Abstract
Objective
To determine the effectiveness of ultrasound-guided aspiration and lavage in the treatment of patients with calcific tendinosis of the shoulder.
Materials and methods
Retrospective chart review resulted in 44 patients who were identified as having received ultrasound-guided aspiration of calcific tendinosis of the shoulder between 2000 and 2003. Of these, 36 patients were interviewed by telephone for pre- and posttreatment assessment of pain, shoulder function, prior shoulder surgery, injury, and prescribed treatment modalities with a follow-up time of 8 months to 3.1 years (mean = 22.5 months). L’Insalata score, numeric rating scale (NRS), and patient satisfaction score served as outcome measures.
Results
Our criteria for a successful outcome included (1) 12-point or greater improvement in the L’Insalata shoulder rating questionnaire, (2) 2-point or greater improvement in the NRS, (3) patient satisfaction rating of “good”, “very good”, or “excellent”, (4) patients’ willingness to undergo the procedure again if they experienced recurrent symptoms, and (5) 1 month or less of analgesic medication use after the aspiration procedure. We determined that ultrasound-guided aspiration of calcific tendinosis of the shoulder resulted in a successful outcome for 75% (27/36) of patients with a mean 20.2-point improvement in the L’Insalata shoulder rating questionnaire score and a mean 6.4-point improvement in the NRS (p < 0.01). Conclusion: This retrospective study suggests that ultrasound-guided aspiration and lavage of calcific shoulder deposits appears to be an efficacious therapeutic modality for treatment of calcific tendinosis. Further studies involving prospective randomized controlled trials would be helpful to further assess the long-term efficacy of this procedure as a minimally invasive treatment for calcific tendinosis of the shoulder.
Key words: ultrasound-guidance, aspiration, lavage, calcification, shoulder tendinosis
Introduction
Calcific tendinosis of the shoulder is a condition characterized by the deposition of calcium hydroxyapatite within the tendons of the rotator cuff. Originally described in 1872 as “maladie de Duplay,” calcific tendinosis involves the supraspinatus tendon in up to 82% [1] of cases, approximately 1–2 cm from its insertion onto the greater humeral tuberosity [2]. The incidence of periarticular calcifications in the distribution of the rotator cuff tendons on radiographs has been cited in 7.5% [3] to 20% [4] of asymptomatic individuals and in 6.8% [5] of symptomatic individuals. About 50% of patients with calcific tendinosis have shoulder pain, with varying degrees of restricted motion and disability with activities or at rest. Individuals between 30 and 60 years old are most commonly affected, [5] with a 57–76.7% incidence range in women as compared to all patients [6].
Conservative treatment has traditionally included oral nonsteroidal antiinflammatory medication, physical therapy, and local corticosteroid injections. Data demonstrating the benefits of conservative therapy or lack thereof are sparse [5, 6]. Although the condition is described as self-limiting and usually resolves spontaneously, some patients experience a cyclic, progressive course that can result in acutely disabling pain and loss of function. Therapeutic interventions for such cases include radiation, needle aspiration and lavage, extracorporeal shock-wave therapy, and open or arthroscopic surgical removal.
Needle aspiration and lavage usually involves direct percutaneous puncture of the calcific deposit using fluoroscopic guidance [7, 8]. Comfort and Arafiles [9] first performed percutaneous needle aspiration of calcific deposits under fluoroscopic guidance in 1978. More recently, ultrasound guidance has become a commonly employed method to perform diagnostic or therapeutic interventions [10]. In 1995, Farin et al. [11] improved the percutaneous needle aspiration and lavage technique by using ultrasound guidance to accurately and reliably localize calcifications.
Studies utilizing the ultrasound-guided aspiration and lavage technique have reported clinical success rates of 60–74%, with follow-up times ranging from 2 weeks to 1 year [12, 13]. Concern for potential injury to rotator cuff tendons after multiple intratendinous punctures using large-bore needles encouraged further improvement to the technique. In 2001, Aina et al. [14] refined their percutaneous approach by utilizing a single (3.81-cm, 22- or 25-gauge) fine needle. This modified approach resulted in statistically significant improvement in the shoulder pain and disability index score (27%), the pain score (30.5%) and the disability score (23.9%) at a mean follow-up time of 53 days.
Despite the apparent utility of this new technique, relatively few data have been published to support or refute its efficacy. We thus undertook this study to evaluate the effectiveness of ultrasound-guided aspiration and lavage of symptomatic rotator cuff calcific tendinosis at our institution. In addition, we address for the first time the potential impact of physical therapy, prior medication, corticosteroid injection, and history of prior shoulder injury and/or surgery on the efficacy and clinical outcomes of ultrasound-guided aspiration.
Materials and methods
In a retrospective case series from August 2000 to January 2003, 44 shoulders of 44 patients were consecutively identified as having received ultrasound-guided aspiration and lavage of calcific tendinosis of the shoulder by the two musculoskeletal radiologists at our institution trained in musculoskeletal sonography (RSA and CMS). Each patient was seen either by an attending physiatrist or orthopedic surgeon in the outpatient practices of a major teaching hospital. Research approval was obtained from our host institution’s institutional review board. All plain radiographs and sonograms were reviewed before patient interview to verify and document the location and size of calcific deposit within the rotator cuff. The mean area of the calcifications was estimated as a simple product of two orthogonal dimensions. The area of calcification was calculated by multiplying the length times the width of the calcification as provided on the ultrasound report. This method of calculation results in a slight overestimation of the cross-sectional area; however, this method was used consistently throughout the study. Patients with confirmed calcific deposits were invited to participate in the study. With their permission, an independent examiner (a fourth year medical student) conducted a 10- to 15-min telephone interview and individually reviewed each patient’s chart.
Inclusion criteria were patients with documented evidence of calcific tendinosis in the shoulder, visualized on sonography as a nodular or curvilinear echogenic focus within a tendon, with or without posterior acoustic shadowing (Fig. 1). Exclusion criteria included evidence of a rotator cuff tear on sonography. All ultrasound examinations were performed using a medium frequency (7.5 MHz) linear transducer on a Siemens Sonoline Elegra ultrasound unit (Siemens/Acuson, Mountain View, CA).
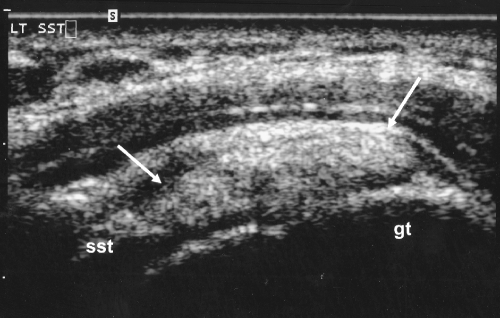
Fig. 1.
Ultrasound of the right shoulder demonstrates a calcification (identified by the arrows) located within the deep portion of the supraspinatus tendon (SST). The greater tuberosity (gt) is labeled. Calcification typically appears bright (echogenic) on ultrasound. In this image, the tendon is viewed in the long axis
All procedures were performed using standard sterile technique with the patient positioned either recumbent or decubitus on the examination table. Aspiration of the calcifications were performed under direct ultrasound guidance using either a single or dual-needle technique, as described by Farin et al. [11, 13]. Mechanical fragmentation and lavage was performed in all patients utilizing a free-hand technique with a 20- or 18-gauge spinal needle (Fig. 2), with injections performed into the calcium deposit initially with 1% lidocaine and subsequently with a sterile saline-filled syringe.
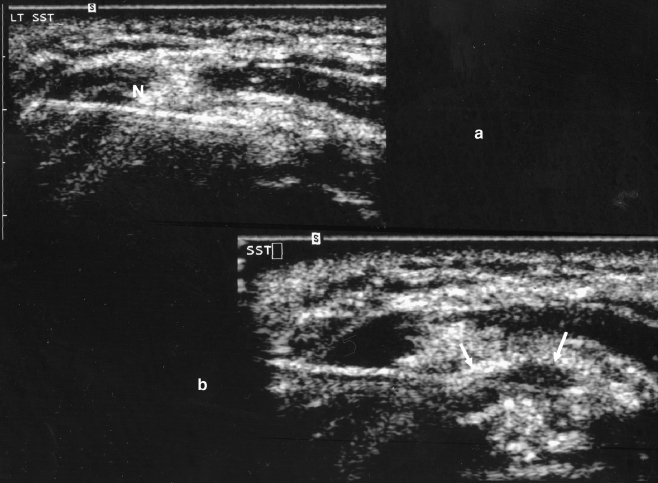
Fig. 2.
a. Ultrasound of the same patient demonstrates needle placement directly into the calcification (N). The tendon is viewed in short axis (rotated 90° relative to its position in Fig. 1). The needle appears linear and echogenic (bright) on ultrasound. b. Ultrasound of the same patient demonstrates the needle, with the tip of the needle demonstrating a smaller area of calcification after mechanical fragmentation and lavage. The boundary of the calcification is depicted by arrows. Fluid replaces some of the echogenic materials after multiple lavages
Whether or not calcification was returned during this process, an anesthetic/corticosteroid mixture was injected into the pericalcific region before the removal of the needle. The therapeutic mixture consisted of a 2-ml volume of 0.5 ml 1% lidocaine (Abbott Laboratories, North Chicago, IL), 0.5 ml 0.5% bupivacaine (Sensorcaine; Astra Pharmaceuticals, Westborough, MA), and 1 ml (40 mg) tramcinolone (Kenalog, Apothecon, a Bristol Myers Squibb Company, Princeton, NJ) or a similar long-acting agent injected into the pericalcific region after the mechanical fragmentation and aspiration. In the majority of cases, a single needle approach was utilized as long as the pseudocapsule surrounding the calcification remained intact. Ultrasound images after the lavage and aspiration documented reductions in the size of the calcification (Fig. 3).
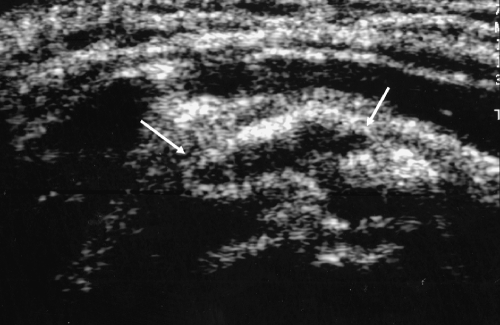
Fig. 3.
Transverse ultrasound image of the supraspinatus tendon in the same patient after the completion of the procedure and after injection of anesthetic and corticosteroid mixture injected into the pseudocapsule of the calcification and pericalcific region. There is marked reduction in size of the calcification (arrow) originally present with a small amount of fluid (anesthetic/steroid mixture) within the space previously occupied by the calcific deposit
A retrospective evaluation of shoulder pain was obtained at an 8-month minimum follow-up time (range, 8 months–3.1 years; mean, 22.5 months) with the L’Insalata shoulder rating questionnaire [15]. This index, developed at our institution, is a well-validated instrument designed to evaluate symptoms and function of the shoulder and consists of 21 items divided into six domains corresponding to global assessment, pain, daily activities, recreational and athletic activities, and satisfaction. Most items were rated along a numeric scale of 1 (poorest) to 5 (best) except for the global assessment domain, which was scored from 0 (very poorly) to 10 (very well). Each domain was scored separately by averaging the scores of individual questions, multiplying by two, and weighting each domain according to relative importance, as determined by L’Insalata et al. Total possible scores ranged from 17 to 100 points. The validity, reliability, and responsiveness to change (psychometric properties) of the L’Insalata questionnaire have been assessed in patients with a variety of shoulder pathologies [15].
Additional outcome measures included a numeric pain scale (NRS) and a patient satisfaction score. All patients were also asked questions regarding history of prior shoulder injury and surgery, duration of symptoms before ultrasound-guided aspiration, history of joint injections and/or prior aspirations, use of physical therapy, NSAIDs or opioids before and after the ultrasound-guided aspiration, pain relief as a result of the aspiration, and willingness to undergo the aspiration again for recurrent symptoms. Willingness to undergo the aspiration again for recurrent symptoms was asked after the initial question “Did you get pain relief from the ultrasound-guided aspiration?”. If the response was yes, patients were then asked, “Would you be willing to have another ultrasound-guided aspiration for recurrent symptoms?”. We defined a successful outcome in this study as (1) 12-point or greater improvement in the L’Insalata shoulder rating questionnaire, (2) 2-point or greater improvement in the numeric rating scale (NRS), (3) patient shoulder satisfaction rating of “good,” “very good”, or “excellent”, (4) patients’ willingness to undergo the procedure again if they experienced recurrent symptoms, and (5) 1 month or less of NSAID or opioid use after the aspiration.
Statistical analysis was performed using nonparametric and two-tailed paired t tests on SPSS Windows version 9.0 software. Significance was assumed for p < 0.05 with 95% confidence intervals. L’Insalata pre- and postinjection scores, medications pre-and postinjection, physical therapy pre- and postinjection, and visual analog scale pre- and postinjection were analyzed with two-tailed paired sample t test. Groups of successes and failures were also compared for significance of shoulder surgery history, shoulder injury history, duration of symptoms preaspiration, and mean area of calcification. Patients who underwent surgery postaspiration were considered as a separate group.
Results
Forty-four patients, 31 women and 13 men, with a mean age of 50.8 years (range, 35–70 years), were identified as having received ultrasound-guided aspiration of calcific tendinosis, meeting our inclusion and exclusion criteria. Complete data at a minimum 8-month follow-up time were obtained for 36 out of 44 patients (81.8%). Eight patients were excluded from the study as they either could not be reached by telephone or post or did not wish to be interviewed. Twenty-three (63.9%) women and 13 (36.1%) men with a mean age of 52.9 years (range, 37–70 years) were interviewed at a mean follow-up time of 22.5 months after the procedure (range, 8 months–3.1 years). The aspiration was performed on the same side as the dominant hand in 21 (58.3%) of our patients.
Ultrasound-guided aspiration was performed in patients referred by clinicians at our institution consisting of physiatrists, rheumatologists, and orthopedic surgeons. The aspiration was utilized both as a primary form of therapy as well as a secondary form of treatment in patients who had undergone additional treatments such as conservative or surgical therapies. Before the ultrasound-guided aspiration, 4 (11.1%) patients reported history of shoulder injury, 20 (55.6%) patients reported history of corticosteroid injection in the shoulder, and 4 (11.1%) patients reported history of shoulder surgery. The mean area of the calcifications, derived from the ultrasound examination, before the procedure in all patients was 148.4 mm2 (range, 13.5–900 mm2). The mean duration of symptoms before ultrasound-guided aspiration was 22.2 months (range, 4 days–2 years). The mean L’Insalata score before aspiration was 51.8 (range, 18.5–79.0). The mean NRS score before aspiration was 8.4 (range, 5–10). Twenty-three (63.9%) patients reported NSAID or opioid use before the procedure for an average period of 2.5 months (range, 6 days–3 years). Fourteen of 36 patients (38.9%) received physical therapy before the procedure for a mean period of 3 months (range, 1 month–2 years).
After the aspiration procedure, the mean L’Insalata score was 72.0 (range, 24.5–100). The mean NRS score after aspiration was 2.1 (range, 0–9.5), the mean improvement in L’Insalata score was 20.2 (range, 0–70.5), and the mean improvement in NRS score was 6.4 (range, 0–10). Thirty (83.3%) of all patients reported pain relief from the ultrasound-guided aspiration. Of those who did experience pain relief, 25 (83.3%) patients reported that they would be willing to undergo another ultrasound-guided aspiration if they experienced recurrent symptoms. Twenty-eight (77.8%) patients rated satisfaction with their shoulder as “good”, “very good”, or “excellent” at a minimum 8-month follow-up after the procedure (range, 8 months–3.1 years). Eight (22.2%) patients rated their shoulder satisfaction as “fair” or “poor” after the aspiration. Seventeen (47.3%) patients reported NSAID or opioid use after aspiration for a mean period of 24 days (range, 2 days–6 months). Twenty-nine (80.6%) patients reported 1 month or less of NSAID or opioid use after aspiration. Twenty-two (61.1%) patients received physical therapy after aspiration for a mean period of 3 months (range, 2 weeks–2 years). There were no adverse complications, such as infection or weakness, reported by the patients.
Using our criteria for a successful outcome described above, at a minimum 8-month follow-up time for 36 patients, we determined that ultrasound-guided aspiration of calcific tendinosis resulted in a statistically significant successful outcome for 75% (27/36) of patients, with a mean 20.2-point improvement in the L’Insalata shoulder rating questionnaire score and a mean 6.4-point improvement in the NRS (p < 0.01).
We also compared groups of successes and failures for significance of (1) history of shoulder surgery, (2) history of shoulder injury, (3) physical therapy use pre- and postaspiration, (4) medication use pre- and postaspiration, (5) duration of symptoms preaspiration, and (6) mean area of calcification before the procedure. Statistical analysis failed to reveal a significant relationship between the above variables and successful outcome. However, a trend was detected in symptom duration before the procedure and outcome. Seven (87.5%) of the eight patients who had acute symptoms (lasting 3 months or less) before the aspiration and lavage procedure had successful outcomes as opposed to 20 (71%) of 28 patients with chronic symptoms (p = 0.38).
Four patients underwent surgical removal of the calcific shoulder deposits after the aspiration procedure due to persistent pain. Of these four patients, two stated that they received pain relief from the aspiration procedure for approximately 3–4 months immediately after the procedure. Of these two patients, one patient stated that he would be willing to undergo the procedure again, and one stated that he would be unwilling. The two remaining patients stated that they would be willing to undergo the aspiration procedure again if they experienced recurrent symptoms.
Three of the four patients who had shoulder surgery before the aspiration and lavage procedure and three of the patients with prior shoulder injury had a successful outcome after the ultrasound-guided procedure. Of the shoulder surgeries, one patient underwent prior shoulder calcification removal, one patient underwent rotator cuff repair, one patient underwent arthroscopic surgery after a softball and weightlifting injury, and one patient underwent an unknown type of surgery. Seventeen (85%) of the 20 patients who had history of corticosteroid injection in the shoulder before the aspiration and lavage procedure had a successful outcome.
Discussion
While the etiology and natural history of symptomatic calcific tendinosis of the shoulder remain unclear, conservative treatment measures result in over 90% improvement [16]. Conservative treatment has traditionally included analgesic medications, physical therapy, and corticosteroid injections although the self-limiting nature of the disease limits the identification of patients who would have spontaneously improved with or without conservative treatment. However, for those patients who do not respond to conservative treatment measures, until recently, surgery remained the only other option. Recalcitrant patients have undergone open or arthroscopic surgical removal, with success rates ranging from 70 to 92% [17–19]. In 1995, ultrasound-guided aspiration and lavage was introduced as a minimally invasive procedure to offer patients an attractive alternative to surgery and its associated morbidity and costs [11].
Data evaluating the efficacy of ultrasound-guided aspiration and lavage are sparse but promising, with success rates ranging from 60 to 74% with a maximum follow-up time of 1 year [12, 13]. The aim of this study was to evaluate the long-term clinical efficacy of ultrasound-guided aspiration and lavage in the treatment of patients with calcific tendinosis using a set of well-validated functional outcome measures. We also specifically addressed the impact of prior shoulder injury, surgery or corticosteroid injection in the shoulder, physical therapy, and NSAID or opioid use on patients’ response to treatment. We found that 75% of our patients had a successful treatment response as identified by a significant improvement in pain and function, satisfaction with treatment, and 1 month or less of analgesic medication use after the aspiration. The average follow-up time for our patients was approximately 2 years. The mean area of the calcific deposits aspirated in this study (148 mm2) was comparable to the mean area (150–162 mm2) of aspirated symptomatic calcific deposits reported in previously published literature [14, 20]. Our results are consistent with the previous studies that had shorter follow-up periods, indicating that the effects of ultrasound-guided aspiration and lavage are both reproducible and lasting.
Patients who had history of shoulder surgery, corticosteroid injection, or shoulder injury had no statistically significant difference in their response to treatment than did other patients. These factors therefore do not appear to be contraindications to treatment. Patients with symptoms lasting for 3 months or less did not have a statistically significant greater response to treatment; however, they did tend to respond better than patients with chronic symptoms before aspiration. The failure of this difference to reach statistical significance may have been related to the small number of patients with acute symptoms before the procedure. The observed trend, however, may have been due to two factors. First, the reported natural history of symptomatic calcific tendinosis of the shoulder is extremely favorable. It is thus possible that most patients with acute symptoms would have spontaneously improved with or without ultrasound-guided aspiration and lavage. Second, patients with acute lesions may have greater existing vascularity surrounding the calcification.
This theory stems from the mechanism underlying the aspiration and lavage procedure. Specifically, aspiration and lavage removes a substantial portion of the calcific deposit and increases vascularity to the area of calcification by traumatizing the shoulder. The remaining calcification is then resorbed via the increased vasculature over the following weeks. It is thus reasonable to speculate that the more acute the calcific lesion, the greater the existing vascularity surrounding that lesion. Once aspirated and lavaged, the acute lesion would more easily renew its vascularity and enhance the resorptive process. Also, more acute lesions are often more liquefied, making them more amenable to aspiration.
This study has presented evidence supporting the efficacy of ultrasound-guided aspiration and lavage in the treatment of symptomatic calcific tendinosis of the shoulder after a minimum follow-up of 8 months. However, there are limitations to our study. While evaluating efficacy at a minimum follow-up of 8 months, we did not evaluate short-term efficacy. Patients may have responded well to treatment but acutely. This is reflected in the fact that of the four patients who required surgery after a failed aspiration, two reported pain relief subsequent to the aspiration and willingness to undergo the aspiration again for recurrent symptoms. It is likely, therefore, that additional patients experienced at least transient relief from ultrasound-guided aspiration and lavage.
Limitations of our study also included small sample size, lack of comparison to other methods, retrospective nature of this study, and heterogeneous patient population. Most importantly, there was a lack of blinding and lack of a control group with which to compare results. The lack of a control group makes it difficult to exclude a placebo effect of the procedure. Although blinding or a control group is not typical of a retrospective analysis, contacting patients retrospectively and asking them to recall symptoms from several months prior may have potentially biased and favorably influenced our results. Our subjects were heterogeneous in that they received different therapies before the aspiration and lavage. Therefore, the efficacies of certain treatments received before our intervention cannot be determined. Furthermore, it is unknown whether our results can be generalized to all outpatient radiology centers with radiologists less experienced with ultrasound-guided procedures.
Significant questions remain regarding the natural history of symptomatic calcific tendinosis and its treatment with ultrasound-guided aspiration and lavage. For example, it is unknown whether patients with more severe acute symptoms have a worse natural history and, if they have, whether aspiration and lavage should be considered earlier in this patient population. Subsequent corticosteroid injection after the lavage may have confounded the results or may play no significant role, and the role of lavage both with and without corticosteroid injection requires further investigation. It also remains unclear whether ultrasound-guided aspiration and lavage is significantly operator dependent. All procedures described in this study were performed by musculoskeletal radiologists with considerable experience performing ultrasound-guided procedures. The role of rehabilitation in ultrasound-guided aspiration and lavage also needs to be clarified. Specifically, it is unknown whether there is greater efficacy if patients are placed in a rehabilitation program immediately after the procedure, possibly secondary to increased vascularity to the lesion and thus improvement in the resorptive process. In addition, it remains to be elucidated what is appropriate rehabilitation for the weakened shoulder tendon after treatment with aspiration, lavage, traumatic injection, and a long-acting corticosteroid.
Prior outcome studies have appropriately addressed the apparent short-term efficacy of ultrasound-guided aspiration and lavage in the treatment of symptomatic calcific shoulder tendinosis. Our present study has confirmed previously reported success rates approximating 75% and has provided important long-term clinical efficacy outcomes. Although difficult to perform, double-blind, placebo-controlled clinical trials could further evaluate this procedure in the treatment algorithm for symptomatic calcific tendinosis and improve the treatment options available to our patients. Furthermore, prospective studies comparing subjects who receive lavage, but without the subsequent corticosteroid, can help to further elucidate the role of corticosteroid injections.
Conclusion
Ultrasound-guided aspiration and lavage of calcific shoulder deposits appears to provide an efficacious therapeutic modality for treatment of symptomatic calcific tendinosis and shows promise in providing an alternative treatment option for patients with this diagnosis
Footnotes
This study was performed by the Departments of Physiatry and of Radiology and Imaging, Hospital for Special Surgery, New York, NY.
References
- 1.Plenck H (1952) Calcifying tendinosis of the shoulder. Radiology 59:384–389 [DOI] [PubMed]
- 2.Uhthoff HK, Loehr JW (1997) Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis and management. J Am Acad Orthop Surg 5(4):183–191 [DOI] [PubMed]
- 3.Welfling J, Kahn MF, Desroy M, Paologgi JB, De Seze S (1965) Calcification of the shoulder. The disease of multiple tendinous calcifications. Rev Rheumatology 32:325–334 [PubMed]
- 4.Ruttimann G, Uber (1959) Die Haufigkeit rontenologischer Veranderungen bei Patienten mit typischer Periarthritis humeroscapularis und Schultergesunden. Inaugural dissertation. University of Zurich, Zurich, Switzerland
- 5.Speed CA, Hazelman BL (1999) Calcific tendinosis of the shoulder. N Engl J Med 340(20):1582–1584 [DOI] [PubMed]
- 6.Rokito AS, Loebenberg MI (1999) Frozen shoulder and calcific tendinosis. Curr Opin Orthop 10(4):294–304 [DOI]
- 7.Chevriere A, Carlier R, Feydy A, Mompoint D, Bayou E, Vallee C (2000) Imaging-guided needle puncture, infiltration and lavage of rotator cuff calcifications. Retrospective evaluation of 50 cases of treated periarthritis. [French] J Radiol 81(9):971–974 [PubMed]
- 8.Pfister J, Gerber H (1997) Chronic calcifying tendinosis of the shoulder—therapy by percutaneous needle aspiration and lavage: a prospective open study of 62 shoulders. Clin Rheumatol 16(3):269–274 [DOI] [PubMed]
- 9.Comfort TH, Arafiles R (1978) Barbotage of the shoulder with image-intensified fluoroscopic control of needle placement for calcific tendinosis. Clin Orthop 135:171–178 [PubMed]
- 10.Adler RS, Sofka CM (2003) Percutaneous ultrasound-guided injections in the musculoskeletal system. Ultrasound Q 19(1):3–12 [DOI] [PubMed]
- 11.Farin PU, Jaroma H, Soimakallio S (1995) Rotator cuff calcifications: treatment with ultrasound-guided technique. Radiology 195:841–843 [DOI] [PubMed]
- 12.Giacomoni P, Siliotto R (1999) Echo-guided percutaneous treatment of chronic calcific tendinosis of the shoulder. [Italian] Radiol Med (Torino) 98:386–390 [PubMed]
- 13.Farin PU, Rasanen H, Jaroma H, Harju A (1996) Rotator cuff calcifications: treatment with ultrasound-guided percutaneous needle aspiration and lavage. Skelet Radiol 25:551–554 [DOI] [PubMed]
- 14.Aina R, Cardinal E, Bureau NJ, Aubin B, Brassard P (2001) Calcific shoulder tendinosis: treatment with modified US-guided fine-needle technique. Radiology 221:455–461 [DOI] [PubMed]
- 15.L’Insalata JC, Warren RF, Cohen SB, Altchek DW, Peterson MGE (1997) A self-administered questionnaire for assessment of symptoms and function of the shoulder. J Bone Jt Surg 79-A(5):738–748 [PubMed]
- 16.Gschwend N, Patte D, Zippel J (1972) Therapy of calcific tendinosis of the shoulder. [German] (Die Therapie der Tendinosis calcarea des Schultergelenks). Arch Orthop Trauma Surg 73(2):120–135 [DOI] [PubMed]
- 17.Gazielly D, Bruyere G, Gleyze P, Thomas T (1997) Open acromioplasty with excision of calcium deposits and tendon suture. In: Gazielly D, Gleyze P, Thomas T (eds) The cuff. Elsevier, Paris, pp 172–175
- 18.Mole D, Kempf J, Gleyze P, Rio B, Bonnomet F, Walch F (1993) Results of arthroscopic treatment of tendinosis of the rotator cuff of the shoulder. Second part: calcified lesions of the rotator cuff. Rev Chir Orthop Repar Apar Mot 79:532–541 [PubMed]
- 19.Ark JW, Flock TJ, Flatow EL, Bigliani LU (1992) Arthroscopic treatment of calcific tendinosis of the shoulder. Arthroscopy 8:183–188 [DOI] [PubMed]
- 20.Bosworth BM (1941) Calcium deposits in shoulder and subacromial bursitis: Survey of 122 shoulders. JAMA 116:2477–2482