Abstract
Nitric oxide (NO), synthesized from l-arginine by NO synthases (NOS), plays an essential role in the regulation of cerebrovascular tone. Adenoviral vectors have been widely used to transfer recombinant genes to different vascular beds. To determine whether the recombinant endothelial NOS (eNOS) gene can be delivered in vivo to the adventitia of cerebral arteries and functionally expressed, a replication-incompetent adenoviral vector encoding eNOS gene (AdCMVNOS) or β-galactosidase reporter gene (AdCMVLacZ) was injected into canine cerebrospinal fluid (CSF) via the cisterna magna (final viral titer in CSF, 109 pfu/ml). Adventitial transgene expression was demonstrated 24 h later by β-galactosidase histochemistry and quantification, eNOS immunohistochemistry, and Western blot analysis of recombinant eNOS. Electron microscopy immunogold labeling indicated that recombinant eNOS protein was expressed in adventitial fibroblasts. In AdCMVNOS-transduced arteries, basal cGMP production and bradykinin-induced relaxations were significantly augmented when compared with AdCMVLacZ-transduced vessels (P < 0.05). The increased receptor-mediated relaxations and cGMP production were inhibited by eNOS inhibitors. In addition, the increase in cGMP production was reversed in the absence of calcium, suggesting that the increased NO production did not result from inducible NOS expression. The present study demonstrates the successful in vivo transfer and functional expression of recombinant eNOS gene in large cerebral arteries. It also suggests that perivascular eNOS gene delivery via the CSF is a feasible approach that does not require interruption of cerebral blood flow.
Keywords: adenoviral vector, caveolae, adventitial fibroblast, gene therapy, nitric oxide synthase
Cerebral blood vessels differ structurally, physiologically, and pharmacologically from extracranial vasculature (1). Gene transfer to cerebral blood vessels represents a promising and novel approach for the prevention and/or treatment of various intracranial vascular disorders, including cerebral vasospasm. Intravascular administration of the vectors, as conventionally used to accomplish gene transfer to the peripheral vasculature, may have major limitations when applied to cerebral blood vessels. The interruption of cerebral blood flow required for luminal administration of the vector, and the possible attenuation by the blood–brain barrier of viral vector in cerebral vessels beyond endothelium constitute some of the major problems with this approach (2). These obstacles, however, may be overcome by perivascular delivery of the vector via cerebrospinal fluid (CSF), either under normal conditions (3) or in the presence of cisternal blood (4). Such an approach may facilitate the delivery of genes of therapeutic potential to the cerebral vasculature.
Nitric oxide (NO), an endogenous vasodilator formed from its precursor l-arginine by a family of NO synthases (NOS) (5–7), plays an essential role in the regulation of cerebrovascular tone (8–10). NO induces vasodilation by activating soluble guanylate cyclase with a consequent elevation of intracellular cGMP levels in vascular smooth muscle cells (11, 12). Thus far, three distinct NOS isoforms, encoded by three distinct genes, have been isolated and purified (7, 13). In cerebral arteries, NO can be produced constitutively by endothelial NOS (eNOS) or neuronal NOS (7), or by an inducible NOS (iNOS) in response to cytokines and endotoxin (13). Thus cerebral vascular tone may be regulated by the expression of constitutive and/or iNOS activities under physiological and pathological conditions.
Adenoviral vectors have been used to achieve efficient transfer and expression of recombinant genes (14) in different vasculatures both in vitro and in vivo (15, 16). Recently, eNOS and iNOS gene transfer to peripheral arteries of different animals mediated by liposome–Sendai viral complex, adenovirus, or retrovirus have been reported (17–19). Our previous ex vivo study has shown that recombinant eNOS gene can be successfully expressed in cerebral arteries (20). Herein we report the first in vivo recombinant eNOS gene transfer via CSF. The results of our study provide direct evidence that adventitial eNOS transgene expression leads to increased local NO activity, with functional effects in major cerebral arteries. They also suggest that perivascular transfer of a functional gene, via the cisterna magna in CSF, is a feasible approach that does not require interruption of cerebral blood flow.
MATERIALS AND METHODS
Adenoviral Vectors.
A replication-incompetent adenoviral vector encoding an eNOS gene (AdCMVNOS), driven by the cytomegalovirus immediate early promoter, was generated through homologous recombination. The generation, propagation, purification, and evaluation of adenoviral vector containing eNOS gene were described in detail (20). Briefly, the early region 1 (E1) of a full-length serotype 5 wild adenovirus was deleted and replaced by a cDNA sequence encoding bovine aortic endothelial cell eNOS (kindly provided by David G. Harrison, Emory University, Atlanta). The recombinant adenovirus encoding an Escherichia coli β-galactosidase (β-gal) (LacZ) reporter gene (AdCMVLacZ), used in all experiments as a control vector, was a generous gift of James M. Wilson (University of Pennsylvania, Philadelphia). The viral vectors were stored at −80°C in dialysis solution containing 10% glycerol.
In Vivo Gene Transfer and Surgery Procedure.
All procedures were performed in accordance with the institutional guidelines of the Mayo Clinic. Adult mongrel dogs of both sexes (15–20 kg) were anesthetized with thiopental (15–25 mg/kg i.v.). A spinal needle (22 gauge) was aseptically inserted into the cisterna magna, and 1 ml of CSF was withdrawn as described (21). Adenoviral vector was mixed with the withdrawn CSF and then injected back into the subarachnoid space. Injections of AdCMVLacZ and AdCMVNOS vectors were always done using two separate dogs in each experiment so that functional expression of the transgene could be assessed in parallel. The dog’s head was maintained in a nose-up position (i.e., the superior plane of the parietal bone was tilted backward by 30°) for 30 min after viral injection (4). Antipyretic (Dipyrone, 1–5 ml, s.c.), antibiotics (penicillin, 55,000 units/kg, i.m.; gentamicin, 2 mg/kg, s.c., bid.), analgesics (Torbugesic, 0.6 mg/kg, i.m.; Acepromazine, 0.1 mg/ml, i.m.), and lactated Ringers solution (500–1,500 ml, i.v.) were administered over 24 h after the vector injection. Rectal temperatures were monitored just before (0 h), and at 6, 10, 12, and 24 h after vector injection.
Twenty-four hours after injection, dogs were killed and the brains with attached cerebral arteries were removed for evaluation of transgene expression. Basilar and middle cerebral arteries were removed for β-gal staining and quantification, eNOS immunohistochemistry, Western immunoblot analysis, and isometric tension recording studies. The remaining portion of basilar arteries were subjected to electron microscopy (EM) immunogold labeling and cGMP radioimmunoassay.
Ex Vivo Gene Transfer.
Ex vivo gene transfer experiments were performed on rings (4 mm long) of normal canine basilar and middle cerebral arteries. Arteries were infected with three different titers [108, 109, and 1010 plaque forming units (pfu)/ml] of AdCMVLacZ in minimal essential medium (MEM) for 30 min, followed by 24-h incubation in fresh MEM, as described (20). The vessels were then subjected to β-gal quantification (see below) to compare with the vessels after in vivo gene transfer.
Optimal Vector Titer for in Vivo Gene Delivery.
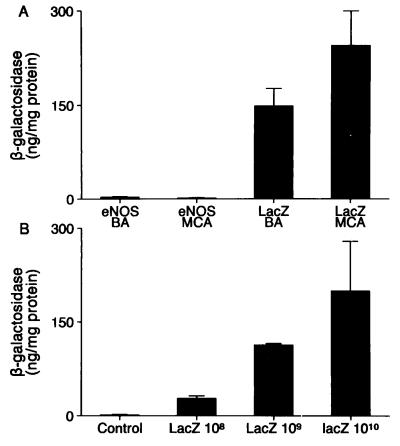
The final viral titer of 109 pfu/ml in the CSF was an estimation based on the CSF volume of 20 ml in adult dogs (22). This optimal titer used for gene transfer in CSF was determined by three approaches. First, a group of six dogs was tested with the injection of three different viral titers in CSF: 5 × 108, 109, and 5 × 109 pfu/ml, followed by β-gal staining and observation of systemic symptoms for a 24-h period. The transgene expression after β-gal staining ranged between just visible with low viral titer (i.e., 5 × 108 pfu/ml) and very dense staining with high viral titer (i.e., 5 × 109 pfu/ml) (data not shown). Second, β-gal protein level in in vivo AdCMVLacZ-transduced vessels was similar to that of ex vivo-transduced vessels with a final titer of 109 pfu/ml in the incubation medium (20) (see Fig. 3 A and B). Finally, rectal temperatures after gene transfer with the titer of 109 pfu/ml were similar to the normal baseline readings prior to gene transfer, and animals showed no signs of systemic or local toxic effects.
Figure 3.
β-Gal levels in AdCMVLacZ- and AdCMVNOS-transduced cerebral arteries 24 h after gene transfer. (A) β-Gal levels in basilar and middle cerebral arteries of same dogs after in vivo gene transfer (final viral titer in CSF, 109 pfu/ml). (B) β-Gal levels in basilar arteries of same dogs after ex vivo gene transfer (final viral titers in the incubation media, 108, 109, and 1010 pfu/ml). Data are expressed as means ± SEM (n = 4 from 4 dogs). ∗, P < 0.05, LacZ vs. eNOS (one-way factorial ANOVA).
Morphological Analysis of Transgene Expression.
Histochemical staining for the detection of β-gal and immunohistochemical studies of eNOS expression were performed as described (20). For control studies, the eNOS specificity was confirmed by (i) omission of the primary eNOS antiserum in the incubation medium, (ii) eNOS immunostaining of AdCMVLacZ-transfected vessels, and (iii) immunostaining of AdCMVNOS-transfected vessels with an isotype-matched primary antibody, mouse IgG1 monoclonal anti-human CD4 antiserum (OPD4).
EM Immunogold Labeling.
A postembedding immunogold cytochemistry technique (23) was modified and used to analyze the ultrastructural localization of recombinant eNOS in basilar arteries after in vivo gene transfer. Arterial segments were fixed, dehydrated, infiltrated, and embedded in LR white resin. The resin was polymerized at 50°C. Ultrathin sections were cut and mounted on uncoated 300 mesh nickel grids. Grids were floated for 15 min in PBS with 0.05% Tween 20 (PBS-T), 0.15 M glycine, 1% ovalbumin, and 1% goat serum, followed by 2-h incubation in eNOS antibody of 1:200 dilution with PBS-T and 1% goat serum, and 1-h incubation in 1:100 goat anti-mouse IgG conjugated to 15 nm colloidal gold. After drying, grids were stained with uranyl acetate and lead citrate for examination by transmission EM.
β-Gal Quantification.
β-Gal measurement in basilar and middle cerebral arteries 24 h after in vivo gene transfer were performed using a Galacto-light chemiluminescent reporter assay kit (Tropix, Bedford, MA). Arterial segments were homogenized and β-gal levels in the supernatant were quantified. A standard curve was generated by serially diluting known amounts of purified β-gal (Sigma). All assays were performed in triplicate with the average being taken for calculation.
Western Blot Analysis of Recombinant eNOS.
Soluble proteins were extracted by homogenization and two cycles of freezing at −70°C and thawing at 37°C. After centrifugation, the supernatant was collected and the total protein concentration was measured by Bradford assay (Bio-Rad). Prestained protein markers (Bio-Rad), 20 μl eNOS positive controls (homogenates of bovine aortic endothelial cells, Transduction Laboratories, Lexington, KY), and 60 μg protein were loaded on 3.75% stacking/7% separating SDS/PAGE. The resolved proteins were transferred to 0.2 μm nitrocellulose membrane on a semi-dry electrophoretic transfer cell (Bio-Rad) for Western blot analysis as described (24). Blots were blocked and incubated with the eNOS mAb (1:1,500) for 12 h at room temperature and after washing with a goat anti-mouse IgG alkaline phosphatase-conjugated secondary antibody (1:50,000, Sigma). Color development was performed using an Immun-Blot Assay Kit (Bio-Rad).
Measurement of Vascular Reactivity.
Functional assessment of transgene expression were performed in parallel rings (4 mm length) of AdCMVLacZ- and AdCMVNOS-transduced cerebral arteries, using isometric tension recording techniques (20). In certain randomly selected rings, endothelium was mechanically denuded. In some experiments, NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 10−4 M) was present in the organ chamber for 15 min before agonist bradykinin application.
Measurement of cGMP.
Basal cGMP production was quantified in both AdCMVLacZ- and AdCMVNOS-transduced basilar arteries as described (20). In some experiments, NOS inhibitor NG-monomethyl-l-arginine (l-NMMA, 10−4 M), or calcium chelators (i.e., EGTA, 10−3 M; bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra-acetate/AM, 2 × 10−5 M) were present in the incubation medium (20).
Statistical Analyses.
Data are expressed as mean ± SEM. Repeated measures ANOVA (RM-ANOVA) was used for comparison of multiple values obtained from the same subject, whereas factorial ANONA was used for comparing data obtained from two independent samples of subjects. Bonferroni’s procedure was also used to control type I error when needed. A two-tail P value of <0.05 was considered as statistically significant.
RESULTS
Transgene Expression After Intracisternal Gene Delivery.
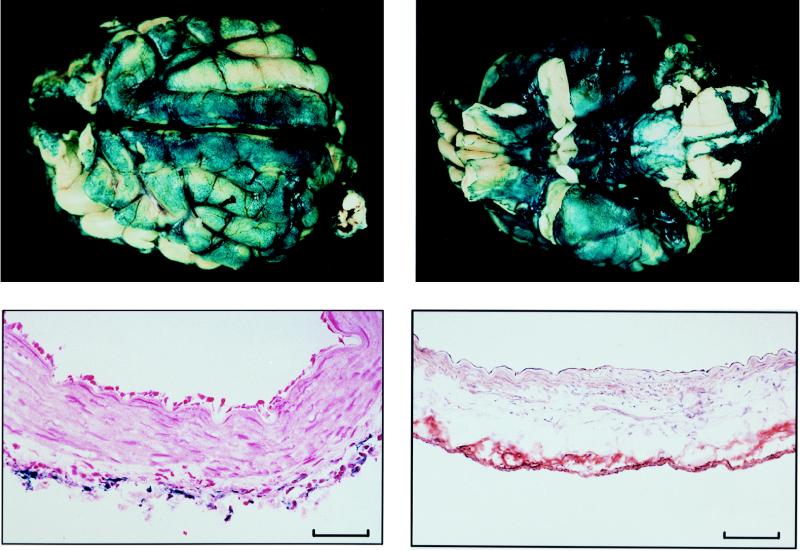
Twenty-four hours after AdCMVLacZ injection into the CSF, recombinant β-gal was observed on leptomeninges overlying the dorsal (Fig. 1A) and the ventral (Fig. 1B) surfaces of the brain, with expression on ventral surface being consistently higher than that of the dorsal surface. Transgene expression was also observed in all major cerebral arteries dissected from the ventral surface after β-gal staining (data not shown). Recombinant β-gal or eNOS protein was expressed in the adventitia (Fig. 1 C and D). A few leukocytes were also observed in the adventitia (Fig. 1C) 24 h after viral injection. In control studies, eNOS immunoreactivity was absent in AdCMVLacZ-transduced vessels, and in AdCMVNOS-transduced vessels when an isotype-matched primary antibody of eNOS was applied. AdCMVNOS-transduced brains and cerebral arteries stained negatively for recombinant β-gal (data not shown).
Figure 1.
Morphological demonstration of transgene expression on canine brain and cerebral arteries 24 h after intracisternal injection of viral vector (final viral titer in CSF, 109 pfu/ml). (A) β-Gal staining on the dorsal surface of the brain. (B) β-Gal staining on the ventral surface of the same brain. Note increased β-gal staining in B compared with A. (C) Microscopic view (cross section) of β-gal staining in a basilar artery after being counterstained with nuclear fast red. (D) Microscopic view (cross section) of immunohistochemical staining of eNOS in a middle cerebral artery 24 h after in vivo eNOS gene transfer. (Bar = 0.1 mm.)
All dogs tolerated a single injection of the vector into the CSF (109 pfu/ml) and showed no major signs of systemic toxic effects with administration of the antipyretic, antibiotics, and lactated Ringers solution over 24 h. Rectal temperatures in a 24-h period before and after gene transfer were similar (data not shown).
Cellular Localization of Recombinant eNOS.
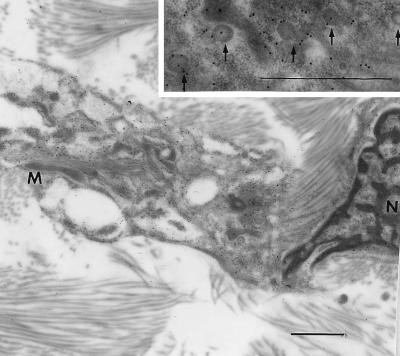
EM immunogold labeling revealed that recombinant eNOS protein was localized in adventitial fibroblasts after in vivo gene transfer. Immunogold particles were observed in the membrane region and within the cytoplasm (Fig. 2). The gold particles were present in the flask-shaped plasma membrane invaginations that are characteristic of plasmalemmal caveolae (arrows in the Inset). Immunogold particles were also observed in the Golgi apparatus and mitochondria. The eNOS immunogold particles were not detected in the adventitia of the nontransduced control vessels.
Figure 2.
EM immunogold labeling showing recombinant eNOS cellular localization in adventitia fibroblast of a basilar artery. Gold particles (15 nm; also see Inset) indicating recombinant eNOS localized along plasma membranes and inside the cytoplasm. N, nucleus; M, mitochondria. (Bar = 1 μm.)
Quantitative Analysis of Recombinant Protein Expression.
β-Gal levels in in vivo AdCMVLacZ-transduced basilar and middle cerebral arteries were significantly higher than in AdCMVNOS-transduced vessels (Fig. 3A) but were comparable with ex vivo AdCMVLacZ-transduced vessels of the same viral titer (i.e., 109 pfu/ml) (Fig. 3B). The presence of recombinant eNOS protein was confirmed in AdCMVNOS- (but not AdCMVLacZ-) transduced basilar and middle cerebral arteries by Western blot analysis (Fig. 4).
Figure 4.
Western blot analysis demonstrating recombinant eNOS expression after in vivo gene transfer. Lanes: 1, homogenate of bovine aortic endothelial cells (positive control); 2 and 3, AdCMVLacZ-transduced basilar and middle cerebral arteries, respectively; 4 and 5, AdCMVNOS-transduced basilar and middle cerebral arteries, respectively. Similar results were seen in duplicate experiments of four dogs.
Effects of in Vivo Gene Transfer on Vascular Reactivity.
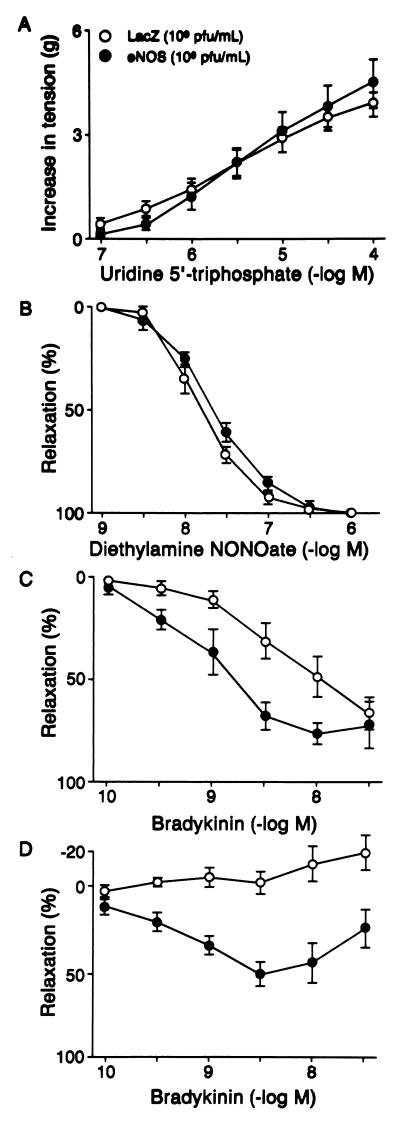
In AdCMVLacZ- and AdCMVNOS-transduced middle cerebral arteries (Fig. 5 A and B) and basilar arteries (data not shown), the receptor-mediated contractile responses to UTP (10−7–10−4 M) (Fig. 5A) and relaxation responses to the NO donor diethylamine NONOate (10−9–10−6 M) (Fig. 5B) were not significantly different from each other 24 h after in vivo gene delivery. In addition, UTP-induced contractions (10−7–3 × 10−5 M) and bradykinin-induced relaxations (10−10–10−6 M) in AdCMVLacZ-transduced arteries were similar to that of the nontransduced control vessels (control vs. LacZ: UTP, EC50 5.6 ± 0.1 vs. 5.6 ± 0.2 g, maximal contraction 4.0 ± 0.6 vs. 3.0 ± 1.0 g, n = 4–6; bradykinin, EC50 8.3 ± 0.4 vs. 8.5 ± 0.3, % maximal relaxation to 3 × 10−4 M papaverine 78.4 ± 6.9 vs. 84.7 ± 3.3, n = 6–8). In contrast, bradykinin-induced relaxations (10−10–3 × 10−7 M) were significantly enhanced in AdCMVNOS-transduced middle cerebral arteries when compared with that of AdCMVLacZ-transduced vessels with intact endothelium (Fig. 5C). These relaxations were readily reversed by l-NAME (10−4 M) in AdCMVNOS-transduced vessels (eNOS vs. eNOS + l-NAME, % maximal relaxation to 3 × 10−4 M papaverine 72.3 ± 5.3 vs. 5.7 ± 12.1, n = 4–6). After the removal of endothelium, however, bradykinin caused relaxations in AdCMVNOS, but not in AdCMVLacZ-transduced middle cerebral arteries (Fig. 5D).
Figure 5.
Effects of transgene expression on contractions to UTP, relaxations to diethylamine NONOate and bradykinin in middle cerebral arteries 24 h after in vivo gene transfer. Shown are concentration-response curves to (A) UTP with endothelium (P > 0.05); (B) diethylamine NONOate with endothelium (P > 0.05); (C) bradykinin with endothelium (P < 0.05); and (D) bradykinin without endothelium (P < 0.05) (two-way RM-ANOVA). Data are expressed as means ± SEM (n = 8 for A and D; n = 4 for B and C). Contractions are expressed in grams, and relaxations are expressed as percentage of the maximal relaxation to papaverine (3 × 10−4 M).
Effects of in Vivo Gene Transfer on Intracellular cGMP Levels.
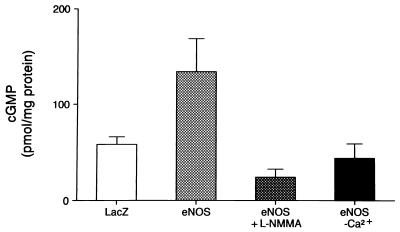
Basal cGMP production was significantly elevated in AdCMVNOS-transduced basilar arteries when compared with that of AdCMVLacZ-transduced vessels (Fig. 6). The increased intracellular cGMP content in AdCMVNOS-transduced vessels was reversed either in the absence of calcium, or by the NOS inhibitor l-NMMA (10−4 M) (Fig. 6). l-Arginine (10−4 M) did not significantly change cGMP production in AdCMVLacZ- or AdCMVNOS-transduced vessels (data not shown).
Figure 6.
Basal cGMP production in AdCMVLacZ- and AdCMVNOS-transduced basilar arteries 24 h after gene transfer, and the effects of NOS inhibitor l-NMMA and calcium depletion. Data are expressed as means ± SEM (n = 7 from 7 dogs each). P < 0.05, eNOS vs. LacZ (one-way factorial ANOVA); eNOS vs. eNOS + l-NMMA (one-way RM-ANOVA); and eNOS vs. eNOS without calcium (one-way RM-ANOVA).
DISCUSSION
In this report we demonstrate in vivo delivery and functional expression of recombinant eNOS gene in cerebral blood vessels. Intracisternal injection of a replication-incompetent adenoviral vector encoding an eNOS gene resulted in efficient expression of recombinant eNOS protein and increased NOS activity in adventitia of major cerebral arteries. The increase in local NO production led to augmentation of cGMP production and enhancement of bradykinin-induced relaxations.
In vivo delivery of an adenoviral vector to the canine CSF via the cisterna magna resulted in transgene expression localized to the adventitia of cerebral arteries. Because CSF administration of vector does not result in direct contact with the lumen or media, transgene expression is absent in both the endothelium and the smooth muscle cells. Adventitial transgene expression may have major advantages in cerebral blood vessels in comparison to the intraluminal approach which requires the interruption of cerebral blood flow and/or disruption of blood–brain barrier function. Transgene expression on the ventral surface was consistently higher than on the dorsal surface. This site of transgene expression is beneficial because major cerebral arteries are located here. There are two possible explanations for this observation. First, the density of the mixture of viral suspension in 10% glycerol with CSF is higher than that of CSF alone, and as a result more viral particles may be in contact with the ventral than the dorsal surface. The second, and more likely explanation, is that the nose-up positioning of the dog’s head for 30 min after vector injection may further facilitate viral particle contact with the ventral surface, where the virus binds to its receptor (25) in cerebral arteries. Thus, physical positioning of the head may target the vector to a specific cerebral site (4).
Quantitative analysis of transgene expression showed that recombinant β-gal levels after in vivo gene transfer are comparable to the levels in vessels subjected to ex vivo gene transfer (i.e., 109 pfu/ml). The expression of recombinant eNOS protein was confirmed by Western blot analysis. Immunohistochemical studies further demonstrated that recombinant eNOS was localized to the adventitia. The function of recombinant eNOS protein was confirmed by the enhancement of cGMP production and augmentation of bradykinin-induced relaxations in AdCMVNOS-transduced cerebral arteries. The enhanced relaxations to bradykinin are best explained by adventitial expression of recombinant eNOS, because relaxations are maintained after endothelial cell removal in AdCMVNOS-, but not AdCMVLacZ-transduced, vessels and are reversed by NOS inhibition. Similar findings were also obtained in our ex vivo gene transfer experiments (M.T. and Z.S.K., unpublished observation). Bradykinin was used in this study because it is an endogenous vasodilator peptide synthesized by cells of the blood vessel wall and is known to activate constitutive eNOS in endothelial cells (26). Our findings suggest that bradykinin is also capable of stimulating recombinant eNOS in the adventitia, leading to increased local NO production.
The expression of recombinant eNOS protein in adventitial fibroblasts after in vivo gene transfer is a novel finding. The eNOS protein was observed in the area of plasma membrane invaginations, which are characteristic of plasmalemmal caveolae (27, 28). Caveolae are clathrin-free cell surface microdomains of the plasma membrane that are abundant in endothelial cells and fibroblasts. Structurally, they consist of a distinct lipid composition and a 21- to 24-kDa protein implicated in transmembrane signaling and transport, caveolin (27, 28). Caveolae play an essential role in eNOS sequestration via caveolin-1 in endothelial cells (29, 30) after the enzyme is palmitoylated (31, 32). Bradykinin, on binding to its receptors on endothelial cells, promotes eNOS depalmitoylation (33), which may lead to the release and translocation of functional eNOS from caveolae to the cytosol (26). Bradykinin receptors are present in fibroblasts (34, 35), and activation of these receptors leads to both extracellular calcium influx and intracellular calcium release (36, 37). In addition, recombinant eNOS has also been detected in the Golgi apparatus and mitochondria of adventitial fibroblasts. Previous studies have demonstrated that both structures are important cellular localization of eNOS protein in endothelial cells (38, 39). Thus, adventitial fibroblasts are well-equipped with the necessary machinery for bradykinin receptor-mediated activation of recombinant eNOS protein.
The possibility of iNOS activation by adenoviral vectors is unlikely, because the increase in basal cGMP production in AdCMVNOS-transduced vessels was calcium-dependent, and cGMP level in AdCMVLacZ-transduced vessels was similar to the level seen in nontransduced control vessels after 24-h incubation in medium (20). Furthermore, contractions to UTP and relaxations to bradykinin were similar between AdCMVLacZ-transduced vessels and nontransduced controls, reinforcing our conclusion of the unlikely involvement of an iNOS. Although the dogs tolerated a single injection of a relatively small amount of virus (i.e., 109 pfu/ml in CSF), leukocytes were observed infiltrating the adventitia (Fig. 1C). The extent and time course of the immune responses following injections of adenovirus remain to be determined. Extensive further studies are also needed regarding viral titer- and time-dependent duration of transgene expression.
NO plays a key role in the regulation of cerebrovascular tone under both physiological and pathological conditions (8–10). Subarachnoid hemorrhage-induced cerebral vasospasm has been shown to be associated with an impaired l-arginine–NO–cGMP pathway (21, 40–42), including a decrease in eNOS mRNA level (43) and loss of NOS immunoreactivity (44). There are few effective therapeutic regimens for this condition (45). The administration of an adenoviral vector via CSF with functional expression of recombinant eNOS in cerebral arteries raises the possibility of providing continuous NO supply to the underlying smooth muscle cells, and may become a potentially feasible therapeutic strategy in alleviating this devastating complication of subarachnoid hemorrhage. Cerebral vasospasm usually occurs between 4 and 12 days after the onset of subarachnoid hemorrhage (45). Thus, short-term transgene expression (i.e., 7–14 days) (15), mediated by an adenoviral vector, may be an advantage in the treatment of cerebral vasospasm. Intracranial delivery and functional expression of recombinant eNOS gene in the cerebral vasculature, therefore, may provide a novel and feasible approach for the treatment of certain cerebrovascular diseases including vasospasm.
Acknowledgments
We thank Mr. Steven C. Ziesmer and Ms. Margaret J. Springett for their help with immunohistochemistry and EM immnunocytochemistry, respectively. We also thank Ms. Adele M. Stelter and Ms. Sharon A. Guy for their invaluable technical support. This work was supported in part by National Institutes of Health Grant HL-53524 (Z.S.K.), Mayo Clinic intramural research grant (T.O.), funds from the Bruce and Ruth Rappaport Program in Vascular Biology and Mayo Clinic Molecular Medicine Program (Z.S.K. and T.O), and the Mayo Foundation. A.F.Y.C. is supported by National Institutes of Health Institutional Training Grant GM08288 and the Mayo Foundation.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: AdCMVLacZ, recombinant adenovirus encoding β-galactosidase gene driven by cytomegalovirus promoter; AdCMVNOS, recombinant adenovirus encoding endothelial nitric oxide synthase gene driven by cytomegalovirus promoter; NOS, NO synthase; eNOS, endothelial NOS; iNOS, inducible NOS; pfu, plaque forming units; CSF, cerebrospinal fluid; EM, electron microscopy; β-gal, β-galactosidase; l-NAME, NG-nitro-l-arginine methyl ester; l-NMMA, NG-monomethyl-l-arginine; RM-ANOVA, repeated measures ANOVA.
References
- 1.Edvinsson L, MacKenzie E T, McCulloch J, editors. Cerebral Blood Flow and Metabolism. New York, NY: Raven; 1993. [Google Scholar]
- 2.Heistad D D, Faraci F M. Stroke. 1996;27:1688–1693. doi: 10.1161/01.str.27.9.1688. [DOI] [PubMed] [Google Scholar]
- 3.Ooboshi H, Welsh M J, Rios C D, Davidson B L, Heistad D D. Circ Res. 1995;77:7–13. doi: 10.1161/01.res.77.1.7. [DOI] [PubMed] [Google Scholar]
- 4.Muhonen M G, Ooboshi H, Welsh M J, Davidson B L, Heistad D D. Stroke. 1997;28:822–829. doi: 10.1161/01.str.28.4.822. [DOI] [PubMed] [Google Scholar]
- 5.Furchgott R F. J Am Med Assoc. 1996;276:1186–1188. [PubMed] [Google Scholar]
- 6.Moncada S, Higgs E A. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 7.Bredt D S, Snyder S H. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 8.Katusic Z S, Cosentino F. News Physiol Sci. 1994;9:64–67. [Google Scholar]
- 9.Faraci F M, Brian J E. Stroke. 1994;25:692–703. doi: 10.1161/01.str.25.3.692. [DOI] [PubMed] [Google Scholar]
- 10.Iadecola C, Pelligrino D A, Moskowitz M A, Lassen N A. J Cereb Blood Flow Metab. 1994;14:175–192. doi: 10.1038/jcbfm.1994.25. [DOI] [PubMed] [Google Scholar]
- 11.Murad F. J Am Med Assoc. 1996;276:1189–1192. [Google Scholar]
- 12.Ignarro L J. Biochem Pharmacol. 1991;41:485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- 13.Nathan C, Xie Q W. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 14.Wilson J M. N Eng J Med. 1996;334:1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]
- 15.Nabel E G. Circulation. 1995;91:541–548. doi: 10.1161/01.cir.91.2.541. [DOI] [PubMed] [Google Scholar]
- 16.Gibbons G H, Dzau V J. Science. 1996;272:689–693. doi: 10.1126/science.272.5262.689. [DOI] [PubMed] [Google Scholar]
- 17.von der Leyen H E, Gibbons G H, Morishita R, Lewis N P, Zhang L, Kaneda Y, Cooke J P, Dzau V J. Proc Natl Acad Sci USA. 1995;92:1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzeng E, Shears L, Robbins P D, Pitt B R, Geller D A, Watkins S C, Simmons R L, Billiar T R. Mol Med. 1996;2:211–225. [PMC free article] [PubMed] [Google Scholar]
- 19.Janssens S P, Bloch K D, Nong Z, Gerard R D, Zoldhelyi P, Collen D. J Clin Invest. 1996;98:317–324. doi: 10.1172/JCI118795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen A F Y, O’Brien T, Tsutsui M, Kinoshita H, Pompili V J, Crotty T B, Spector D J, Katusic Z S. Circ Res. 1997;80:327–335. doi: 10.1161/01.res.80.3.327. [DOI] [PubMed] [Google Scholar]
- 21.Katusic Z S, Milde J H, Cosentino F, Mitrovic B S. Stroke. 1993;24:392–399. doi: 10.1161/01.str.24.3.392. [DOI] [PubMed] [Google Scholar]
- 22.Fankhauser R. In: Comparative Neuropathology. Innes J R M, Saunders L Z, editors. New York: Academic; 1962. pp. 40–47. [Google Scholar]
- 23.Aliev G, Ralevic V, Burnstock G. Circ Res. 1996;79:317–323. doi: 10.1161/01.res.79.2.317. [DOI] [PubMed] [Google Scholar]
- 24.Jiang S, Eberhardt N L. J Biol Chem. 1995;270:13906–13915. doi: 10.1074/jbc.270.23.13906. [DOI] [PubMed] [Google Scholar]
- 25.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 26.Sase K, Michel T. Trends Cardiovasc Med. 1997;7:28–37. doi: 10.1016/S1050-1738(96)00121-1. [DOI] [PubMed] [Google Scholar]
- 27.Anderson R G. Proc Natl Acad Sci USA. 1993;90:10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parton R G. Curr Opin Cell Biol. 1996;8:542–548. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- 29.Feron O, Belhassen L, Kobzik L, Smith T W, Kelly R A, Michel T. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Cardena G, Fan R, Stern D F, Liu J, Sessa W C. J Biol Chem. 1996;271:27237–27240. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- 31.Shaul P W, Smart E J, Robinson L J, German Z, Yuhanna I S, Ying Y, Anderson R G W, Michel T. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Cardena G, Oh P, Liu J, Schnitzer J E, Sessa W C. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson L J, Michel T. Proc Natl Acad Sci USA. 1995;92:11776–11780. doi: 10.1073/pnas.92.25.11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hess J F, Borkowski J A, Young G S, Strader C D, Ransom R W. Biochem Biophys Res Commun. 1992;184:260–268. doi: 10.1016/0006-291x(92)91187-u. [DOI] [PubMed] [Google Scholar]
- 35.Menke J G, Borkowski J A, Bierilo K K, MacNeil T, Derrick A W, Schneck K A, Ransom R W, Strader C D, Linemeyer D L, Hess J F. J Biol Chem. 1994;269:21583–21586. [PubMed] [Google Scholar]
- 36.Byron K L, Babnigg G, Villereal M L. J Biol Chem. 1992;267:108–118. [PubMed] [Google Scholar]
- 37.Baumgraten L B, Lee H C, Villereal M L. Cell Calcium. 1995;17:41–52. doi: 10.1016/0143-4160(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 38.Sessa W C, Garcia-Cardena G, Liu J, Keh A, Pollock J S, Bradley J, Thiru S, Braverman I M, Desai K M. J Biol Chem. 1995;270:17641–17644. doi: 10.1074/jbc.270.30.17641. [DOI] [PubMed] [Google Scholar]
- 39.Bates T E, Loesch A, Burnstock G, Clark J B. Biochem Biophys Res Commun. 1996;218:40–44. doi: 10.1006/bbrc.1996.0008. [DOI] [PubMed] [Google Scholar]
- 40.Cosentino F, Sill J C, Katusic Z S. Am J Physiol. 1993;264:H413–H418. doi: 10.1152/ajpheart.1993.264.2.H413. [DOI] [PubMed] [Google Scholar]
- 41.Kim P, Sundt T M, Jr, Vanhoutte P M. J Neurosurg. 1988;69:239–246. doi: 10.3171/jns.1988.69.2.0239. [DOI] [PubMed] [Google Scholar]
- 42.Kim P, Sundt T M, Jr, Vanhoutte P M. Circ Res. 1992;70:248–256. doi: 10.1161/01.res.70.2.248. [DOI] [PubMed] [Google Scholar]
- 43.Hino A, Tokuyama Y, Weir B, Takeda J, Yano H, Bell G I, Macdonald R L. Neurosurgery. 1996;39:562–568. doi: 10.1097/00006123-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 44.Pluta R M, Thompson B G, Dawson T M, Snyder S H, Boock R J, Oldfield E H. J Neurosurg. 1996;84:648–654. doi: 10.3171/jns.1996.84.4.0648. [DOI] [PubMed] [Google Scholar]
- 45.Weaver J P. In: Stroke Therapy. Fisher M, editor. Newton, MA: Butterworth-Heinemann; 1995. pp. 399–433. [Google Scholar]