Abstract
The clinical goal of spinal fusion is to reduce motion and the associated pain. Therefore, measuring motion under loading is critical. The purpose of this study was to validate four-point bending as a means to mechanically evaluate simulated fusions in dog and rabbit spines. We hypothesized that this method would be more sensitive than manual palpation and would be able to distinguish unilateral vs bilateral fusion. Spines from four mixed breed dogs and four New Zealand white rabbits were used to simulate posterolateral fusion with polymethyl methacrylate as the fusion mass. We performed manual palpation and nondestructive mechanical testing in four-point bending in four planes of motion: flexion, extension, and right and left bending. This testing protocol was used for each specimen in three fusion modes: intact, unilateral, and bilateral fusion. Under manual palpation, all intact spines were rated as not fused, and all unilateral and bilateral simulated fusions were rated as fused. In four-point bending, dog spines were significantly stiffer after unilateral fusion compared with intact in all directions. Additionally, rabbit spines were stiffer in flexion and left bending after unilateral fusion. All specimens exhibited significant differences between intact and bilateral fusion except the rabbit in extension. For unilateral vs bilateral fusion, significant differences were present for right bending in the dog model and for flexion in the rabbit. Unilateral fusion can provide enough stability to constitute a fused grade by manual palpation but may not provide structural stiffness comparable to bilateral fusion.
Key words: lumbar spinal fusion, biomechanics, animal model, spine
Introduction
Lower back pain affects 65 million people in the USA. Surgical treatment results in 150,000–300,000 lumbar spinal fusions annually [1], of which a substantial number are treated by posterolateral intertransverse process fusion with iliac crest autograft. The use of this surgery, originally for severe scoliosis, spinal tuberculosis, and fractures, has expanded to a range of disorders, including spondylosis, disk derangement, and spinal stenosis [2]. The clinical goal of posterolateral fusion is to eliminate motion and to ultimately reduce pain of the involved spinal segment.
While successful fusion can reduce pain for the patient, a nonunion (pseudoarthrosis) may result in serious morbidity over time [3]. Despite the extensive experience with this surgery, the frequency of pseudoarthrosis has been found to be as high as 10–40% [4–6]. Pseudoarthrosis can be asymptomatic but more frequently results in a significant decrease in quality of life and morbidity leading to complicated revision surgeries [7, 8]. The link between solid fusion and pain relief, however, is not well characterized. This lack of understanding of the fundamental goals of this surgery should result in a more critical view of its use. In turn, research efforts should be directed toward a closer examination of these interactions.
Fusion is a multifactorial process with many contributing variables. As these variables are coupled clinically, animal models are a tool to focus on specific contributing factors. In addition, animal models can be assessed using destructive methods not available clinically, such as mechanical testing and histology. Rat, rabbit, dog, and sheep spines have been used to represent human spinal fusion [9–15]. While each has its advantages, many animal models have a 100% fusion rate unless specific steps are taken to reduce the fusion mass [15] to simulate clinical fusion rates of 60–90% [4–6]. The New Zealand white rabbit posterolateral fusion model produces clinically relevant fusion rates of 66% [14]. Different graft materials, including autograft, allograft, and synthetic biomaterials, have been evaluated in this model, as well as growth factor and gene therapy treatment intended to enhance healing [16–19].
Clinical assessments of spinal fusion include noninvasive imaging [3]. In animal models, imaging techniques overestimate the rate of fusion compared to manual palpation [20]. Manual palpation and mechanical testing can directly measure the motion and stiffness of the spine to determine fusion success. However, manual palpation only provides a qualitative measure, whereas mechanical testing allows quantification of fusion stiffness and can better reproduce the loading experienced in vivo. When considering different testing approaches, an ideal mechanical testing method should be simple to implement, sensitive to the measure of interest, and reproducible. An additional consideration in spine testing is the ability to distinguish between unilateral and bilateral fusion.
The purpose of our study was to validate four-point bending as a means to mechanically assess intact and unilateral and bilateral simulated fusions in dog and rabbit spines. Manual palpation and multidirectional mechanical testing were directly compared. We hypothesized that this method would be more sensitive than manual palpation and would be able to distinguish unilateral vs bilateral fusion.
Materials and methods
Spines from four mixed breed dogs and four New Zealand white rabbits were used to simulate posterolateral spine fusion. The dog model was chosen based on the availability of specimens and previous studies evaluating the mechanics of spinal fusion using four-point bending [21]. The rabbit model was chosen based on extensive experience and development of this animal as a model for posterolateral fusion [14]. Polymethyl methacrylate (PMMA) was used to represent the fusion mass. PMMA has been used previously in cadaveric spines to simulate fusion [22]. Three conditions, intact, unilateral fusion, and bilateral fusion, were modeled sequentially within each specimen, allowing for within-subject comparisons. Each specimen went through a repeated testing protocol for each condition: manual palpation, fusion, and mechanical testing.
Specimens were prepared for mechanical testing of the L5–L6 motion segment, which is comparable to the L4–L5 segment in humans because of the extra lumbar vertebrae in the rabbit. The intact spines were dissected of all surrounding muscle and soft tissue, leaving only the joint capsule and ligaments intact. The specimens were wrapped in saline-soaked gauze and stored in double plastic bags at −20°C until testing. Before testing, the L4–L7 segment of each specimen was potted with PMMA in aluminum tubing, leaving only the adjoining halves of L5 and L6 exposed. The transverse processes of the rabbit extend across the adjacent vertebrae and would interfere with motion across the L5–L6 segment. Therefore, the transverse processes were reduced to expose L5–L6 and not constrain motion of the isolated segment.
Prior to mechanical testing, the spines were randomized and manually palpated by a veterinary surgeon familiar with this technique [14]. Each spine was graded as either fused or not fused. The evaluator was presented with mixed batches that included intact and simulated fusions to reduce potential observer bias and was blind to fusion status.
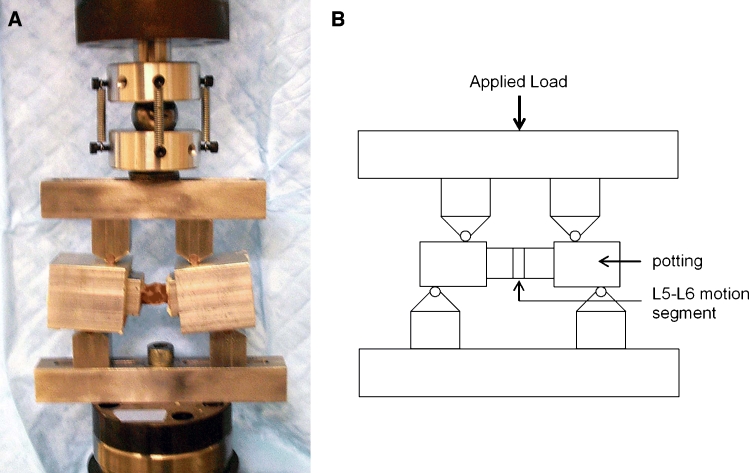
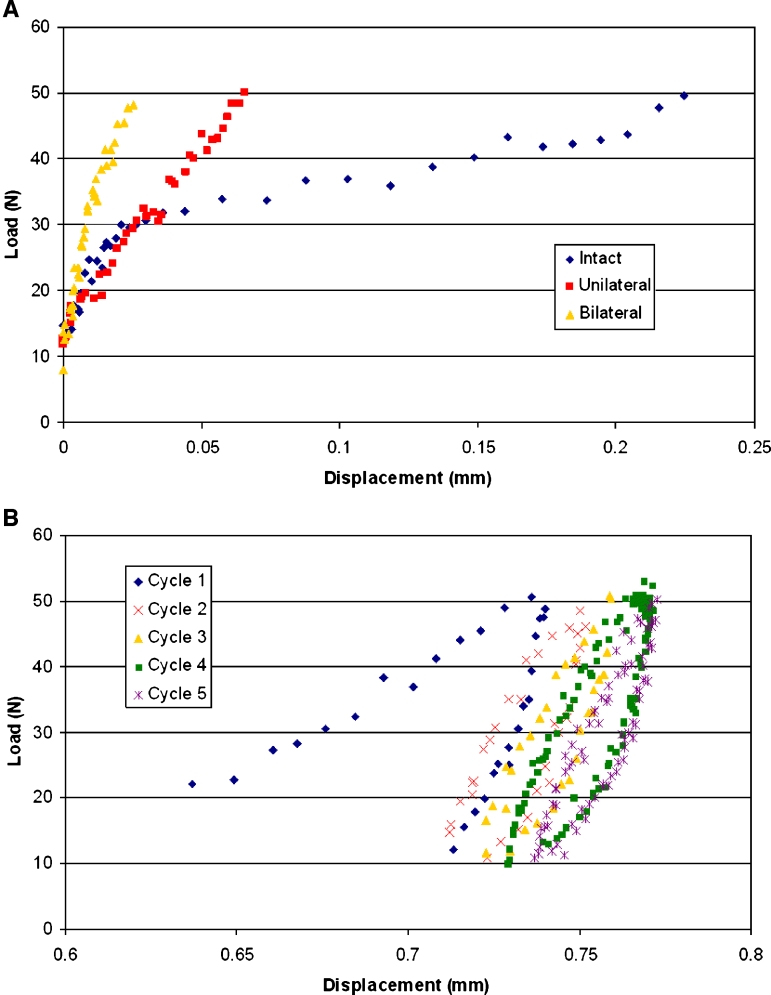
The intact spines were then tested nondestructively in a biaxial servohydraulic test machine (MiniBionix 858, MTS Systems Corporation, Eden Prairie, MN) in four-point bending in all planes of motion: flexion, extension, and right and left bending (Fig. 1). Custom fixtures were designed with an exterior support span of 95.2 mm with the interior span offset by 19.0 mm on each side. The order of the testing modes was randomized for each specimen and direction. The spines were loaded with five load–unload cycles to 50 N at 5-N/s rate corresponding to a moment of 1.43 N m. These values were based on preliminary testing to ensure a nondestructive load level. The first four cycles were used to precondition the sample, and data were analyzed from the fifth and final cycle. Pilot tests showed that three cycles were sufficient for preconditioning the data, and further cycles were nearly identical (Fig. 2). Load-displacement data from the fifth loading cycle and the test geometry were used to calculate bending stiffness (EI, N m2) [23].
Figure 1.
(a) Photograph and (b) schematic of four-point bending fixtures with potted L5–L6 specimen from a rabbit spine.
Figure 2.
Representative load-displacement data points from (a) four-point bending of a single rabbit specimen tested intact and unilaterally and bilaterally fused in flexion and from (b) five load–unload cycles of four-point bending of a single canine specimen bilaterally fused and tested in extension.
After intact testing, the spines were fused with 2 cm3 of PMMA [14]. Unilateral fusions were randomly assigned to left or right sides. PMMA (COE Tray Plastic Fast Set, GC America Inc., Chicago, IL) was applied to the posterolateral side of L5 and L6 to simulate a fusion mass. The PMMA was allowed to harden for 30 min before testing by manual palpation. After the PMMA hardened, the same testing method was repeated: spines were manually palpated followed by nondestructive testing. The fusion process was repeated on the opposite side to form a bilateral fusion, and the procedure was repeated once again. The identical protocol was used for both dogs and rabbits.
A repeated-measures analysis of variance was performed to determine if loading direction, fusion, and species had a significant effect on stiffness (Systat 8.0, SPSS Inc.). Contrast tests were performed to test for differences between degrees of fusion. The type I error rate α was set at 0.05.
Results
Under manual palpation, all intact spines, dog and rabbit, were graded as not fused, whereas all unilateral and bilateral simulated fusions were graded as fused. Thus, manual palpation was successful in determining fusion vs intact but was not sensitive to differences between unilateral and bilateral fusion.
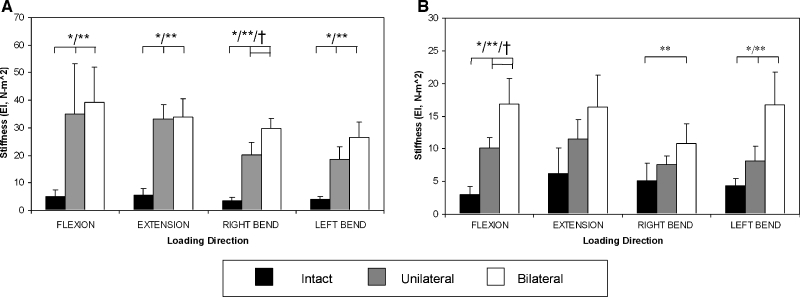
Dog spines were significantly stiffer in four-point bending after unilateral fusion compared to intact in all directions (p<0.05; Fig. 3A). Additionally, rabbit spines were stiffer in flexion and left bending after unilateral fusion (p<0.05; Fig. 3B). All specimens exhibited significant differences between intact and bilateral fusion except the rabbit specimens in extension. For unilateral vs bilateral fusion, significant differences were present for right bending in the dog model (p = 0.02) and for flexion in the rabbit (p = 0.01).
Figure 3.
Bending stiffness (mean ± standard deviation) in four-point bending of intact and unilateral and bilateral fusion of (a) dog and (b) rabbit spines. Scales are different between (a) and (b). Significance represented by * = intact vs. unilateral, ** = intact vs. bilateral, and † = unilateral vs. bilateral.
These significant increases in stiffness were large in the simulated bilateral fusion group compared to the intact spines (Table 1). In the rabbit, the bilateral fusion group exhibited a 5.5-fold increase in flexion, 2-fold in right bending, and 4-fold in left bending over intact values. In the dog spines, these differences were magnified: bilateral fusion was about 8-fold stiffer in flexion, 6-fold in extension, 8.5-fold in right bending, and 6.5-fold in left bending when compared to intact.
Table 1.
Mean percent difference in bending stiffness between intact and unilateral, intact and bilateral, and unilateral and bilateral in all directions for both rabbit and dog specimens
| Flexion | Extension | Right bend | Left bend | |||||
|---|---|---|---|---|---|---|---|---|
| Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | |
| Rabbit | ||||||||
| Intact | 268 ± 119a | 512 ± 219a | 159 ± 163 | 309 ± 404 | 124 ± 217 | 163 ± 148a | 89 ± 42a | 299 ± 140a |
| Unilateral | 65 ± 19a | 53 ± 64 | 46 ± 43 | 125 ± 101 | ||||
| Dog | ||||||||
| Intact | 539 ± 153a | 704 ± 273a | 457 ± 213a | 513 ± 160a | 463 ± 366a | 734 ± 295a | 332 ± 230a | 530 ± 120a |
| Unilateral | 28 ± 43 | 16 ± 30 | 67 ± 42a | 69 ± 63 | ||||
Values are means ± standard deviation as percentages.
aSignificant differences in bending stiffness between degrees of fusion.
When comparing the two species, the intact stiffness values were similar despite the differences in animal size. The effect of fusion was significantly different between the dog and the rabbit. The canine model exaggerated the increase in stiffness between intact and unilateral fusion.
Testing was confirmed to be nondestructive in all cases except during the final loading cycle of one unilateral fused dog specimen. This damage was confirmed by visual inspection of a small fracture in the PMMA and evaluation of load-displacement data. Testing was continued for the two remaining directions (flexion and right bending) and bilaterally fused. To prevent exclusion of this whole specimen from the repeated-measures design, values were imputed for the two directions with compromised data [24]. The two imputed values were calculated based on a linear regression of the stiffness data from the other dog specimens for flexion vs extension and left bending vs right bending. Stiffness data were also collected for the damaged left bending direction by using the cycle prior to failure, which was identical to the previous preconditioning cycle.
Discussion
The primary goals of this study were to develop a measurement approach for assessing experimental spinal fusion, establish sensitivity of the measurement to simulated fusion, and compare with manual palpation. Mechanical testing by four-point bending detected significant differences between intact and bilateral simulated fusion in all loading directions and species except for rabbit in extension. Even in our small sample, four-point bending was able to distinguish differences between unilateral and bilateral fusion in right bending in the dog and in flexion in the rabbit. Manual palpation distinguished between fused and unfused spines; however, palpation was unable to detect the extent of this fusion (unilateral vs bilateral). Flexion bending stiffness was 100% sensitive and 100% specific for fusion in both species as assessed by manual palpation; bending stiffness values for specimens graded as fused did not overlap with unfused values.
Bending stiffness, as measured in our mechanical tests, is a continuous variable that includes all information given by manual palpation but adds quantitative information about more subtle degrees of fusion. Experimental animal studies of spine fusion may benefit by assessing stiffness with four-point bending. This study confirms that unilateral fusion can provide enough stability to constitute a fused grade by manual palpation, but unilateral fusion may not provide structural stiffness comparable to bilateral fusion. For example, bilateral simulated fusion was 1.7 times stiffer than unilateral in the rabbit spine in flexion.
This study explored the correlation between degree of fusion and stiffness in two different species (dog and rabbit). As a model for spinal fusion, the rabbit model replicates the human surgical technique and produces a clinically comparable nonunion rate of 33% [14]. The dog, however, is not an ideal posterolateral fusion model as nonunions are rare [21]. Despite anatomical differences, the range of motion of the intact rabbit lumbar spine has previously been demonstrated to be comparable to that of humans [25]. We have now demonstrated with the same nondestructive load that the bending stiffness of the intact dog spine is comparable to the rabbit, although the fusion effects were different in the two species.
Clinically, surgeons rely on radiographic evaluation, physical exam, and surgical exploration to determine fusion. However, radiographs are not able to always correctly predict fusion. A 69% agreement was found between radiographic assessment and surgical outcome [26, 27]. Manual palpation can detect fusion in animal studies, but to what degree is unknown. Posterolateral fusion techniques may not always result in a solid bilateral fusion mass. At times, a solid fusion may only be achieved on one side (unilateral); however, the spinal segment will often still be graded as fused by manual palpation and X-ray. Therefore, mechanical testing protocols are a necessity to obtain quantitative assessment of stiffness to determine fusion. Differences in right and left bending could also result following unilateral fusion, allowing fusion location to be detected with mechanical testing. A larger sample size is needed to address this question.
Several loading modes have been used to evaluate fusion including tension [28, 29], pure moment flexibility testing [25, 30], and three- [31–33] and four-point bending [21, 34]. One consideration in choosing a loading mode is the ability to represent in vivo loading conditions experienced by the spine. Tensile testing has been used to examine fusion mass failure strength and could be incorporated with our nondestructive testing protocol in the future. However, only a single direction of loading is possible. Flexibility testing has been widely applied to the rabbit lumbar spine. This test produces a constant moment across the fused segment of interest and can be applied in multiple loading directions. However, this method evaluates multiple motion segments using optoelectronic markers that can result in variability and difficulty in measuring small degrees of motion. Three-point bending applies a point load directly to the intervertebral disc on the ventral surface, potentially causing damage at the loading point and indenting the disc, producing artificially high displacements and low stiffnesses. Applying loads in multiple directions is difficult with this configuration. The applied moment decreases linearly from the point of load application, inducing shear across the loaded segment. In contrast, four-point bending generates a constant moment and can be applied to all four in vivo loading directions. The symmetric boundary conditions apply a constant maximum moment between the two interior loading points.
Our experimental design has several limitations. Theoretically, pure bending is present along the entire loaded segment in four-point bending. However, shear forces may have been present because our specimen dimensions did not comply with the ASTM standard for bending tests of manmade materials, which requires a support span-to-depth ratio of 16:1 [35]. Another limitation is the use of PMMA to simulate fusion, which resulted in a rating of 100% fusion by manual palpation. Animal studies using graft materials typically have more variable fusion ratings by manual palpation [14]. Compared to a fully fused spine, PMMA is less stiff than bone. The Young's modulus for PMMA is about 3 GPa, whereas human cortical bone is 18 GPa [36, 37]. Therefore, using PMMA should give conservative values for stiffness, possibly comparable to the fusion mass formed in vivo. The reduction of the transverse processes performed here is not typically performed in vivo in the rabbit fusion surgical model; however, this procedure more closely models the human surgical technique, whereby the processes are often removed and used for graft material. Despite these simplifications, this cadaver model removes many unknowns, allowing for a more direct understanding of the mechanics involved in spinal fusion.
The ultimate clinical goal of posterolateral fusion is to increase stiffness and decrease motion to reduce pain. While the correlation between decreased pain and stiffness is debated, the need for an assessment of fusion is imperative. We validated four-point bending as a quantitative approach to evaluate fusion. The bending stiffness depended on the extent of fusion, a question generally not addressed in mechanical testing studies. Unilateral and bilateral fusions both had firm manipulation so that the examiner could not detect the difference, but the four-point bending test was able to distinguish the two conditions. This finding has important implications for studies employing side-by-side comparisons of two fusion materials [38]. In such bilateral fusion studies, the enhancement of a single side by one material may impact conclusions for the material used on the other side when based on manipulation alone. The added sensitivity of four-point bending in preclinical spinal testing is important when comparing treatment groups during healing to better understand the load-bearing function of spinal fusion.
Acknowledgments
We thank Kai Zhang, MD, for help with specimen collection and Alex Aguila, DVM, for assistance with specimens and manual palpation. We would also like to thank Dr. Timothy Wright for his help in reviewing the manuscript.
This work was supported by the College of Engineering, Cornell University, and an unrestricted educational gift from Synthes USA.
References
- 1.Lipson SJ. Spinal-fusion surgery—advances and concerns. N Engl J Med. 2004;350:643–644. doi: 10.1056/NEJMp038162. [DOI] [PubMed] [Google Scholar]
- 2.Katz JN. Lumbar spinal fusion. Surgical rates, costs, and complications. Spine. 1995;20:78S–83S. [PubMed] [Google Scholar]
- 3.Lee C, Dorcil J, Radomisli TE. Nonunion of the spine: a review. Clin Orthop Relat Res. 2004;419:71–75. doi: 10.1097/00003086-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807–2812. doi: 10.1097/00007632-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 5.Steinmann JC, Herkowitz HN. Pseudarthrosis of the spine. Clin Orthop Relat Res. 1992;284:80–90. [PubMed] [Google Scholar]
- 6.DePalma AF, Rothman RH. The nature of pseudarthrosis. Clin Orthop Relat Res. 1968;59:113–118. [PubMed] [Google Scholar]
- 7.Kornblum MB, Fischgrund JS, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726–733. doi: 10.1097/01.BRS.0000119398.22620.92. [DOI] [PubMed] [Google Scholar]
- 8.Sengupta DK, Herkowitz HN. Degenerative spondylolisthesis: review of current trends and controversies. Spine. 2005;30:S71–S81. doi: 10.1097/01.brs.0000155579.88537.8e. [DOI] [PubMed] [Google Scholar]
- 9.Hidaka C, Goshi K, Rawlins B , et al. Enhancement of spine fusion using combined gene therapy and tissue engineering BMP-7-expressing bone marrow cells and allograft bone. Spine. 2003;28:2049–2057. doi: 10.1097/01.BRS.0000091661.11228.C3. [DOI] [PubMed] [Google Scholar]
- 10.Muschler GF, Negami S, Hyodo A, et al. Evaluation of collagen ceramic composite graft materials in a spinal fusion model. Clin Orthop Relat Res. 1996;328:250–260. doi: 10.1097/00003086-199607000-00039. [DOI] [PubMed] [Google Scholar]
- 11.Kotani Y, Cunningham BW, Cappuccino A, et al. The effects of spinal fixation and destabilization on the biomechanical and histologic properties of spinal ligaments. An in vivo study. Spine. 1998;23:672–682. doi: 10.1097/00007632-199803150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Steffen T, Marchesi D, Aebi M. Posterolateral and anterior interbody spinal fusion models in the sheep. Clin Orthop Relat Res. 2000;371:28–37. doi: 10.1097/00003086-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Grauer JN, Bomback DA, Lugo R , et al. Posterolateral lumbar fusions in athymic rats: characterization of a model. Spine J. 2004;4:281–286. doi: 10.1016/j.spinee.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine. 1995;20:412–420. doi: 10.1097/00007632-199502001-00003. [DOI] [PubMed] [Google Scholar]
- 15.Sama AA, Khan SN, Myers ER, et al. High-dose alendronate uncouples osteoclast and osteoblast function: a study in a rat spine pseudarthrosis model. Clin Orthop Relat Res. 2004;425:135–142. doi: 10.1097/00003086-200408000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Grauer JN, Vaccaro AR, Kato M , et al. Development of a New Zealand white rabbit model of spinal pseudarthrosis repair and evaluation of the potential role of OP-1 to overcome pseudarthrosis. Spine. 2004;29:1405–1412. doi: 10.1097/01.BRS.0000115137.11276.0E. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Viggeswarapu M, Boden SD , et al. Overcoming the immune response to permit ex vivo gene therapy for spine fusion with human type 5 adenoviral delivery of the LIM mineralization protein-1 cDNA. Spine. 2003;28:219–226. doi: 10.1097/01.BRS.0000042417.37236.3F. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Takahashi K, Yamagata M , et al. Spatial and temporal collagen gene expression in lumbar intertransverse fusion in the rabbit. J Bone Joint Surg Br. 2001;83:760–766. doi: 10.1302/0301-620X.83B5.10792. [DOI] [PubMed] [Google Scholar]
- 19.Liao SS, Guan K, Cui FZ, et al. Lumbar spinal fusion with a mineralized collagen matrix and rhBMP-2 in a rabbit model. Spine. 2003;28:1954–1960. doi: 10.1097/01.BRS.0000083240.13332.F6. [DOI] [PubMed] [Google Scholar]
- 20.Yee AJ, Bae HW, Friess D, et al. Accuracy and interobserver agreement for determinations of rabbit posterolateral spinal fusion. Spine. 2004;29:1308–1313. doi: 10.1097/01.brs.0000127184.43765.61. [DOI] [PubMed] [Google Scholar]
- 21.Muschler GF, Huber B, Ullman T, et al. Evaluation of bone-grafting materials in a new canine segmental spinal fusion model. J Orthop Res. 1993;11:514–524. doi: 10.1002/jor.1100110406. [DOI] [PubMed] [Google Scholar]
- 22.Szivek JA, Roberto RF, Slack JM, et al. An implantable strain measurement system designed to detect spine fusion: preliminary results from a biomechanical in vivo study. Spine. 2002;27:487–497. doi: 10.1097/00007632-200203010-00009. [DOI] [PubMed] [Google Scholar]
- 23.Beer F, Johnston E. Mechanics of Materials. New York: McGraw-Hill; 1992. [Google Scholar]
- 24.Tabacknick B, Fidell L. Using Multivariate Statistics. New York: Harper and Row; 1989. pp. 63–65. [Google Scholar]
- 25.Grauer JN, Erulkar JS, Patel TC, et al. Biomechanical evaluation of the New Zealand white rabbit lumbar spine: a physiologic characterization. Eur Spine J. 2000;9:250–255. doi: 10.1007/s005860000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenthal SL, Gill K. Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second surgical look at lumbar fusions. Spine. 1993;18:1186–1189. doi: 10.1097/00007632-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Brodsky AE, Kovalsky ES, Khalil MA. Correlation of radiologic assessment of lumbar spine fusions with surgical exploration. Spine. 1991;16:S261–S265. doi: 10.1097/00007632-199106001-00017. [DOI] [PubMed] [Google Scholar]
- 28.Minamide A, Kawakami M, Hashizume H, et al. Evaluation of carriers of bone morphogenetic protein for spinal fusion. Spine. 2001;26:933–939. doi: 10.1097/00007632-200104150-00017. [DOI] [PubMed] [Google Scholar]
- 29.Boden SD, Martin GJ, Morone M , et al. The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spine fusion. Spine. 1999;24:320–327. doi: 10.1097/00007632-199902150-00003. [DOI] [PubMed] [Google Scholar]
- 30.Erulkar JS, Grauer JN, Patel TC , et al. Flexibility analysis of posterolateral fusions in a New Zealand white rabbit model. Spine. 2001;26:1125–1130. doi: 10.1097/00007632-200105150-00006. [DOI] [PubMed] [Google Scholar]
- 31.Spiro RC, Thompson AY, Poser JW. Spinal fusion with recombinant human growth and differentiation factor-5 combined with a mineralized collagen matrix. Anat Rec. 2001;263:388–395. doi: 10.1002/ar.1119. [DOI] [PubMed] [Google Scholar]
- 32.Glazer PA, Heilmann MR, Lotz JC, et al. Use of electromagnetic fields in a spinal fusion. A rabbit model. Spine. 1997;22:2351–2356. doi: 10.1097/00007632-199710150-00007. [DOI] [PubMed] [Google Scholar]
- 33.Tay BK, Le AX, Heilman M , et al. Use of a collagen–hydroxyapatite matrix in spinal fusion. A rabbit model. Spine. 1998;23:2276–2281. doi: 10.1097/00007632-199811010-00005. [DOI] [PubMed] [Google Scholar]
- 34.Muschler GF, Nitto H, Matsukura Y , et al. Spine fusion using cell matrix composites enriched in bone marrow-derived cells. Clin Orthop Relat Res. 2003;407:102–118. doi: 10.1097/00003086-200302000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ASTM (1997) Standard test methods for flexural properties of unreinforced and reinforced plastics and electrical insulating materials. 790–797
- 36.Carter DR, Spengler DM. Mechanical properties and composition of cortical bone. Clin Orthop Relat Res. 1978;135:192–217. [PubMed] [Google Scholar]
- 37.Lewis G. Properties of acrylic bone cement: state of the art review. J Biomed Mater Res. 1997;38:155–182. doi: 10.1002/(sici)1097-4636(199722)38:2<155::aid-jbm10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 38.Cammisa FP, Lowery G, Garfin SR , et al. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine. 2004;29:660–666. doi: 10.1097/01.BRS.0000116588.17129.B9. [DOI] [PubMed] [Google Scholar]