Abstract
Purpose
To describe the procedure of ultrasound-guided Morton’s neuroma and recurrent stump neuroma injections and early clinical outcomes after a single injection.
Materials and Methods
Retrospective review of 44 percutaneous ultrasound-guided neuroma injections in 24 patients who had completed clinical outcomes questionnaires. A 10-point pain scale [scale of 1 (no pain) to 10 (severe pain)] in a 7-day pain log format was distributed to patients at the time percutaneous neuroma injection was performed.
Results
Neuromas were clearly visualized with sonography as hypoechoic nodules and were distinguishable from other causes of forefoot pain, such as metatarsophalangeal joint synovitis and intermetatarsal bursae. The sizes of the neuromas injected ranged between 4 and 19 mm. Postinjection, all neuromas displayed increased echogenicity and/or the appearance of fluid surrounding it, confirming localization of the therapeutic mixture. We arbitrarily subdivided the pain ratings into symptomatic (greater than 4) and asymptomatic (less than or equal to 4) for statistical analysis. Average pain level pre injection was 5.2 and average pain level was 3.7 at 7 days post single injection, with 62% of the initially symptomatic patients asymptomatic on day 7 (p < 0.000001). Overall, 76% of the total number of neuromas injected once were asymptomatic on day 7.
Conclusion
Ultrasound can be used to accurately target Morton’s neuromas and, therefore, appropriately direct therapeutic interventions, with good short-term clinical results.
Introduction
Morton’s neuroma is a common cause of forefoot pain, often presenting with pain and paresthesias radiating to the toes [1, 2]. Morton’s neuromas have been histologically proven to represent a proliferation of scar tissue and perineural fibrosis about the interdigital nerve, usually at the level of the metatarsal heads [2–4]. Compression of the interdigital nerve beneath the transverse intermetatarsal ligament, often from ill-fitting shoes, has been identified as one of the major factors in the development of interdigital neuromas [3–5]. Neuromas most often involve the third web space (between the third and fourth metatarsal heads), and slightly less frequently, the second web space [3, 6].
Treatment of Morton’s neuromas traditionally includes altering shoe wear and administration of anti-inflammatory medications [3]. Excision of the neuroma reportedly has a variety of clinical results, and surgical technique is important to prevent painful neuroma recurrence (“transection” or “stump” neuroma) [5, 7]. “Blind” percutaneous diagnostic or therapeutic injections have been performed with relatively poor overall clinical outcome [2, 6, 8]. Rasmussen et al. demonstrated that only 15% of patients in their series experienced greater than 50% improvement after a single anesthetic-steroid injection [2]. Traditionally, “blind” injections of neuromas are performed with direction of the needle toward the site of patient’s maximal symptomatology within the web space, and the tip of the needle directed plantarly toward the intermetatarsal ligament [2, 6]. Unfortunately, the site of maximum symptomatology may not always correlate with the location of the neuroma.
The use of imaging guidance for soft tissue steroid injections in the musculoskeletal system has determined that accurate placement of therapeutic anesthetic-steroid mixture correlated with improvement in patients with de Quervain’s tenosynovitis in comparison to those where the needle tip was guided only by clinical palpation [9]. In this study, contrast injected under fluoroscopy within the first extensor compartment tendon sheath correlated with improvement in patient symptomatology [9]. More recently, ultrasound has been employed for guiding diagnostic and therapeutic injections in the musculoskeletal system [10–12]. Ultrasound guidance allows for accurate needle placement within the neuroma, the location of which may not always correspond to where is it is clinically suspected. Ultrasound can accurately identify soft tissue lesions and distinguish between other causes of metatarsalgia with similar clinical symptoms to Morton’s neuromas (e.g., metatarsophalangeal synovitis or intermetatarsal bursae), which, if left undiagnosed, may lead to persistent clinical symptomatology [13].
We evaluated our experience using ultrasound-guidance for Morton’s neuroma injections and reviewed the early clinical outcomes.
Materials and methods
Our Institutional Review Board approved this study. Procedures were performed over an 8-month period (December 2002 through August 2003). There were 24 patients, two men and 22 women, ranging in age from 28 to 79 years. A total of 44 injected neuromas, equally divided between right and left, with clinical follow up, were reviewed. Thirteen patients had more than one neuroma injected at one time, four patients had had prior excision of neuromas, one of which was injected twice (patient #2).
Pain logs are used in our Department for all image-guided injections. Pain logs are designed to quantitate patient symptomatology. These are given to patients immediately after the procedure and the patients were instructed to complete the questionnaire and to mail the results back to the Department (they were given a self-addressed stamped envelope). All clinical outcomes questionnaires returned from patients having undergone ultrasound-guided neuroma injections were reviewed by the principal investigator. Injected material (see below) consisted of an anesthetic/corticosteroid mixture with a typical anesthetic duration of approximately 4 to 5 h. The pain logs employ a 10-point pain scale (1 reflecting no pain and 10, severe (maximal) pain). The pain logs track patient symptomatology for 7 days, inclusive of the day of the procedure, and a baseline assessment prior to injection. There are 17 total data entry points: immediately pre-injection, immediately postinjection, 2 h postinjection, 4 h postinjection, 8 h postinjection, and then the morning and afternoon for days 2–7. If patients circled two adjacent numbers on the pain logs, the lower of the two numbers was used. For two ipsilateral injections, patients filled out one form per foot (N = 16 patients) as patients often cannot separate pain from two adjacent webspaces. For bilateral injections, separate forms for right and left feet were always utilized.
Neuromas injected ranged in size between 4 and 19 mm. Procedures were performed by an attending radiologist trained in musculoskeletal radiology and a musculoskeletal radiology fellow. All ultrasound procedures were performed using a Siemens Sonoline Elegra (Mountainview, CA) machine and a medium frequency (7.5 MHz) linear transducer. Phase inversion tissue harmonic imaging was utilized in most cases. Occasionally, photopic imaging™ (Siemens) was employed both for diagnosis and well as the imaging guided procedure depending on operator preference [13]. Patients were referred for injection by one of our Podiatric or Foot and Ankle services.
A mixture of 25% 1% Lidocaine (Abbot Laboratories, North Chicago, IL), 25% 0.5% bupivacaine (Sensorcaine, Astra Pharmaceuticals, Westborough, MA), and 50% triamcinolone (Kenalog, 40 mg/1 mL) (Apothecon, a Bristol-Myers Squibb Company, Princeton, NJ) was used to inject the neuromas. Topical iodine solution is used for antisepsis and a short (1.5″) 25-gauge needle is used. A small amount of topical anesthesia (1% lidocaine) is administered through this needle and then the same needle is directed into the neuroma. We find that the addition of a longer acting anesthetic (bupivacaine) in the mixture helps to reduce the incidence of a painful “flare response” that occasionally accompanies percutaneous steroid injections. Approximately 0.75 cc total volume (15 mg Kenalog) was utilized in the majority of cases. The volume injected depended on a variety of factors including lesion size and associated adventitial bursal formation and the individual neuroma “end point.” (see Table 1). Two neuromas were injected with vitamin B12 in lieu of 1% lidocaine; at the referring physician’s request and unsupported belief that vitamin B12 results in more rapid decrease in neuroma size. Patients in general tolerate the procedure well, with minimal discomfort when the local anesthesia is administered, and, in general, no further pain for the duration of the procedure.
Table 1.
Sonographic findings in patients who underwent ultrasound-guided Morton’s neuroma injections with clinical follow-up
| Pt # | Neuroma Location | Size | Other Findings/Comments |
|---|---|---|---|
| 1 | RT 2 | 11 × 6 mm | Small bursa associated with 3WS neuroma |
| RT 3 | 12 × 8.5 mm | ||
| 2A | LT 2 | 9 × 9 mm | Postop: stump neuroma in 2WS |
| Small stump neuroma also present in 3WS | |||
| 2B | LT 2 | Same patient as 2A; same webspace injected 4 months later | |
| 3 | LT 2 | 10 mm | 1 cc solution injected; was seen to fill associated plantar bursa |
| 4 | RT 2 | 7 × 7 mm | |
| RT 3 | 7 × 6 mm | ||
| LT 2 | 5 mm | ||
| LT 3 | 5 mm | ||
| 5 | RT 2 | 7.6 × 6 mm | 0.5 cc solution injected |
| postop 3 and 4 WS neuroma excisions, ipsilateral foot | |||
| 6 | LT 2 | ||
| LT 3 | 7 × 5 mm | ||
| 7 | RT 2 | 13 × 12 mm | Small bursa in right 1WS; small neuroma in left 1WS |
| RT 3 | 13 × 5 mm | ||
| LT 2 | 11 × 11 mm | ||
| LT 3 | 5 × 6 mm | ||
| 8 | LT 2 | 6.5 × 6.5 mm | 0.5 cc Vit B12 included in mixture instead of 1% lidocaine |
| 9 | LT 2 | ||
| LT 3 | |||
| 10 | RT 2 | 10 × 7 mm | Small neuroma also in right 1WS |
| LT 2 | 12 × 7 mm | ||
| 11 | RT 2 | 15 × 10 mm | Small adventitial bursa with LT 2WS neuroma |
| RT 3 | 18 × 12 mm | ||
| LT 2 | 9 × 9 mm | ||
| LT 3 | 17 × 9 mm | ||
| 12 | RT 2 | ||
| 13 | RT 2 | 11.5 × 10 mm | 0.5 cc Vit B12 included in mixture instead of 1% lidocaine |
| RT 3 | 8.6 mm | ||
| 14 | RT 2 | 17.5 × 11.5 mm | |
| RT 3 | 12 × 7 mm | ||
| 15 | LT 2 | 16.5 × 12 mm | 2MTP joint synovitis present; also injected |
| Small 3 WS neuroma also present | |||
| 16 | LT 2 | 9.6 mm | Pt symptomatic when pressure applied to both webspaces |
| LT 3 | 6 mm | ||
| 17 | LT 2 | 15 × 9 mm | Stump neuroma |
| Small neuroma also in 3WS | |||
| 18 | RT 2 | 11.7 mm | Bursa associated with 2WS |
| RT 3 | 6.5 × 6 mm | ||
| 19 | RT 3 | 18 × 10 mm | Synovitis 3 and 4 MTP; these joints also injected |
| 20 | RT 2 | 19 mm | DJD right 1 MTP joint |
| RT 3 | 13 mm | ||
| 21 | LT 3 | 10 mm | Stump neuroma; also seen on recent MRI |
| Large 2WS neuroma (14 mm) with associated bursa also present | |||
| 22 | RT 2 | 11.5 × 7.5 mm | Small neuroma also in ipsilateral 3WS |
| 23 | LT 2 | 10 × 14 mm | |
| LT 3 | 9 × 7 mm | ||
| 24 | LT 3 | 15 mm | 16 m neuroma also in ipsilateral 2WS |
Pt 2A: “extreme pain when walking”
Pt 6: believes “pain related to level of activity” and “shoes [she] wears”
Pt 12: noted pain “depends on level of activity”
Pt 14: “sharp pain with walking”
Pt 16: “with sneakers still hurts”
Pt 24: wore “heels, pain returned”
Pt = patient, RT = right, LT = left, 2 = second web space, i.e., between the second and third toes), 3 = third webspace, i.e., between the third and fourth toes), mm = millimeter, WS = webspace, MTP = metatarsophalangeal, DJD = degenerative joint disease, MRI = magnetic resonance imaging
We arbitrarily subdivided the pain ratings into symptomatic (greater than 4) and asymptomatic (less or equal to 4) for statistical analysis. We realize that for some patients a higher value may be considered asymptomatic and for others that a lower value may be employed; however, we chose a number less than the median for statistical analysis. Qualitative and quantitative data analysis was performed with statistical analysis of the data entry points including a paired t-test.
Results
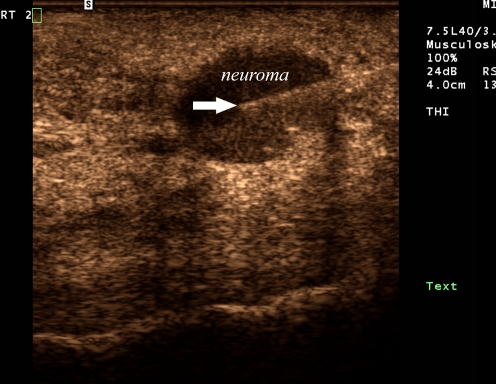
With sonography, neuromas are clearly visualized as incompressible hypoechoic nodules replacing the normally hyperechoic web space fat (see Fig. 1) [14]. Neuromas were distinguishable from other causes of forefoot pain, such as metatarsophalangeal joint synovitis and intermetatarsal bursae. The sizes of the neuromas injected ranged between 4 and 19 mm. Postinjection, all neuromas displayed increased echogenicity and/or the appearance of fluid surrounding it, confirming localization of the therapeutic mixture (see Figs. 2 and 3).
Fig. 1.
A 35-year-old female (patient #1) with a second web space neuroma. Longitudinal ultrasound image of the second web space with photopic and tissue harmonic imaging demonstrates a discrete round hypoechoic mass within the web space consistent with a neuroma. Linear echogenic needle tip (arrow) is within the center of the neuroma for purposes of therapeutic injection
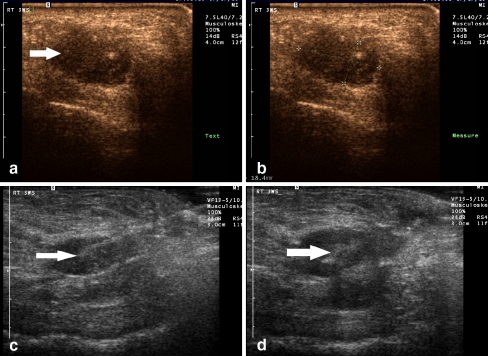
Fig.2.
A 52-year-old female (patient #19) with pain over both the third and fourth metatarsophalangeal joints. A. Longitudinal ultrasound image of the third web space with photopic imaging demonstrates a discrete hypoechoic mass consistent with a neuroma B. Digital calipers indicate the size of the neuroma to be 18.4 × 9.8 mm C. Longitudinal gray scale image demonstrates a linear echogenic needle entering the neuroma for purposes of therapeutic injection (arrow). D. During real-time evaluation, echogenic microbubbles of injected steroid/anesthesia mixture can be seen filling the neuroma (arrow)
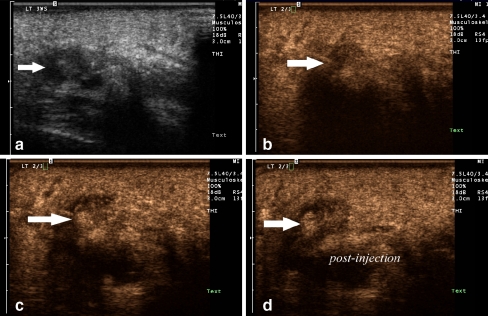
Fig. 3.
A 43-year-old female (patient #23) with a third web space neuroma. A. Longitudinal gray scale image of the third web space with tissue harmonic imaging demonstrates a hypoechoic mass consistent with a neuroma B. During the injection, the linear echogenic needle tip can be seen entering the hypoechoic mass (arrow) C. Echogenic microbubbles can be seen filling the neuroma during real-time evaluation, confirming accurate delivery of the medication. D. Scanning over the web space with photopic imaging postinjection after the needle is removed demonstrates the confluent echogenic material within the neuroma (arrow)
The average pre-procedure pain score was 5.2. Immediately postinjection, the average pain score was 2.2, a 42% average decrease in reported pain. The lowest average pain levels were immediately postinjection and the morning of day 3 (approximately 36 to 48 h postinjection). Sixty two percent of initially symptomatic patients were asymptomatic the morning of day 7 (p < 0.000001). Seventy six percent of all neuromas injected were asymptomatic the morning of day 7.
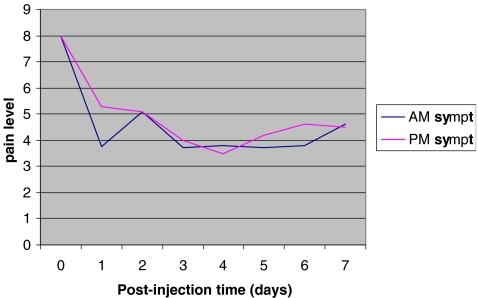
Patients experienced a fluctuation of reported pain levels over time (see Fig. 4). The patients themselves occasionally realized and noted this on their pain log sheets, commenting, on average, that their pain levels fluctuated with their degree of activity and shoe wear (see Table 1). We also noted a trend in slight increased symptomatology on the afternoon rating, as opposed to the corresponding morning scale.
Fig. 4.
Graph of mean patient morning and afternoon symptomatology during the week after injection
Discussion
At ultrasonography, the appearance of Morton’s neuromas are hypoechoic nodules replacing the normally hyperechoic interdigital web space fat [3, 15] (see Fig. 1). Occasionally, an enlarged feeding interdigital nerve can be seen associated with the neuroma [15]. One series in the literature with surgical correlation demonstrated a 98% accuracy in preoperative diagnosis of interdigital neuromas with ultrasound [16]. Masala et al. reported on the utility of ultrasound guidance in percutaneous alcohol injections of interdigital neuromas that involved a series of three to seven injections at 7–10 days intervals [17]. More recently, Gruber et al. and Fanucci et al. also reported on a series of patients treated with sonographically guided alcohol-sclerosing therapy into symptomatic stump neuromas with promising clinical results [18]. To our knowledge, this is the largest reported clinical outcomes study from a single ultrasound-guided neuroma injection using corticosteroids.
Our results documented good to excellent initial pain relief after one ultrasound-guided injection for treatment of interdigital neuromas, with 62% of symptomatic patients asymptomatic 7 days after a single injection. This pain response is better than previous published reports in the orthopedic literature with only 30% of patients exhibiting complete relief of symptoms after a series of 3–4 “blind” injections [6]. In a retrospective study by Greenfield et al., only 14% of patients had total pain relief after only one injection, similar to other published results with only 15% of patients demonstrating greater than 50% improvement in their symptoms after a single “blind” injection [2, 6].
Extensive preoperative work-up with diagnostic injections (1% lidocaine only), clinical examination, as well as surgical excision, does not always result in a good clinical outcome [8]. Twenty-four percent of primary excisions and 43% of re-excision of stump neuromas were clinical failures in one series, with pain, persistent need for shoe modification and cosmetic issues with scar formation accounting for these clinical failures [8]. Surgical excision of neuromas has yielded variable results. Overall, 20% of patients experience less than 50% pain relief after surgical excision [7]. Various technical innovations in surgical treatment of neuromas have demonstrated some improvement in long-term pain relief and avoidance of painful stump neuroma recurrence. Stump neuroma formation usually occurs after resection and are generally only symptomatic when they recur close to the skin, a joint capsule, or under the metatarsal heads [7]. Placing the transected nerve edge into muscle has been shown to have a better clinical outcome, with smaller recurrent stump neuroma formation and their location of recurrence away from a symptomatic area [7].
Clinical outcomes in our series were better than those previously published and are contributed to the improved accuracy of targeting the neuromas using real-time sonographic guidance. Some limitations of this study include the subjective nature of the pain logs. The duration of the patient’s symptoms pre-procedure is not taken into account. The pain logs were designed to promote patient compliance, so the patient may have been more aware of their pain levels in the short term after the procedure. Limiting patient pain responses to 1 week may not be sufficient. It may take a longer time for maximum pain response post steroid injection to allow for a complete anti-inflammatory effect. A modest decrease in symptomatology post procedure may, in fact, improve over time. Greenfield et al. utilized a 1- to 2-year follow-up time to evaluate the clinical outcome of their series of percutaneous injections [6]. In the study by Greenfield et al., the 50% of patients exhibited only “temporary” pain relief after a series of injections; follow up 2 years later demonstrated that 48% of these patients were no longer symptomatic [6]. Coughlin and Pinsonneault had a 5.8-year follow-up interval after surgical excision of Morton’s neuromas [5]. We also have not performed a controlled series study comparing ultrasound-guided steroid neuroma injections with alcohol-sclerosing therapy; however, ideally, the smallest number of interventions providing satisfactory pain relief would likely be ideal. Follow-up evaluation of the patients in our series at 2 years, we propose, would likely demonstrate an even greater percentage of good to excellent responses.
The degree of patients’ pain is usually related to the degree of activity (walking, running), as well as the kind of footwear they wear. For our patients, instructions were given for them not to engage in strenuous walking or running for 2 h; after that, they were free to undergo their usual activity. Even after surgical excision of Morton’s neuromas, modification of shoe wear and activities are often needed [5]. Some of the patients in our study actively recognized this and so indicated on the forms: “extreme pain when walking” (patient 2); “sharp pain when walking” (patient 14); “wore heels, pain returned” (patient 24) (see Table 1).
The ability of ultrasound to localize and differentiate the various causes of forefoot pain has great clinical utility [19]. It has been demonstrated that patients with metatarsalgia may have more than one finding accounting for their pain, such as metatarsophalangeal joint synovitis, degenerative arthrosis, and intermetatarsal bursae. It may be that the Morton’s neuromas themselves were not, or were only partially, responsible for the patient’s symptoms. Given the incidence of concomitant second- and third-web space neuromas, undertreatment may also be a factor in limited clinical response. The identification of these other causes of metatarsalgia is important for clinical treatment, for if ultrasound-guided Morton’s neuroma injections do not reduce the patient’s symptomatology, one of these other causative factors may be responsible for the patient’s symptoms.
Future work includes correlating of the size and number of neuromas and ultimate clinical outcome, as well as follow-up imaging studies to document change, if any, in the size or morphology of neuromas posttreatment. We have noted, anecdotally, a change in the morphology of a few percutaneously treated neuromas, with increased echogenicity within the centers of these neuromas.
In conclusion, ultrasound is a quick, reliable method for guiding for percutaneous Morton’s neuroma injections. The ability to directly visualize the neuroma and needle tip placement allows for confident placement and verification of the anesthetic-steroid mixture into the neuroma. It is this improved injection accuracy that we feel contributed to our good to excellent short-term clinical outcome following a single ultrasound-guided injection.
References
- 1.Morton T (1876) A peculiar and painful affection of the fourth metatarsophalangeal articulation. J Med Sci 71:37–45
- 2.Rasmussen MR, Kitaoka HB, Patzer GL (1996) Nonoperative treatment of plantar interdigital neuroma with a single corticosteroid injection. Clin Orthop Relat Res 326:188–193 [DOI] [PubMed]
- 3.Kaminsky S, Griffin L, Milsap J, Page D (1997) Is ultrasonography a reliable way to confirm the diagnosis of Morton’s neuroma? Orthopaedics 20:37–39 [DOI] [PubMed]
- 4.Oliver TB, Beggs I (1998) Ultrasound in the assessment of metatarsalgia: a surgical and histological correlation. Clin Radiol 53:287–289 [DOI] [PubMed]
- 5.Coughlin MJ, Pinsonneault T (2001) Operative treatment of interdigital neuroma. JBJS 83-A:1321–1328 [PubMed]
- 6.Greenfield J, Rea J Jr, Ilfeld SW (1984) Morton’s interdigital neuroma: indications for treatment by local injections versus surgery. Clin Orthop Relat Res 185:142–144 [PubMed]
- 7.Colgrove RC, Huang EY, Barth AH, Greene MA (2000) Interdigital neuroma: intermuscular neuroma transposition compared with resection. Foot Ankle Int 21:206–211 [DOI] [PubMed]
- 8.Younger ASE, Claridge RJ (1998) The role of diagnostic block in the management of Morton’s neuroma. Can J Surg 41:127–130 [PMC free article] [PubMed]
- 9.Zingas C, Failla JM, van Holsbeeck M (1998) Injection accuracy and clinical relief of de Quervain’s tendinitis. J Hand Surg 23:89–96 [DOI] [PubMed]
- 10.Sofka CM, Collins AJ, Adler RS (2001) Use of ultrasonographic guidance in interventional musculoskeletal procedures: a review from a single institution. J Ultrasound Med 20:21–26 [DOI] [PubMed]
- 11.Sofka CM, Adler RS (2002) Ultrasound-guided interventions in the foot and ankle. Semin Musculoskelet Radiol 6:163–168 [DOI] [PubMed]
- 12.Adler RS, Sofka CM (2003) Percutaneous ultrasound-guided injections in the musculoskeletal system. Ultrasound Q 19:3–12 [DOI] [PubMed]
- 13.Sofka CM, Lin D, Adler RS (2005) Advantages of Color B-mode imaging with contrast optimization in sonography of low-contrast musculoskeletal lesions and structures in the foot and ankle. J Ultrasound Med 24:215–218 [DOI] [PubMed]
- 14.Redd RA, Peters VJ, Emery SF, Branch HM, Rifkin MD (1989) Morton neuroma: sonographic evaluation. Radiology 171:415–417 [DOI] [PubMed]
- 15.Okafor B, Shergill G, Angel J (1997) Treatment of Morton’s neuroma by neurolysis. Foot Ankle Int 18:284–287 [DOI] [PubMed]
- 16.Quinn TJ, Jacobson JA, Craig JG, van Holsbeeck MT (2000) Sonography of Morton’s neuromas. Am J Roentgenol 174:1723–1728 [DOI] [PubMed]
- 17.Shapiro PP, Shapiro SL (1995) Sonographic evaluation of interdigital neuromas. Foot Ankle Int 16:604–606 [DOI] [PubMed]
- 18.Masala S, Fanucci E, Ronconi P et al (2001) Treatment of intermetarsal neuromas with alcohol injection under ultrasound guidance. Radiol Med (Torino) 102:370–373 [PubMed]
- 19.Gruber H, Kovacs P, Peer S, Frischhut B, Bodner G (2004) Sonographically guided phenol injection in painful stump neuroma. Am J Roentgenol 182:952–954 [DOI] [PubMed]
- 20.Iagnocco A, Coari G, Palombi G, Valesini G (2001) Sonography in the study of metatarsalgia. J Rheumatol 28:1338–1340 [PubMed]
- 21.Fanucci E. Masala S, Fabiano S, Perugia D, Squillaci E, Varrucciu V, Simonetti G (2004) Treatment of intermetatarsal Morton’s neuroma with alcohol injection under US guide: 10-month follow up. Eur Radiol 14(3):514–518 [DOI] [PubMed]