken into account, such as the increasing costs relating to prescription drugs, over-the-counter (OTC) and self-care products, hospitalizations, productivity loss due to absence from work for sick leave, and time spent by patients in medical consultations, treatments, or diagnostic procedures (Braathen et al 2001; Dehkharghani et al 2003).
It has recently been demonstrated that psoriasis has a relevant impact, and some cost implications, on the patients’ family members and/or caregivers (Eghlileb et al 2007). Since little information about the economic impact of psoriasis in European countries is available (Sohn et al 2006), we conducted a study to evaluate the direct and indirect costs related to moderate and severe plaque psoriasis in an Italian population and to assess the correlation of cost with the different degrees of severity of the disease and to measure the impairment in QoL.
Patients and methods
Design
We carried out a 3-month, national, multicenter, observational, prospective cost-of-illness study of moderate and severe plaque psoriasis. Direct, indirect costs, and intangible costs (QoL) were considered from the patient, third-party payer (National Health Service, NHS – Servizio Sanitario Nazionale, SSN) and societal perspectives. QoL data were also collected. The study design included 2 medical examinations, at baseline and after 3 months.
Recruitment
From November 2003 through October 2004, patients with moderate and severe psoriasis attending 6 departments of dermatology were invited to participate in the study. The involved centers were: Clinica di Dermatologia, Policlinico Tor Vergata, Roma; Clinica Dermatologica Università degli Studi de l’Aquila; Istituto Ortopedico Galeazzi, Dipartimento di Scienze Cliniche Luigi Sacco, Milano; Clinica di Dermatologia Azienda U.S.L.2, Lucca, Monte San Quirico; Presidio ospedaliero Vito Fazi U.O. di Dermatologia, Lecce.
Consecutive patient enrolments occurred over 1 year. In order to minimize seasonal fluctuations in disease severity the study period was divided in 4 consecutive periods (Q1: Nov 2003/Jan 2004; Q2: Feb/Apr2004; Q3: May/Jul 2004; Q4: Aug/Oct 2004).
Inclusion criteria
Patients aged ≥18 with moderate or severe plaque psoriasis were included in the study. Disease severity was classified as ≥12 according to the PASI. Disease severity was scored as moderate when PASI was 12–20 and severe when PASI score was >20. PASI score is the most accepted and widely used measure to assess disease severity and improvement in clinical trials and clinical practice. The study was approved by the local ethical committees and the Declaration of Helsinki protocols were followed. Written informed consent was obtained from all participants.
Data collection
Patients were invited to participate in the study at the time of their dermatological examination. Data on resource utilization between Visit 1 and Visit 2 were collected using a 2-part semi-structured questionnaire: the “dermatologist questionnaire” and the “patient questionnaire”. At the dermatological examination (Visit 1), the evaluating physician completed the “dermatologist questionnaire”, containing data on medical history, physical examination, and any concomitant drug both topic and systemic. The patient was given a self-administered questionnaire (“patient questionnaire”), to be completed on the first and second examinations. The “patient questionnaire” registered data on socio-demographic characteristics and QoL, by means of the 2 self-administered questionnaires, DLQI and SF-36. During the first examination (Visit 1), patients were asked to keep all drug packs (including cream tubes) that would be used in the next quarter, in order to verify real drug consumption. Patients were evaluated again after 3 months (±15 days) at Visit 2. At Visit 2, the following data were collected: results of physical and laboratory examinations as well as diagnostic procedures, the number and time of hospitalizations, concomitant drugs, non-conventional treatments, concurrent visits by dermatologists and other medical specialists, and working days lost in the previous quarter.
Costing
The direct, indirect, and intangible costs (QoL) of the present study were assessed as follows:
Direct costs
Medical costs related to: a) hospitalization, b) day-hospital admissions, c) specialist medical examinations, d) laboratory tests and instrumental investigations, e) phototherapy, f) drug therapies. The costs for hospitalization and day-hospital admission were evaluated on the basis of the national DRG system available from the Italian Ministry of Health (Health Ministry 2006a). Specialist medical examinations, laboratory tests, diagnostic procedures, and phototherapy sessions were based on the “2006 National Tariff Nomenclator” (Health Ministry 2006b). Health care services provided by the private sector and other private costs were evaluated based on the actual expenses incurred in by the patients. All pharmacological therapies (topical, systemic) were considered in the questionnaire. Non conventional treatments were also assessed. The annual treatment pattern was obtained through the assessment of the total drug consumption over a 1-year period, in order to minimize possible seasonal fluctuations in disease severity. To assess the costs of prescribed pharmacological therapies, units of consumed resources were multiplied by the prices reported in the official Italian price list (Informatore Farmaceutico 2007), taking into consideration the dosage and duration of the treatment. The costs of non-prescription topical medications and non-conventional treatments were determined by averaging the costs of the most representative drugs recorded in the patients’ notebooks, based on the official retail price list. All costs are expressed in Euros and were updated to 2006 values according to official inflation rates (Italian inflation rates 2007).
Indirect costs
The human capital approach was used to estimate the productivity loss due to plaque psoriasis. Travel costs were not collected. Indirect costs included the value of lost production due to leaving work earlier than usual in the day, sick leave and loss of leisure time for non-employees during the time of the study. Patients’ time off work (lost working days, permanent reduction, or loss of work activities) was measured in terms of salary evaluation, with the assumption that income reflects productivity. The monetary value of 1 lost working day for patients was calculated as €98.44, equal to the gross domestic product (GDP) per capita/day (Banca d’Italia 2006). National income data were subsequently updated to 2006 according to official inflation rates and represented a mean value for all of Italy.
The analysis was done from the society and NHS perspectives.
Intangible costs
In order to assess the functional status and QoL in their broad aspects, patients were asked to fill in the SF-36 and the DLQI questionnaires.
The SF-36 is a multi-purpose, short-form health survey with 36 questions. It yields an 8-scale profile of functional health and well-being scores, as well as psychometrically-based physical and mental health summary measures, and a preference-based health utility index. It is a generic measure, as opposed to one that targets a specific age, disease, or treatment group (Apolone et al 1997).
The DLQI is a dermatology-specific measure which consists of 10 questions related to patient’s symptoms and feeling, daily activities, leisure, work or school, personal relationship and treatment over the previous 1 week. The total DLQI ranges from 0 (no impairment of QoL) to 30 (maximum impairment of QoL) (Finlay et al 1994).
Statistics
First exploratory data analyses were performed in order to provide all the frequencies on all variables of the questionnaire. These descriptive analyses included distribution patterns (ranges, means, medians, skewness), missing values, and outliers on quantitative variables. The statistical methods included: analysis of variance (ANOVA) taking the center effect into account, applied to continuous variables: age at first diagnosis and mean duration of disease; and Chi-square test for categorical variables such as employment status, marital status, gender, stratified by center.
Results
Study population
Table 1 shows the main socio-demographic and disease characteristics. The mean age of the 150 patients examined, 99 males (66.0%) and 51 females (34.0%), was 48.3 years. The majority (53.3%) were employed, while 46.7% were unemployed or retired.
Table 1.
Demographic and disease characteristics in moderate and severe psoriasis
| Moderate PASI ≤ 20 |
Severe PASI > 20 |
Total |
P value* | ||||
|---|---|---|---|---|---|---|---|
| N or % | N or SD | N or % | N or SD | N or % | N or SD | ||
| N | 49.3% | 74 | 50.7% | 76 | 100.0% | 150 | |
| Mean age in years | 49.1 | 15.2 | 47.4 | 13.8 | 48.3 | 14.5 | 0.478 |
| Sex (male) | 64.9% | 48 | 67.1% | 51 | 66.0% | 99 | |
| Mean age at first diagnosis (years) | 30.4 | 15.2 | 28.7 | 15.5 | 29.5 | 15.3 | 0.505 |
| Mean duration of disease (years) | 18.8 | 12.9 | 18.5 | 11.7 | 18.7 | 12.3 | 0.901 |
| PASI | |||||||
| Mean PASI | 14.7 | 2.2 | 28.0 | 6.4 | 21.4 | 8.2 | 0.000000 |
| BSA | |||||||
| Mean BSA | 31.4 | 11.4 | 48.0 | 13.7 | 39.8 | 15.1 | 0.000000 |
| DLQI | |||||||
| ≤ 10 | 70.3% | 52 | 59.2% | 45 | 64.7% | 97 | |
| > 10 | 29.7% | 22 | 40.8% | 31 | 35.3% | 53 | 0.157 |
| Mean DLQI | 8.2 | 5.7 | 9.6 | 6.3 | 8.9 | 6.0 | 0.153 |
| Occupation | |||||||
| Employed | 51.4% | 38 | 55.3% | 42 | 53.3% | 80 | |
| Retired, unemployed | 48.6% | 36 | 44.7% | 34 | 46.7% | 70 | 0.631 |
Moderate vs Severe.
Abbreviations: PASI, Psoriasis Area Severity Index; BSA, body surface area; DLQI, Dermatology Life Quality Index.
The mean age at first diagnosis was 29.5 years (30.4 years for patients with moderate psoriasis, 28.7 years for patients with severe psoriasis). The mean time of disease duration was 18.7 years. Disease severity, as assessed by PASI score, was moderate in 49.3% of patients (64.9% males) and severe in 50.7% of patients (67.1% males).
Patients were also asked to complete the DLQI and SF-36 questionnaires. The DLQI score at the baseline examination was >10 for 53 patients (35.3%). DLQI scores correlated to the patients’ classification on the basis of PASI, showing a worsening in the patients’ conditions, since 29.7% of the patients with moderate psoriasis and 40.8% of the patients with severe psoriasis had a DLQI higher than 10. Average DLQI score was 8.2 for patients with moderate psoriasis, and 9.6 for patients with severe psoriasis. Body surface area (BSA) score also confirmed the classification of disease severity based on the PASI parameters.
Treatment pattern
Treatment patterns at baseline (Visit 1) are shown in Table 2. At the time of recruitment, the majority of patients received topical treatment (72.0%) and phototherapy (58.0%) or multiple therapy (52%). At 3 months, the number of patients receiving topical treatment increased (86.0%), and also the number of hospitalizations and medical examinations is significantly high, involving 66 (44.0%) and 136 (90.7%) patients respectively (Table 3).
Table 2.
Demographic and disease characteristics in moderate and severe psoriasis
| Moderate PASI ≤ 20 |
Severe PASI > 20 |
Total |
P value* | ||||
|---|---|---|---|---|---|---|---|
| % | N | % | N | % | N | ||
| 49.3 (%) | 74 | 50.7 (%) | 76 | 100 (%) | 150 | ||
| Drug therapy | |||||||
| Topic | 68.9 (%) | 51 | 75.0 (%) | 57 | 72.0 (%) | 108 | |
| Systemic | 23.0 (%) | 17 | 15.5 (%) | 11 | 18.7 (%) | 28 | 0.005 |
| Phototherapy | 35.1 (%) | 26 | 80.3 (%) | 61 | 58.0 (%) | 87 | |
| Adoption of combination vs monotherapy | |||||||
| Combination | 37.8 (%) | 28 | 65.8 (%) | 50 | 52.0 (%) | 78 | |
| Non-conventional treatments | |||||||
| Balneotherapy | 13.5 (%) | 10 | 6.6 (%) | 5 | 10.0 (%) | 15 | |
| Homeopathy | 13.5 (%) | 10 | 19.7 (%) | 15 | 16.7 (%) | 25 | |
| Bach’s flowers | 2.7 (%) | 2 | 2.6 (%) | 2 | 2.7 (%) | 4 | 0.377 |
| Other therapies | 4.1 (%) | 3 | 6.6 (%) | 5 | 5.3 (%) | 8 | |
Moderate vs Severe.
Abbreviation: PASI, Psoriasis Area Severity Index.
Resource utilization and absence from work
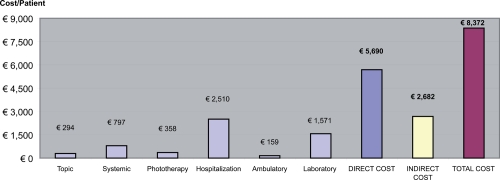
The consumption of medical services and medications at Visit 1 and in following 3 months is shown in Table 4 and Figure 1. The mean total cost (direct and indirect) per patient was 8,371.61 per year.
Table 4.
Direct and indirect costs per year
| Total | Per patient | % | |
|---|---|---|---|
| Direct cost | |||
| Topic | € 44,168.75 | € 294.46 | 4% |
| Systemic | € 119,597.24 | € 797.31 | 10% |
| Phototherapy | € 53,768.72 | € 358.46 | 4% |
| Hospitalization | € 376,535.64 | € 2,510.24 | 30% |
| Ambulatory visit | € 23,780.92 | € 1,578.54 | 2% |
| Laboratory examinations | € 235,664.28 | € 1,571.10 | 19% |
| Subtotal direct cost | € 853,515.55 | € 5,690.10 | 68% |
| Indirect cost (loss of productivity) | € 402,225.84 | € 2,681.51 | 32% |
| Total cost | € 1,255,741.39 | € 8,371.61 | 100% |
Figure 1.
Direct and indirect costs per year.
Direct costs accounted for 68% of the total estimated disease costs for moderate and severe plaque psoriasis. The most significant direct costs pertained to hospitalization (44%) followed by laboratory examinations (28%), while costs due to topical or systemic therapy only accounted for 5% and 14%. Significantly, while the majority of patients at Visit 2 were using a topical therapy (129), this represents only 4% of the total cost of medical and pharmacological therapy (Tables 3 and 4). On the other hand, hospitalization costs at Visit 2 account for 30% of total costs, but they are attributed to less than half of the patients (66), as reported in Table 3.
Table 3.
Treatment patterns at Visit 2 by disease severity
| Moderate PASI ≤ 20 |
Severe PASI > 20 |
Total |
P value* | ||||
|---|---|---|---|---|---|---|---|
| % | N | % | N | % | N | ||
| 49.3 (%) | 74 | 50.7 (%) | 76 | 100 (%) | 150 | ||
| Pharmacological treatment | |||||||
| Topic | 78.4 (%) | 58 | 93.4 (%) | 71 | 86.0 (%) | 129 | 0.228 |
| Systemic | 36.5 (%) | 27 | 59.2 (%) | 45 | 48.0 (%) | 72 | |
| Phototherapy | 35.1 (%) | 26 | 68.4 (%) | 52 | 52.0 (%) | 78 | |
| Hospitalization | 21.6 (%) | 16 | 65.8 (%) | 50 | 44.0 (%) | 66 | 0.003 |
| Medical examinations | 83.8 (%) | 62 | 97.4 (%) | 74 | 90.7 (%) | 136 | |
Moderate vs Severe.
Abbreviation: PASI, Psoriasis Area Severity Index.
Costs related to loss of productivity amounted to €2,681.51, accounting for 32% of total costs (Figure 1).
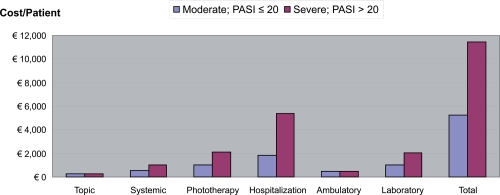
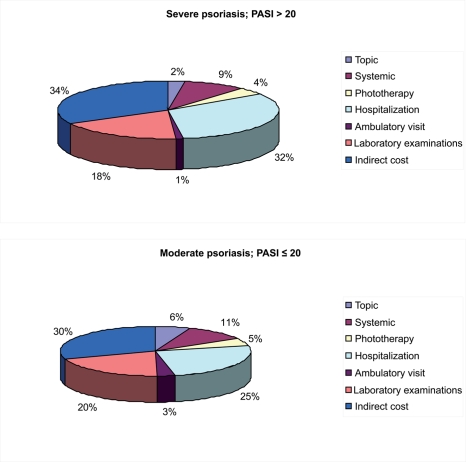
The expenses incurred for psoriasis treatment were significantly higher in those patients with more severe psoriasis (PASI > 20) than in patients with moderate plaque psoriasis (PASI ≤20) (PASI 10–20) (Table 5 and Figure 2). While the annual costs per patient with moderate plaque psoriasis amounted to 5,226.04, the costs per patient with a severe condition were 11,434.40. The cost differences between moderate and severe psoriasis were predominantly due to the higher hospitalization costs, phototherapy, laboratory examinations, systemic drugs, and costs related to loss of productivity. The most significant item is hospitalization, which represents 30% of the total expenses, with differences between patients with moderate psoriasis (25%) and severe psoriasis (32%) as shown in Figure 3.
Table 5.
Direct and indirect costs for disease severity per year
| Moderate PASI ≤ 20 | Severe PASI > 20 | |
|---|---|---|
| Number of patients | 74 | 76 |
| Avg PASI | 14.7 | 28 |
| Per-patient cost | ||
| Direct cost | ||
| Topic | € 304.69 | € 284.49 |
| Systemic | € 555.46 | € 1,032.81 |
| Phototherapy | € 261.50 | € 452.86 |
| Hospitalization | € 1,311.40 | € 3,677.52 |
| Ambulatory visit | € 159.36 | € 157.74 |
| Laboratory examinations | € 1,050.60 | € 2,077.89 |
| Subtotal direct cost | € 3,643.02 | € 7,683.32 |
| Indirect cost (loss of productivity) | € 1,583.02 | € 3,751.08 |
| Total | € 5,226.04 | € 11,434.40 |
Moderate vs Severe P value 0.005.
Abbreviation: PASI, Psoriasis Area Severity Index.
Figure 2.
Direct and indirect costs for disease severity per year.
Abbreviation: PASI, Psoriasis Area Severity Index.
Figure 3.
Distribution of costs (%) at different degrees of severity of plaque psoriasis.
Intangible costs
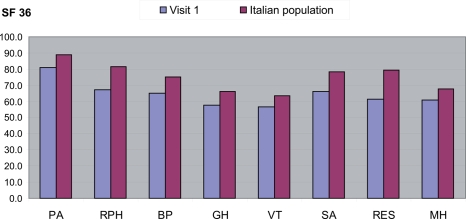
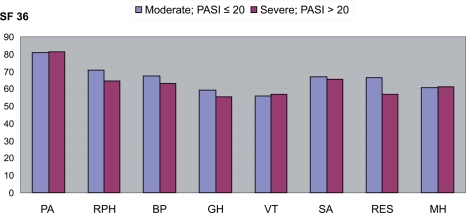
Figure 4 compares the results of the SF-36 questionnaire in the study population with the mean values of the Italian general population (Apolone et al 1997). All the measurements performed at Visit 1 are below the mean, thus confirming the high impact of plaque psoriasis on the QoL of patients. In particular, patients reported worsening for role and emotional status (RES), role and physical health (RPH), and social activities (SA). Table 6 shows the SF-36 score as reported at Visit 1 for each of the health items investigated by the SF-36, stratified by patients’ disease severity. The psychological impact recorded the lowest score, while the physical components seemed to be less affected, as reported in Figure 5.
Figure 4.
SF-36: quality of life measurements at visit 1 compared to the mean for the Italian population.
Abbreviations: PASI, Psoriasis Area Severity Index; BP, body pain; GH, general health; MH, mental health; PA, physical activity; RPH, Role-physical; RES, role emotional status; SA, social activity; VT, vitality.
Table 6.
SF-36: quality of life measurements at Visit 1* per different degrees of severity
| Moderate PASI ≤ 20 | Severe PASI > 20 | |
|---|---|---|
| Physical activity (PA) | 80.8 | 81.4 |
| SD | 24.7 | 23.6 |
| Role-physical (RPH) | 70.5 | 64.5 |
| SD | 35.9 | 40.3 |
| Body pain (BP) | 67.4 | 62.8 |
| SD | 28.4 | 30.1 |
| General health (GH) | 59.4 | 55.6 |
| SD | 24.2 | 21.9 |
| Vitality (VT) | 55.9 | 56.8 |
| SD | 21.0 | 21.8 |
| Social activity (SA) | 67.1 | 65.3 |
| SD | 26.5 | 24.5 |
| Role emotional status (RES) | 66.2 | 56.6 |
| SD | 39.9 | 40.7 |
| Mental health (MH) | 60.6 | 61.3 |
| SD | 20.5 | 22.0 |
Measurements are given as a mean (SD).
Abbreviation: PASI, Psoriasis Area Severity Index.
Figure 5.
SF-36 scores according to severity of disease.
Abbreviations: PASI, Psoriasis Area Severity Index; BP, body pain; GH, general health; MH, mental health; PA, physical activity; RPH, Role-physical; RES, role emotional status; SA, social activity; VT, vitality.
Discussion
Plaque psoriasis is a very common cutaneous disease which may have a profound impact on the affected person’s QoL and a substantial economic impact for the patients and the health care system. In spite of this, only a few studies have been performed so far presenting an overall picture of the costs of care for patients with psoriasis. This also applies to Italy, where little is known about the epidemiology and the economics of psoriasis (Marchetti et al 1998; Galadari et al 2001). Our national multicenter prospective study on the cost of illness of moderate and severe plaque psoriasis in Italy was performed according to the bottom-up approach on patients stratified by severity of disease (estimated by PASI score). Its results provide descriptive data on the pattern of resources used and show that:
The more severe plaque psoriasis, the higher the direct and indirect costs for its management;
Direct costs are higher than indirect costs; hospitalization is the most significant item, accounting for 30% of total expenses;
QoL in moderate and severe plaque psoriasis is low compared with the population at large.
The more severe the psoriasis, the higher the cost of care, as reported by Feldman et al (1997), and disease severity is related to the cost of treatment, time required for treatment, and time lost from work. The social cost of moderate and severe plaque psoriasis varies significantly for each stage of the disease, increasing until it more than doubles and goes from moderate to severe, according to the PASI score. It has recently been demonstrated that psoriasis is also associated with unemployment (Horn et al 2007). The annual costs calculated for a patient with moderate plaque psoriasis in Italy amounted to €5,226.04, whereas the costs for a patient with a severe condition were €11,434.40. The German Cost-of-Illness study (Finlay et al 1998; Berger et al 2005) found a total annual cost per treated patient (direct + indirect costs) in a range of €4,985.00–6,709.00, with a mean PASI score of 18.2, a reasonably similar value to our study. Moreover, the German study highlights a correlation between PASI score growth and annual cost per patient, backing up the Italian study findings.
In the present analysis, direct costs are higher than indirect costs both for patients with moderate and severe plaque psoriasis. It should be pointed out that hospitalization represents the most significant item contributing to the total cost, and also among direct costs, as reported in a previous cost-of-care study conducted in Italy (Rapp et al 1999). Indeed, hospitalization represents 30% of the total expenses, with differences between patients with moderate psoriasis (25%) and severe psoriasis (32%). Using population census data and considering the prevalence of psoriasis in the Italian population in the year 2006 (Naldi et al 2006), the total cost for this disease in our country was estimated to be approximately €2,403 million per year on a total national health expenditure of €128,914 million (1.8% of the total national health expenditure in 2006).
Psoriasis also causes significant psychosocial problems, as the results of the SF-36 questionnaire from this study show, which means that improvement in the quality of life should be a major issue in economic considerations. These results confirm the findings of Sampogna et al (2005): a heavy burden on mental health and social functioning was observed, comparable to depression. As previously reported, skin diseases can substantially diminish QoL, restricting work, social, and family relationships (Finlay et al 1998). Patients may find skin diseases more disabling than other diseases that are usually considered more serious, as they often feel that the disease alters their lives far more adversely than patients with basal cell carcinomas, warts, and moles (Koo et al 1996; Nevitt et al 1996; Lamberg et al 1997; Finlay 2001).
There are some limitations to this study and the results are likely to underestimate total disease costs for various reasons. The first possible limitation regards the extrapolations of 3-month expenses to 1 year, as patients’ expenses are not strictly related to their current condition (such as a flare), but are influenced by routinely used items, especially in case of moderate or severe disease. Some cost items, such as out-of-pocket expenses, were not included in the study, since it adopted the NHS perspective. The use of the human capital approach in calculating indirect costs in the form of overall productivity losses can discriminate against people with no market value such as the unemployed, retired people, and housewives. Overall, this study underestimated some psoriasis costs, not included in our protocol, such as co-morbidities and reduction of productivity by families and caregivers. These expenses might represent an important cost driver on the total burden of disease (Sander et al 1993; Feldman et al 1997; Jenner et al 2002; Sampogna et al 2005; Horn et al 2007). Further research work is needed to understand the reasons for this association.
In this analysis no patient was treated with new biologics drugs (efalizumab, etanercept, infliximab, alefacept) for psoriasis; in Italy these therapies were introduced on to market only in June 2005, out of our enrollment period. An increasing use of innovative treatments in dermatology has been noted recently, and with the advent of this new biologics drugs psoriasis costs will become even higher. However, the prescription of these new costly drugs could be associated with a decrease in loss of productivity and a reduction in diagnostic procedures and medical care, with a great benefit for society (Finlay 2001). These investments in innovative treatments for psoriasis might also provide future benefits, due to the avoidance of recurrent problems, improved compliance, and a better QoL (Berger et al 2005). Adequate analysis of outcomes and costs, as performed for biologics in current use, should be assessed in terms of incremental cost-effectiveness ratio.
In conclusion, moderate and severe plaque psoriasis represents an important cause of economic burden in Italy, both to the society and the NHS. The data presented here show that public payers and the individuals themselves afford a total cost ranging from €5,226.04 to €11,434.40. The most significant costs were due to hospitalization, followed by medical activities and costs for prescription drugs. This study provides evidence of the association between psoriasis severity and cost of illness. This cost-of-illness study provides basic data for further decision making, dealing especially with economic assessment of innovative therapies for moderate and severe plaque psoriasis. Further data on outcomes need to be collected over a long time frame.
Acknowledgments
The study was financially supported by MerkSerono S.p.a. Rome, Italy. The authors would like to thank Marta Vinci for editorial assistance and Sergio Di Matteo for statistical support.
References
- Altobelli E, Maccarone M, Petrocelli R, et al. Analysis of health care and actual needs of patients with psoriasis: a survey on the Italian population. BMC Public Health. 2007;7:59. doi: 10.1186/1471-2458-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolone G, Mosconi P, Ware JEJ. Questionario sullo stato di salute SF-36. Mauale d’uso e guida all’interpretazione dei risultati. Guerini e Associati. 1997 [Google Scholar]
- Banca d’Italia. Italian household budgets in year 2002. 2006 [online] Accessed Febr 2006. URL: www.bancaditalia.it/pubblicazioni.
- Berger K, Ehlken B, Kugland B, et al. Cost-of-illness in patients with moderate and severe chronic psoriasis vulgaris in Germany. J Dtsch Dermatol Ges. 2005;3:511–8. doi: 10.1111/j.1610-0387.2005.05729.x. [DOI] [PubMed] [Google Scholar]
- Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157:1249–51. doi: 10.1111/j.1365-2133.2007.08190.x. [DOI] [PubMed] [Google Scholar]
- Braathen LR. Cost of caring for hospital-based patients in dermatology in Europe. J Eur Acad Dermatol Venereol. 2001;15:292. [PubMed] [Google Scholar]
- Dehkharghani S, Bible J, Chen JG, et al. The economic burden of skin disease in the United States. J Am Acad Dermatol. 2003;48:592–9. doi: 10.1067/mjd.2003.178. [DOI] [PubMed] [Google Scholar]
- Del Giglio M, Gisondi P, Girolomoni G. The need for long-term continuous therapyin moderate to severe chronic plaque psoriasis. G Ital Dermatol Venereol. 2007;142:269–81. [Google Scholar]
- Eghlileb AM, Davies EEG, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Clinical and Laboratory Investigation. 2007 doi: 10.1111/j.1365-2133.2007.07881.x. [DOI] [PubMed] [Google Scholar]
- Feldman SR, Fleischer AB, Reboussin DM, et al. The economic impact of psoriasis increases with psoriasis severity. J Am Acad Dermatol. 1997;37:564–9. doi: 10.1016/s0190-9622(97)70172-5. [DOI] [PubMed] [Google Scholar]
- Finlay AY. Current severe psoriasis and the Rule of Tens. Br J Dermatol. 2005;152:861–7. doi: 10.1111/j.1365-2133.2005.06502.x. [DOI] [PubMed] [Google Scholar]
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): A simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–16. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Finlay AY. Psoriasis from the patient’s point of view. Arch Dermatol. 2001;137:352–3. [PubMed] [Google Scholar]
- Finlay AY. Skin disease disability: measuring its magnitude. Keio J Med. 1998;47:131–4. doi: 10.2302/kjm.47.131. [DOI] [PubMed] [Google Scholar]
- Finzi AF, Mantovani LG, Belisari A. The expenses of hospital-related care of patients with psoriasis in Italy based on the AISP study. J Eur Acad Dermatol Venereol. 2001;15:320–4. [PubMed] [Google Scholar]
- Galadari I, Rigel E, Lebwohl M. The cost of psoriasis treatment. J Eur Acad Dermatol Venereol. 2001;15:320–4. [PubMed] [Google Scholar]
- Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53:573. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- Gisondi P, Girolomoni G, Sampogna F, et al. Prevalence of psoriatic arthritis and jot complaints in large population of Italian patients hospitalized for psoriasis. Eur J Dermatol. 2005;15:279–83. [PubMed] [Google Scholar]
- Gisondi P, Girolomoni G. Aggiornamento sui trattamenti biologici della psoriasi. G Ital Dermatol Venereol. 2007;142(Suppl 1):1–11. [Google Scholar]
- Gisondi P, Tessari G, Conti A, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital based case-control study. Br J Dermatol. 2007;157:68–73. doi: 10.1111/j.1365-2133.2007.07986.x. [DOI] [PubMed] [Google Scholar]
- Griffiths EMC, Barke JNWN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–71. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- Health Ministry. Nomenclatore delle Prestazioni di assistenza specialistica ambulatoriale. Ministero della Salute. 2006 National Tariff Nomenclature. 2006a [Google Scholar]
- Health Ministry. Hospital DRG tariffs. Tariffa Unica Convenzionale, National DRG Tariff; 2006b. [Google Scholar]
- Horn EJ, Fox KM, Patel V, et al. Association of patient-reported psoriasis severity with income and employment. J Am Acad Dermatol. 2007;57:963–71. doi: 10.1016/j.jaad.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Informatore Farmaceutico. Medicinali: Masson-Elsevier; 2007. [Google Scholar]
- Italian inflation rates. 2007 [online] Accessed July 2007. URL: http://www.istat.it/prezzi/precon/rivalutazioni.
- Javitz HS, Ward MM, Farber E, et al. The direct cost of care for psoriasis and psoriatic arthritis in the United States. J Am Acad Dermatol. 2002;46:850–60. doi: 10.1067/mjd.2002.119669. [DOI] [PubMed] [Google Scholar]
- Jenner N, Campbell J, Plunkett A, et al. Cost of psoriasis: a study on the morbidity and financial effects of having psoriasis in Australia. Australas J Dermatol. 2002;43:255–61. doi: 10.1046/j.1440-0960.2002.00611.x. [DOI] [PubMed] [Google Scholar]
- Kimball AB, Jacobson C, Weiss S, et al. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6:383–92. doi: 10.2165/00128071-200506060-00005. [DOI] [PubMed] [Google Scholar]
- Kirby B, Fortune DG, Bhushan M, et al. The Salford Psoriasis Index: an holistic measure of psoriasis severity. Br J Dermatol. 2000;142:728–32. doi: 10.1046/j.1365-2133.2000.03418.x. [DOI] [PubMed] [Google Scholar]
- Koo J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin. 1996;14:485–96. doi: 10.1016/s0733-8635(05)70376-4. [DOI] [PubMed] [Google Scholar]
- Lamberg L. Dermatologic disorders diminish quality of life. JAMA. 1997;277:1663. [PubMed] [Google Scholar]
- Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51:563–9. doi: 10.1016/j.jaad.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Malerba M, Gisondi P, Radaeli A, et al. Psoriasis and risk of myocardial infarction. JAMA. 2007;297:361–2. doi: 10.1001/jama.297.4.361-b. [DOI] [PubMed] [Google Scholar]
- Malerba M, Gisondi P, Radaeli A, et al. Plasma homocysteine and folate levels in patients with chronic plaque psoriasis. Br J Dermatol. 2006;155:1165–9. doi: 10.1111/j.1365-2133.2006.07503.x. [DOI] [PubMed] [Google Scholar]
- Mallbris L, Akre O, Granath F, et al. Increased risk for cardiovascular mortality in psoriasis inpatients but not in outpatients. Eur J Epidemiol. 2004;19:225–30. doi: 10.1023/b:ejep.0000020447.59150.f9. [DOI] [PubMed] [Google Scholar]
- Mallbris L, Akre O, Stahle M. Metabolic disorders in patients with psoriasis and psoriatic arthritis. Curr Rheumatol Rep. 2006;8:355–63. doi: 10.1007/s11926-006-0065-8. [DOI] [PubMed] [Google Scholar]
- Marchetti A, Lapensee K, An P. A pharmacoeconomic analysis of topical therapies for patients with mild-tomoderate stable plaque psoriasis: a US study. Clin Ther. 1998;20:851–69. doi: 10.1016/s0149-2918(98)80146-x. [DOI] [PubMed] [Google Scholar]
- Naldi L, Chatenaud L, Castelli E, et al. Trattamento della psoriasi con farmaci sistemici in Italia, Psocare, Primo Rapporto 2006. 2006 [online] URL: http://www.psocare.it/epidemiologia.php.
- Naldi L, Colombo P, Benedetti Placchesi E, et al. Sutudy design and preliminary results from the pilot phase of the Praktis Study: Self-reported diagnoses of selected skin diseases in a representative sample of the italian population. Dermatology. 2004;208:38–42. doi: 10.1159/000075044. [DOI] [PubMed] [Google Scholar]
- Neimann AL, Shin DB, Wang X, et al. Prevalence of cardiovascular risks factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–35. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Nevitt GJ, Hutchinson PE. Psoriasis in the community: prevalence, severity and patients’ beliefs and attitudes towards the disease. Br J Dermatol. 1996;135:533–7. [PubMed] [Google Scholar]
- Parisier DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation Clinical Consensus on Disease Severity. Arch Dermatol. 2007;143:239–42. doi: 10.1001/archderm.143.2.239. [DOI] [PubMed] [Google Scholar]
- Plunkett A, Marks R. A review of the epidemiology of psoriasis vulgaris in the community. Australas J Dermatol. 1998;39:225–32. doi: 10.1111/j.1440-0960.1998.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41:401–7. doi: 10.1016/s0190-9622(99)70112-x. [DOI] [PubMed] [Google Scholar]
- Sampogna F, Tabolli S, Soderfeldt B, et al. Measuring quality of life of patients with different clinical types of psoriasis using the SF-36. Clinical and Laboratory Investigation. 2005 doi: 10.1111/j.1365-2133.2005.07071.x. [DOI] [PubMed] [Google Scholar]
- Sander HM, Morris LF, Phillips CM, et al. The annual expenses of psoriasis. J Am Acad Dermatol. 1993;3:422–5. doi: 10.1016/0190-9622(93)70062-x. [DOI] [PubMed] [Google Scholar]
- Saraceno R, Mannheimer R, Chimenti S. Regional distribution of psoriasis in Italy. J Eur Acad Dermatol Venereol. 2008 doi: 10.1111/j.1468-3083.2007.02423.x. In press. [DOI] [PubMed] [Google Scholar]
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629–34. doi: 10.1016/j.jaad.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Shikiar R, Bresnahan BW, Stone SP, et al. Validity and reliability of patient reported outcomes used in psoriasis: results from two randomized clinical trials. Health Qual Life Outcomes. 2003;1:53. doi: 10.1186/1477-7525-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn S, Schoeffski O, Prinz J, et al. Cost of moderate to severe plaque psoriasis in Germany: a multicenter cost-of-illness study. Dermatology. 2006;212:137–44. doi: 10.1159/000090654. [DOI] [PubMed] [Google Scholar]
- Stern RS. Epidemiology of psoriasis. Dermatol Clin. 1995;13:717–22. [PubMed] [Google Scholar]
- Sterry W, Barker J, Boehncke W-H, et al. Biological therapies in the systemic management of psoriasis: International Consensus Conference. Br J Dermatol. 2004;151(Suppl 69):3–17. doi: 10.1111/j.1365-2133.2004.06070.x. [DOI] [PubMed] [Google Scholar]