Case presentation
A 78-year-old African-American woman was diagnosed with rheumatoid arthritis (RA) in 1996 after she presented with a symmetrical polyarthritis of the hands. Over the next 4 years, she was treated with multiple disease-modifying antirheumatic drugs including methotrexate, hydroxychloroquine, gold, and leflunomide, all of which were stopped due to side effects or lack of response. She was started on low-dose prednisone in 1998, and was also maintained on low-dose azathioprine, which had been initiated in June.
In October 2000, the patient developed a cervical myelopathy, manifested by ataxia and hyperreflexia. Magnetic resonance imaging of the cervical spine showed compression of the ventral cord by an enhancing epidural soft-tissue mass behind the odontoid, which was thought to be pannus, as well as multilevel spondylosis with cord compression at C5–6. In December 2000, she underwent cervical spine decompression at the C5–6 level but was left with residual ataxia.
In early 2000, the patient had begun to lose weight, and this worsened after her spine surgery; she lost 20 lb between August 1999 and April 2001. Computerized tomography (CT) scan of the chest, abdomen, and pelvis without contrast was normal in April 2001. Magnetic resonance imaging of the abdomen was unremarkable except for thickening of the gastric antrum. Endoscopy was normal. Bone-marrow biopsy showed no evidence of malignancy. Testing for antigliadin antibodies and human immunodeficiency virus (HIV) were negative.
In May 2001, the patient was started on infliximab for the likelihood that RA activity was contributing to her weight loss and also because of the myelopathy due to the presence of pannus behind the odontoid process. She received the first three infusions but was lost to follow-up.
She presented again in September 2001 with a further weight loss of 10 lb and was admitted for further evaluation. The patient’s prior medical history was significant for hypertension, osteoporosis, and a pancreatic cystic mass which, when aspirated in 1998, had revealed no malignant cells. There also was an allergy to contrast dye. Medications at the time of admission were Premarin 0.625 mg daily (qd), prednisone 5 mg qd, furosemide 20 mg qd, and atenolol 50 mg qd.
On physical examination, the patient was cachectic, weighing 66 lb. Blood pressure was 110/80 mmHg, and she was afebrile. Significant findings included: chronic swan neck and boutonniere’s deformities of the hands but no active synovitis; hyperreflexia in the lower extremities with mild left-sided dysmetria; and negative Babinski signs. Abdominal examination revealed mild left upper-quadrant abdominal tenderness without masses. Cardiopulmonary examination was within normal limits, and hemoccult stool test was negative.
The initial laboratory investigation demonstrated a leukocyte count of 3.7 × 109/l (normal 3.4–11.2 × 109/l) with a normal differential, hemoglobin 9.5 g/dl (normal 12–16 g/dl), and platelet count of 201 × 109/l (normal 150–450 × 109/l). Liver, renal, and thyroid function tests, as well as urinalyses, were normal. Serum albumin was 2.9 g/dl, and amylase was 153 mg/dl (normal: 30–110 mg/dl). Rheumatoid factor was persistently negative.
On the first day of the hospitalization, the patient developed a low-grade fever. Blood and urine cultures were negative. Echocardiogram revealed no vegetations. Colonoscopy was normal. A percutaneous endoscopic gastrotomy tube was placed to optimize the patient’s nutritional status.
Radiological findings
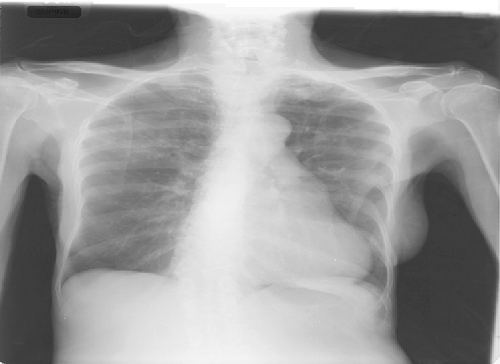
A posteroanterior view of the chest (Fig. 1) showed small patchy nodular densities in both lungs (upper more than lower, right more than left), which were new compared to prior chest radiographs. No hilar adenopathy or pleural effusions were noted.
Fig. 1.
Posteroanterior view of the chest. Patchy nodular densities in both lungs (upper more than lower, right more than left)
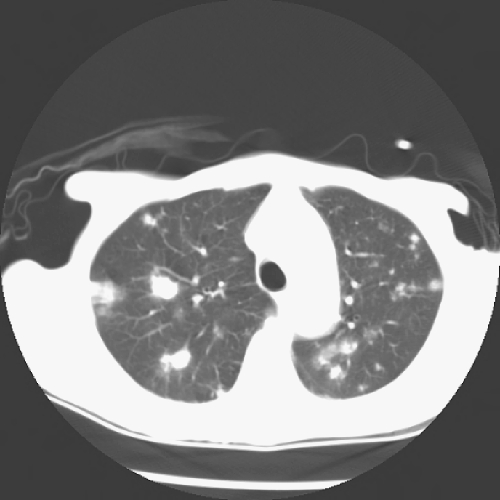
The CT scan of the chest (Fig. 2) showed prominent interstitial markings throughout the chest, with more confluent abnormalities in the right lung apex. There were multiple pulmonary nodules of various sizes throughout the lungs (most prominent in the upper lungs) and fibrolinear scarring in the lung apex and bases, which were also new since April 2001. No cavitation, calcification, or consolidation was noted. Precarinal adenopathy was present.
Fig. 2.
Computerized tomography of the chest. Multiple pulmonary nodules throughout the lungs
The differential diagnosis of multiple pulmonary nodules in RA includes metastatic disease, infectious processes, inflammatory diseases, or rheumatoid nodules. Metastatic diseases do not develop within weeks and are an unlikely diagnosis in this patient because the pulmonary nodules occurred very quickly. A specific diagnosis could not be made based on radiographic findings.
Differential diagnosis
This case has a broad differential diagnosis process, falling into three categories that must be considered: malignancy, autoimmune diseases, and infection.
With regard to malignancy, multiple pulmonary nodules occur primarily as a manifestation of metastatic disease, which can come either from an adenocarcinoma of the lung or from a distant primary. Although it is not always recognized during life, 30 to 40% of cancer patients have pulmonary metastases at autopsy. Parenchymal metastases, as opposed to endobronchial metastases, are often asymptomatic. This patient had had a negative noncontrast CT of the chest, abdomen, and pelvis 5 months before her admission—arguing against malignancy.
At the same time, she had a history of pancreatic cyst. Although the cyst was benign on prior aspiration and was not seen on recent scans, the patient did have increased amylase levels on admission, as well as left upper-quadrant abdominal tenderness, suggesting that the cyst might be relevant to her illness. Could this cyst have transformed into a malignant lesion and have metastasized? Benign mucinous cystic neoplasms of the pancreas can proliferate, and 10% become malignant over time. They occur predominantly in women, and the malignant potential is high enough that the resection is recommended [1]. This was a diagnostic possibility in this patient.
Lung involvement can be seen in hematologic as well as solid tumors. In the case of lymphoma, however, lymphangitic spread is far more common than multiple nodules in the lung. Lymphoproliferative disorders can cause pulmonary nodules, particularly lymphomatoid granulomatosis (LG). Half of patients with LG also have skin involvement in the form of nodules or rashes, and many have central nervous system disease. Pathology shows a granulomatous angiitis but, unlike that seen in Wegener’s granulomatosis (WG), the inflammatory infiltrate in LG consists of immature, atypical B cells. Some 10 to 25% of these patients develop lymphoma. The best arguments against LG in this patient are the radiographic findings. In LG, pulmonary nodules tend to be quite large and occur in the lower and peripheral lung fields, not at the apices. You can see cavitation in the lung in LG, but adenopathy, such as seen in this patient, is rare [2].
Autoimmune diseases, including WG, can also produce pulmonary nodules. Half of WG patients have pulmonary disease at the time of presentation, and also 87% of patients over the course of their disease. Pulmonary nodules in these patients can be multiple and bilateral, and they can cavitate. Arguments against this diagnosis in the presented case are the absence of renal or respiratory disease and the patient’s relatively indolent course. There is no link between WG and RA, so one would have to posit a second autoimmune disease.
Sarcoidosis can also involve the lungs. There is usually prominent hilar and mediastinal adenopathy, which was not the case in our patient, and chest radiographs usually show interstitial infiltrates. Small pulmonary nodules can be seen in sarcoid, but they usually follow a bronchovascular distribution.
Finally, RA itself can produce lung disease. In addition to nodular lung disease, one can see pleural disease, interstitial fibrosis, bronchiolitis, arteritis with pulmonary hypertension, and small airway disease. Rheumatoid nodules generally occur in the lower lung zones rather than being scattered throughout the lungs. The diffuse nature of this patient’s pulmonary nodules and the nodules’ upper lobe localization argue against the possibility that they are rheumatoid nodules.
Many infectious diseases can cause pulmonary nodules, including bacterial (embolic or nonembolic), fungal, and mycobacterial infections. Embolic disease is unlikely in this patient given her normal echocardiogram, but other types of infection must be seriously considered given her immunosuppression.
Bacterial infections to consider include nocardia, a Gram-positive aerobic bacteria of the Actinomyces family. Patients with nocardia tend to have impaired cell-mediated immunity, and pulmonary disease is seen in 75% of cases. Patients can be asymptomatic or have bronchopneumonia, but occasionally, patients present with solitary or multiple nodules or abscesses. Generally, there is no hilar lymphadenopathy and no calcification of the nodules [3].
Histoplasmosis is the most common endemic mycosis in the United States, but it is primarily seen in the south and north central states. The organism exists in the soil, is inhaled, multiplies in pulmonary macrophages, and migrates to neighboring lymph nodes, the reason why these patients generally have prominent lymphadenopathy. Patients can be asymptomatic, have a flu-like illness, or they can have chronic cavitary lung disease [4]. The most severe disease is seen primarily in immunocompromised patients, particularly those with HIV. Lung nodules are generally a late radiographic finding, however. Initially, patients develop an interstitial pneumonitis. Later, there can be organization of tissue into round nodules and cavitation. This is not consistent with this patient’s history. This patient had a normal CT of her lungs several months before, and other than weight loss, she was essentially asymptomatic until she presented with pulmonary nodules. In addition, pulmonary nodules in histoplasmosis generally involve the lower lung zones.
Blastomycosis can be seen not only in immunocompromised patients but also in immunocompetent patients. The geographical distribution of blastomycosis is similar to that of histoplasmosis: central, south central, and southeastern United States. Patients can have a variety of clinical presentations ranging from no symptoms to a flu-like illness to the gradual onset of fever, cough, or weight loss. Patients generally do not have hilar adenopathy. Forty to eighty percent of patients have skin involvement in the form of subcutaneous nodules. These develop not from direct inoculation but from bacteremic spread from the pulmonary focus. On chest radiographs, these patients can have chronic pneumonia, fibronodular infiltrates, or single or multiple nodules; however, nodules are generally located in the lower lung fields [4].
Coccidioidomycosis (desert fever) is generally seen in the deserts of southwestern United States. Patients can have a flu-like illness, pneumonitis, or the slowly progressive disease seen in this patient. This infection is of interest to rheumatologists because it can be associated with erythema nodosum and arthritis. Pulmonary infiltrates can evolve into spherical lesions, which do not calcify and can mimic cancer [4]. Apical cavitary disease can be seen and can mimic tuberculosis (TB). This patient’s radiographic findings are consistent with coccidioidomycosis, but the absence of recent travel to an endemic area is against the diagnosis.
Tuberculosis can certainly present with multiple pulmonary nodules. Reactivation of Mycobacterium tuberculosis (M. tuberculosis) usually involves the upper lobes, in contrast to primary TB, which is usually a lower lobe disease. Calcification is common. Recent reports suggest an increased incidence of TB in recipients of infliximab. An article published in October 2001, 1 month after this patient’s admission, reported 70 cases of TB after treatment with infliximab for a median of 12 weeks [5]. Forty-eight out of 70 patients developed TB after three or fewer infusions, and 40/70 had extrapulmonary disease (17 disseminated, 11 lymph-node disease, 4 peritoneal, 2 pleural, 1 each of meningeal, enteric, paravertebral, bone, genital, and bladder). The authors estimated that 147,000 patients had received infliximab in the U.S. at the time of the study, and they calculated the rate of TB in RA patients receiving infliximab to be 24.4 cases/100,000 compared to a 6.2/100,000 background rate.
At the time of this patient’s hospitalization, TB or malignancy was felt to be the most likely diagnosis. The upper lobe predominance of the patient’s pulmonary nodules argued against histoplasmosis and blastomycosis. Her geographic location was against these infections and coccidioidomycosis. The precarinal lymphadenopathy was more suggestive of TB than nocardia.
Diagnostic procedure
This is a situation in which an immunocompromised patient with weight loss and fever was found to have new, small pulmonary nodules. Pulmonary evaluation was requested. Our differential diagnosis was similar to that of the referring physicians: infection, malignancy, or inflammation. A diagnostic procedure was recommended because the patient was unable to produce sputum for analysis.
Open lung biopsy is most likely to be diagnostic; specific diagnosis is expected in 60% of those with underlying malignancy and infiltrates. Complications, including mortality, vary from 11 to 20% in different series with different patient groups. We reserve this procedure for those in whom less invasive procedures are not diagnostic.
Transthoracic needle aspiration performed under fluoroscopic, CT, or occasionally ultrasonographic guidance can be used to evaluate pulmonary nodules or infiltrates. In those with carcinoma, sensitivity varies from 70 to 95% with up to 30% false-negative results. Infections can be diagnosed in 70%, and inflammatory disease or other benign processes can also be identified. A diagnostic yield of 70% has been reported in immunocompromised patients. Complications such as bleeding or pneumothorax can occur in nearly one-third of patients.
Flexible bronchoscopy with bronchoalveolar lavage and transbronchial biopsy can be performed with an expectation of diagnosis in 50–96% of patients, depending on their underlying disease and pulmonary problem. In acquired immunodeficiency syndrome (AIDS) patients with Pneumocystis carinii pneumonia (PCP), the diagnosis can be expected in more than 95% of patients. In non-AIDS patients with PCP, the diagnosis is expected in 50–80%. Other infectious diseases or nonmalignant conditions, such as drug toxicity or inflammation, are less likely to be diagnosed by this procedure. In those with negative sputum smears for tuberculosis, bronchoscopy may provide the diagnosis in up to 50% of patients. Complications such as hypoxemia, bleeding, or pneumothorax are uncommon. This was the procedure we initially chose.
Pathological findings
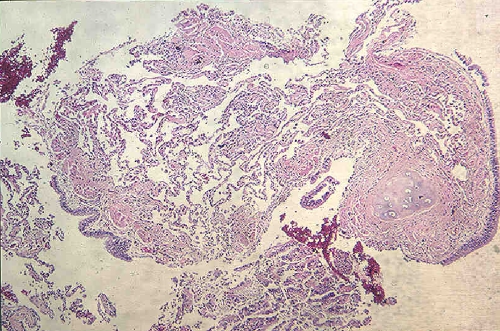
An adequate transbronchial biopsy showed alveolar parenchyma without significant histopathologic changes (Fig. 3). No granulomas, vasculitis, or tumor was seen. Special stains for fungi including P. carinii (Grocott methenamine silver) and acid-fast organisms (Ziehl–Neelsen) were negative.
Fig. 3.
Transbronchial lung biopsy. Transbronchial biopsy demonstrates normal bronchial wall and underlying alveolar parenchyma. Note the open airspaces and absence of interstitial inflammation
Further hospital course
Pathology and cytology were both normal. Acid-fast smears and routine cultures on the bronchoscopy specimen were negative. DNA probes on the bronchoscopy specimen, however, were positive for M. tuberculosis, and, ultimately, cultures were also positive for M. tuberculosis.
Immunological discussion
The immunology of tumor necrosis factor (TNF) as it relates to the intersection of RA and TB involves three important points: (a) like many cytokines, TNF has complex effects, which include induction of proinflammatory actions followed by antiinflammatory actions; (b) exacerbation of latent tuberculosis by neutralization of TNF is predictable based on what we know; and (c) if that logic is convincing, then it falls on us to try to predict what other consequences might be less common but might also be expected in the setting of immunosuppression.
The adverse effects of TNF (see Table 1) that eventually led to the development of etanercept and infliximab historically began with the systemic effects. These were discovered at the Sloan-Kettering Institute by Elizabeth Carswell and Lloyd Old, and then at Rockefeller University and Cornell University Medical College by Anthony Cerami, Bruce Beutler, Steven Lowry, Kevin Tracey, and their colleagues [6–8]. These effects include the hemorrhagic necrosis of tumors, cachexia, and mediation of septic shock, and systemic inflammatory response syndrome. However, the inhibition of TNF in RA is really directed more to the local effects, and, of those, the best known is the central role in driving a cytokine cascade that perpetuates inflammation and proteolysis as shown by Feldman [9] and others.
Table 1.
Adverse effects of tumor necrosis factor (TNF)
| Adverse Effect |
|---|
| Systemic |
| Cachexia |
| Systemic inflammatory response syndrome |
| Shock |
| Hemorrhagic necrosis |
| Local |
| Drives a cytokine cascade that perpetuates inflammation and proteolysis |
| Triggers neutrophils to degranulate and undergo a massive respiratory burst |
| Releasing serine proteases |
| Inactivating protease inhibitors |
| Activating metalloproteinases |
Another set of effects has been studied for the last 15 years in our laboratory, among others, and that is the ability of TNF to trigger neutrophils to degranulate and undergo a massive respiratory burst [10]. When neutrophils do so, they release proteases such as elastase and cathepsin G. The respiratory burst-derived oxidants can inactivate proteinase inhibitors such as alpha-2 trypsin inhibitors and the secretory leukocyte protease inhibitor. The oxidants can also activate metalloproteinases through oxidizing the cysteine residues which coordinate the zinc ion that normally helps hold the enzymes in their inactive form.
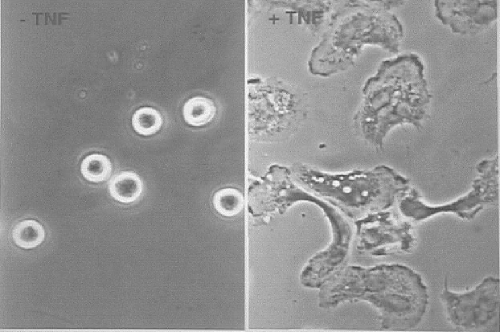
Some photomicrographs of normal human neutrophils in culture make this vivid. What happens when you add TNF to them? The photomicrographs in Figure 4 were taken 30 min apart at the same magnification. The cells spread out, bring granules to the surface, discharge their contents, and have a massive respiratory burst. Thus, neutralizing TNF in RA not only works but it also makes sense. It is gratifying when efficacy and rationale come together.
Fig. 4.
Photomicrographs of human neutrophils in culture. A: Normal. B: The cells spread out, bring granules to the surface, discharge their contents, and have a massive respiratory burst 30 min after addition of tumor necrosis factor
Among the toxicities of TNF we are focusing on is the reactivation of TB. What is extraordinary about reactivation of TB in the setting of TNF neutralization is that about 80% of the cases are not confined to the lung: 56% are extrapulmonary and 24% are disseminated. This is not the only toxicity of TNF-neutralizing interventions that have been suspected; others such as demyelinating disease, systemic and cutaneous lupus, and lymphoma are far less well established and will require careful attention as clinical experience accumulates [11].
What is special about TB infection? First, consider the enormous magnitude of TB on the global scale. The fact that the incidence of TB in American patients treated with infliximab went up fourfold does not really tell you what you might expect if this kind of treatment were more widespread. M. tuberculosis, the causative agent of TB, is such a successful pathogen first of all because its infectivity is extremely high, as distinct from the disease rate. About a third of people in the world are infected, that is, carrying the organism in a viable form in the body. When M. tuberculosis enters the body, whether or not the disease develops, our best understanding is that infection is usually lifelong. The incidence of death has been estimated at almost three million per year. Although this reflects only 5–10% of infected people developing disease and then failing to receive effective treatment, it is nonetheless the largest burden of death from any single bacterium. The pathology of TB is central to the life cycle of M. tuberculosis because it provokes tissue destruction and cough, which disseminates infectious aerosols [12].
After inhalation of M. tuberculosis, the bacteria are taken up by alveolar macrophages and dendritic cells that migrate to the hilar nodes. The mycobacteria win the first round of the battle because there has not been an immunologic activation. M. tuberculosis disseminates hematogenously all over the body, including back to the lung by the hematogenous route. By then, adaptive immunity develops, and most often the bacteria disappear from notice although some viable organisms persist. In 90–95% of people, this whole process is asymptomatic and remains so throughout life. The macrophages, which ingest the TB everywhere it goes, seem to control it well in most organs but less well in the lung.
Anything that suppresses the immune system can lead to reactivation of M. tuberculosis infection. Usually, reactivation occurs in the lung, but if the immune system is profoundly suppressed, which seems to be the case with inhibition of TNF, then the dormant M. tuberculosis at other sites can also resume replication. Other immunosuppressive factors can include HIV infection, age, silicosis probably via elevation of TGF-B, and corticosteroid therapy.
In this patient, M. tuberculosis may have reactivated before the infliximab in response to corticosteroid therapy. The ensuing course of infliximab may just have let the TB progress more rapidly.
What is it about TNF that helps the host to resist tuberculosis? We can get some answers from mice, where the literature goes back to the 1980s [13]. In wild-type mice infected with M. tuberculosis, it is typical to see well-structured granulomas with epithelial macrophages in the center and a mantle of monocytes and CD4 and CD8 lymphocytes surrounding. But in the setting of TNF or TNF receptor deficiency, the granulomas are ill formed. The same number of cells is present, if not more, but they do not form a tightly organized structure. While wild-type mice survive the infection over the study period, TNF knockout mice die within a few weeks of infection. Early in the infection, the TNF knockout mice had a deficiency in the ability to make chemokines. Later on, they make more chemokines than the wild-type mice, probably reflecting that the bacterial burden is so much greater [14, 15]. Initially, the chemokine response of TNF-deficient mice is not brisk enough, and that may be why these granulomas are not well formed. Thus, tuberculous pneumonitis is much more severe in TNF-deficient than in wild-type mice. It is a paradoxical picture; TNF is proinflammatory, yet, in this study, the lack of TNF is proinflammatory.
To summarize immunologic defects in the absence of TNF: (a) initials steps generating a new immune response are impaired as dendritic cells do not mature and migrate normally [16]; (b) granuloma formation in response to mycobacteria is impaired [14, 15]; (c) an intrinsic bactericidal defect of macrophages is manifest in vitro, which we cannot explain yet [17]; (d) impaired apoptosis of neutrophils and its attendant prolonged retention of neutrophils can exacerbate the inflammatory response [18]; and (e) prolonged production of IL-12 and interferon-G, which leads to failure to resolve inflammation [19]. Thus, impaired survival of mice after infection with M. tuberculosis when they do not have TNF is the result of at least two things: (a) inadequate killing of M. tuberculosis and (b) exaggerated pneumonitis and excessive inflammation [20].
Thus, patients on anti-TNF therapy should be considered to be immunodeficient. One of the implications of this is that it has led to routine pretreatment purified protein Derivative (PPD), chest X-ray, and history relevant to possible latent infections, as should be done before corticosteroid therapy. In the anti-TNF-treated patient, one should anticipate possible recrudescence of other latent infections besides M. tuberculosis, such as Leishmania donovani, Trypanosoma cruzi, or Listeria monocytogenes. Finally, in an era when we are contemplating live virus vaccines such as against smallpox, patients on anti-TNF therapy should be considered potentially susceptible to severe complications.
Conclusions
We are taught that when we hear hoof beats, we should think “horse” before we think “zebra.” But that which is “horse” may evolve over time, as our understanding of a disease and the drugs used to treat it evolve as evidenced by this case.
This patient was not screened for TB before initiation of infliximab; indeed, such screening was not standard in May 2001 when the therapy was initiated although it became so before the end of the year. The U.S. Food and Drug Administration’s Arthritis Drugs Advisory Committee heard updated postmarketing adverse event data in August 2001 from the manufacturers of infliximab and etanercept—reports that reflected updated information since FDA’s approval of these drugs in 1999 and 1998, respectively, and that led to new recommendations [21].
Thus, had infliximab been prescribed for the patient in this case in the fall rather than the spring of 2001, in all likelihood, a PPD would have been performed. In the presence of a positive PPD, the patient would have been put on INH for 3 months before initiating infliximab.
At the time of TB diagnosis, she was placed on INH and her RA is being treated with low-dose prednisone. The pulmonary nodules were slightly smaller on follow up CT scan in January 2002. The patient’s clinical status has improved slightly, with a resolution of fevers and a weight increase to about 80 lbs., which has stabilized. Overall, the patient is stable but may never return to her pre-c spine surgery baseline.
References
- 1.Sarr MG, Kendrick ML, Nagorney DM (2001) Cystic neoplasms of the pancreas: benign to malignant epithelial neoplasms. Surg Clin North Am 81(3):497–509 [DOI] [PubMed]
- 2.Luce JA (2000) Lymphoma, lymphoproliferative diseases and other primary malignant tumors. In: Murray J, Nadel J (eds) Textbook of respiratory medicine, 3rd edn. Saunders, Philadelphia, PA, pp 1453–1468
- 3.Bullock WE (2000) Nocardiosis. In: Goldman L, Bennett JC (eds) Cecil textbook of medicine, 21st edn. Saunders, Philadelphia, PA, pp 1715–1717
- 4.Dismukes WE (2000) Histoplasmosis, coccidiomycosis, blastomycosis. In: Goldman L, Bennett JC (eds) Cecil textbook of medicine, 21st edn. Saunders, Philadelphia, PA, pp 1860–1866
- 5.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J (2001) Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 345(15):1098–1104 [DOI] [PubMed]
- 6.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B (1975) An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A 72(9):3666–3670 [DOI] [PMC free article] [PubMed]
- 7.Beutler B, Mahoney J, Le Trang N, Pekala P, Cerami A (1985) Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med 161(5):984–995 [DOI] [PMC free article] [PubMed]
- 8.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A (1987) Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330(6149):662–664 [DOI] [PubMed]
- 9.Feldmann M (2002) Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol 2(5):364–371 [DOI] [PubMed]
- 10.Nathan CF (1987) Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. Clin Invest 80(6):1550–1560 [DOI] [PMC free article] [PubMed]
- 11.Criscione LG, St Clair EW (2002) Tumor necrosis factor-alpha antagonists for the treatment of rheumatic diseases. Curr Opin Rheumatol 14(3):204–211 [DOI] [PubMed]
- 12.Nathan CF, Ehrt S (2003) Nitric oxide and tuberculosis. In: Rom W, Garay S (eds) Tuberculosis, 1st edn. Lippincott, New York
- 13.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P (1989) The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56(5):731–740 [DOI] [PubMed]
- 14.Bean AG, Roach DR, Briscoe H, France MP, Korner H (1999) Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol 162(6):3504–3511 [PubMed]
- 15.Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ (2002) TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol 168(9):4620–4627 [DOI] [PubMed]
- 16.Trevejo JM, Marino MW, Philpott N, Josien R, Richards EC, Elkon KB, Falck-Pedersen E (2001) TNF-alpha-dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc Natl Acad Sci U S A 98(21):12162–12167 [DOI] [PMC free article] [PubMed]
- 17.Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S (1999) Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10(1):29–38 [DOI] [PubMed]
- 18.Maianski NA, Roos D, Kuijpers TW (2002) Tumor necrosis factor {alpha} induces a caspase-independent death pathway in human neutrophils. Blood 101(5):1987–1995 [DOI] [PubMed]
- 19.Hodge-Dufour J, Marino MW, Horton MR, Jungbluth A, Burdick MD (1998) Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc Natl Acad Sci U S A 95(23):13806–13811 [DOI] [PMC free article] [PubMed]
- 20.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K et al (1995) Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2(6):561–572 [DOI] [PubMed]
- 21.Paget SA (2001) Postmarketing adverse event data for TNF-alpha antagonists. <http://www.hss.edu/Professionals/Conditions/Rheumatoid-Arthritis/Postmarketing-Adverse-Event-TNF-Alpha> (Accessed on December 7, 2006)