Abstract
Technical advances in imaging have increased their applicability to diagnosing conditions of the musculoskeletal system, especially in the postoperative setting, where traditionally metallic artifacts have hindered evaluation. Advances in computed tomography (CT), magnetic resonance (MR) imaging, ultrasound, and nuclear medicine have resulted in improved overall image quality. Specific modifications of imaging parameters, especially in CT and MRI, have improved the radiologist's ability to diagnose potential hardware complications such as loosening and osteolysis. Sonography can evaluate the periprosthetic soft tissues and enables both diagnostic information and therapeutic treatment at the same sitting. Lastly, diagnostic scintigraphic applications such as positron emission tomography (PET) have increased specificity in diagnosing potential infection in the arthroplasty setting. This review discusses some of the current applications of CT, MRI, ultrasound, and nuclear medicine in evaluating the postoperative orthopedic patient, concentrating on the appropriate imaging evaluation for the painful arthroplasty patient.
Introduction
Evaluation of the postoperative orthopedic patient has formally been limited to plain film radiographs and clinical evaluation. Plain film radiographs are often noncontributory with regards to possible regional soft tissue pathology or even subtle intramedullary osseous changes [1, 2]. Advanced imaging modalities such as computed tomography (CT) and magnetic resonance (MR) imaging have until recently been limited by metal artifacts, which have severely degraded the diagnostic image quality [1]. Parameter modifications and image optimization can dramatically improve CT and MR image quality, yielding diagnostic information about the regional osseous structures as well as the surrounding soft tissues [3]. Sonography, with its portability, dynamic capabilities, and relatively low cost, can evaluate the periprosthetic soft tissues, and guide for therapeutic or diagnostic injections at the same sitting, although this imaging modality is moderately operator-dependent. Nuclear medicine examinations have been hindered by radiotracer agents with relatively poor specificity, often not being able to differentiate aseptic loosening from infection. Newer scintigraphic agents, however, have greatly improved specificity, especially with regards to evaluating infection in the setting of a painful arthroplasty [4].
Advanced imaging modalities, when performed with specific attention and protocol modification, can result in diagnostic information, which is often clinically unsuspected, aiding the orthopedic surgeon in clinical management.
Computed tomography (CT)
Artifacts generated at CT imaging are related to the type of metal used. Titanium implants result in the least amount of CT artifact, whereas denser materials such as cobalt–chrome result in stronger beam attenuation and resultant increased metallic streak artifact, thus limiting evaluation of the immediate periprosthetic tissues due to x-ray beam attenuation by the hardware [3].
Metal artifact can be reduced with specific parameter settings and image modification with CT imaging. The patient is positioned within the CT scanner such that the x-ray beam crosses the shortest transverse dimension of the metal implant [3]. Metal artifact is further decreased by increasing the number of x-rays crossing the metal (increasing the kVp). However, there is a relative limit as to the absolute percent increase in the kVp, especially in the pediatric population. The advent of multidetector CT scanners, which allow multiple images to be acquired at the same time, thus resulting in an effective increase in the number of x-rays crossing the metal, without a significant increase in total radiation dose [3].
Postprocessing workstations allows meticulous adjustment to modify the ultimate image quality to adequately evaluate the structure of interest [3]. Postprocessing workstations also allow for image reformatting into multiple orthogonal planes, improving evaluation of hardware with the adjacent bone and soft tissues. Multiplanar reformatting (MPR) of the image data can allow for a three-dimensional rendering of an area of interest.
Various “window settings” are used to evaluate certain anatomic structures, for example, the osseous structures, the lung parenchyma, and the soft tissues. Smaller metal implants (such as a Herbert screw in the scaphoid) are best viewed with an edge-enhancement filter or “bone algorithm” setting [3]. In the setting of bulky metal artifacts, such as the presence of a total hip arthroplasty, viewing images with a smooth reconstruction filter or “soft tissue windows,” which is the CT imaging setting normally used to evaluate the visceral soft tissues such as the abdomen or mediastinum, allows for improved detection of focal areas of osteolysis [3].
Typically, osteolysis is seen as multilobulated, hypodense foci, which can be expansile and is occasionally seen in association with cortical abnormalities such as periosteal reaction [5, 6]. As a tomographic imaging modality, CT is better able to determine the total osteolysis volume present and can be used to better evaluate progression of osteolysis, in contrast to plain film radiographs that frequently can only demonstrate definitive evidence of osteolysis when serially examined over time [6–8]. An additional advantage of CT is its ability to definitively characterize periprosthetic lucencies as osteolysis, as opposed to preexisting degenerative pseudocysts, by identifying capsular communication [9].
In conclusion, proper image parameter modification, meticulous attention to x-ray dosage and appropriate postprocessing of image data result can result in diagnostic CT images, even in the setting of bulky metal implants.
Magnetic resonance imaging
Magnetic resonance (MR) imaging is an integral part of evaluating the patient with the painful arthroplasty, where clinical findings are nonfocal, and laboratory diagnosis is negative for infection. As with CT, the generated artifact is dependent on the type of metal implants used, with titanium yielding less artifact compared to implants composed of cobalt chromium. The quality is dependent on the MR imaging pulse sequence parameters prescribed by the musculoskeletal radiologist, who will determine the overall quality of the MR images produced. Routine techniques used in the preoperative setting cannot be employed in the postoperative setting, as the resultant metal artifact is too great, yielding nondiagnostic images. In the setting of metal implants, artifact is reduced if the patient is positioned with the long axis of the hardware parallel to the long axis of the bore of the magnet, but this is often not feasible due to the size restriction of the imaging bore.
Clinical applications of postoperative MR imaging include evaluation of the painful shoulder, knee, or hip arthroplasty [1, 10, 11]. The integrity of the rotator cuff tendons can be evaluated with MR in the setting of a total shoulder arthroplasty, with metal artifact appropriately reduced [10]. In the setting of a painful total knee or hip arthroplasty, the supporting ligamentous structures can be evaluated, as well as the quality of the surrounding musculature and tendons [11].
Because of its superior soft tissue contrast and tomographic capabilities, MR imaging can better delineate the extent and volume of osteolysis involvement compared to plain film radiographs and has been shown (in a cadaveric model) to be more sensitive and specific than optimized CT or plain radiographs in the detection of focal osteolytic lesions [1, 12, 13] (Fig. 1). In contrast to plain film radiographs, MR can evaluate potential extraosseous foci of osteolysis, noting the location and the volume of osteolysis and the relationship of the osteolysis burden to the surrounding soft tissues (Fig. 2). MR can diagnose osteolysis involvement in the posterior osseous structures in the setting of periacetabular osteolysis, such as the posterior column and ischium, areas that are routinely poorly evaluated with routine anteroposterior and Lauenstein lateral views of the hip [1] (Fig. 3).
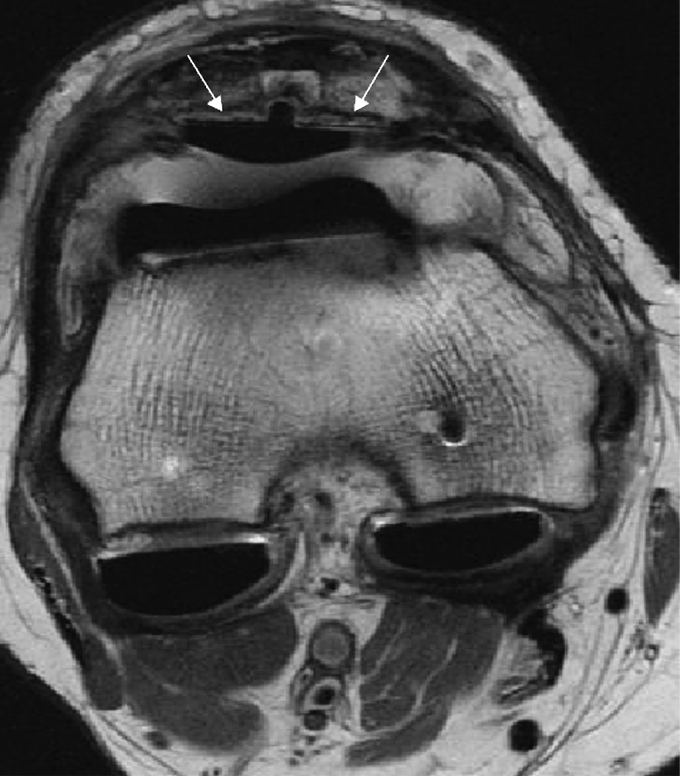
Fig. 1.
Axial fast spin echo proton density weighted MR image of the knee demonstrates a total knee arthroplasty with loosening of the patellar resurfacing component (arrows). A moderately dense synovitis within the pseudocapsule is also present
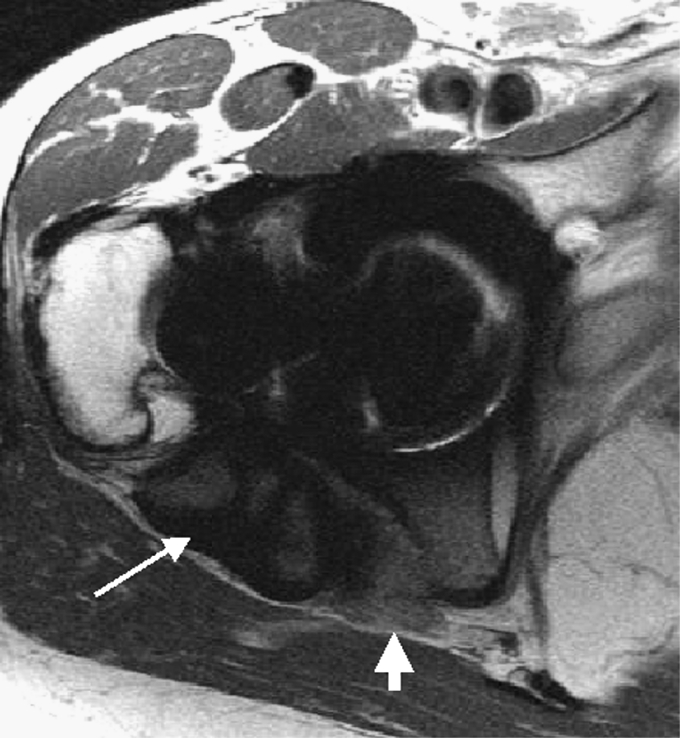
Fig. 2.
Coronal fast spin echo surface coil image of a total hip arthroplasty demonstrates marked fluid distention of the pseudocapsule (arrow) incited by particle reaction with osteolysis in the acetabulum and greater trochanter
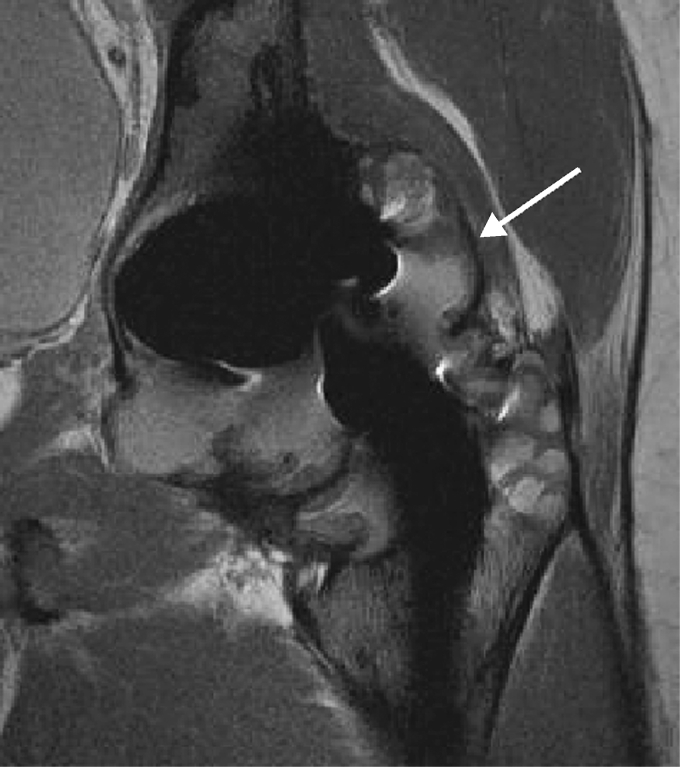
Fig. 3.
Axial fast spin echo surface coil image of a total hip arthroplasty demonstrating marked osteolysis distending the posterior pseudocapsule (arrow). Extension of the osteolysis burden posteromedially results in effacement of the fat planes about the sciatic nerve (short thick arrow)
Despite the demonstration of the efficacy of these techniques in a clinical cohort, validation of these techniques is necessary. Inspection of the joint at the time of revision surgery is often an imperfect standard by which to judge the accuracy of imaging in determining the location and total volume of bone loss. We have thus studied a cadaveric pelvic model, comparing the ability of optimized radiographs and MR imaging to locate and quantify simulated osteolytic lesions. In this nonclinical model, MRI was 95% sensitive in the detection of lesions, with a specificity of 98% and an accuracy of 96%, and lesion detection was not statistically dependent on lesion location [12].
In a comparative nonclinical model of modified MR imaging, optimized plain radiographs, and optimized computerized tomography, MRI was determined to be the most sensitive technique in detecting osteolytic lesions, with a sensitivity of 95%, compared to 75% for CT and 52% for radiographs. In this study, MRI emerged as the most effective tool for detecting small periacetabular osteolytic lesions measuring less than 3 cm [13]. These newly available MR techniques provide an effective means to prospectively assess the synovial and intraosseous burden of particle disease, thus serving as a means by which to noninvasively monitor disease progression. Although improved sensitivity in the detection of osteolysis will facilitate preoperative planning for revision arthroplasty, improved quantification of particle disease (in both the soft tissues and osseous structures) is needed for serial evaluation of nonoperative treatments, such as the use of oral bisphosphonates.
Other applications of MR imaging in the setting of the painful arthroplasty include the evaluation of surrounding soft tissue structures in the setting of recurrent dislocation, the depth and extent of infection, regional neurovascular pathology such as postoperative neuromas, as well as the location of heterotopic ossification relative to adjacent neurovascular structures.
With the advent of faster and more robust MR gradient platforms, wider readout bandwidths and faster scanning techniques can be employed, while maintaining acceptable scan times and signal to noise. Fast scanning techniques (fast spin echo imaging) result in overall decreased metal artifacts in contrast to routine spin echo imaging, and are essential in the postoperative setting [14]. At least one “water-sensitive” pulse sequence is needed to evaluate for areas of bone marrow edema, (e.g., possible fracture, infection, or other infiltrative marrow pathology such as osteolysis). In the postoperative setting, at least one fast inversion recovery sequence is indicated. A routine “fat suppression” sequence, often applied in the preoperative setting, is ineffective in the postoperative setting as it is more susceptible to regional field inhomogeneities, such as the presence of metal [14, 15]. At our institution, one large field of view (imaging this entire pelvis, inclusive of the sacrum and lower lumbar spine) is included to evaluate for any possible areas of referred pain, for example, an ipsilateral sacral insufficiency fracture. The inversion recovery sequence aids in identifying areas of osseous, soft tissue, and prosthetic abnormalities, including fractures, periprosthetic tendon, or muscle abnormalities [1, 16].
Intravenous gadolinium has been reported to be helpful in the postoperative setting; however, the use of concomitant frequency-selective fat suppression techniques, which are commonly used following contrast injection, will be hampered in the presence of the metallic components [1, 14].
Ultrasound
Ultrasound lacks ionizing radiation, but has dynamic capabilities, and is relatively low-cost, and has a major role in evaluation of the orthopedic patient.
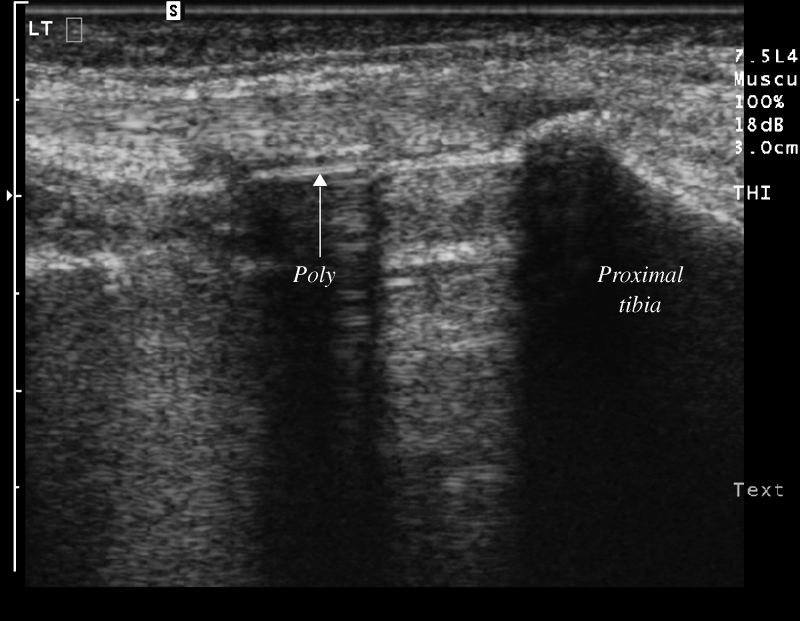
There is a characteristic reverberation artifact produced by orthopedic hardware at sonographic evaluation, similar to that generated by a needle during sonographic-guided procedures. The sonographic appearance of the polyethylene liners used in joint replacements produces a sharp echogenic linear interface with posterior acoustic shadowing, similar to the sonographic appearance of bone (Fig. 4).
Fig. 4.
Longitudinal ultrasound image along the medial joint line of the knee demonstrating the normal sonographic appearance of a total knee arthroplasty. The polyethylene (arrow, labeled) is seen as a linear echogenic interface with posterior acoustic shadowing. Polyethylene demonstrates similar acoustic characteristics as bone (proximal tibia is labeled). The metallic tibial tray is seen between the polyethylene and the proximal tibia
There is essentially no metal artifact degradation of image quality with the use of sonography and in the patient with the painful arthroplasty, the integrity of the surrounding tendons as well as the presence of possible muscle atrophy. The relationship of the arthroplasty components can be evaluated because polyethylene and metal have different sonographic appearances and the possibility of subluxation or polyethylene wear can be observed (Fig. 4). The use of ultrasound for postarthroplasty evaluation has been described in the shoulder and in the knee, evaluating the status of the rotator cuff and the appearance of the polyethylene liner in total knee prostheses, respectively [17, 18].
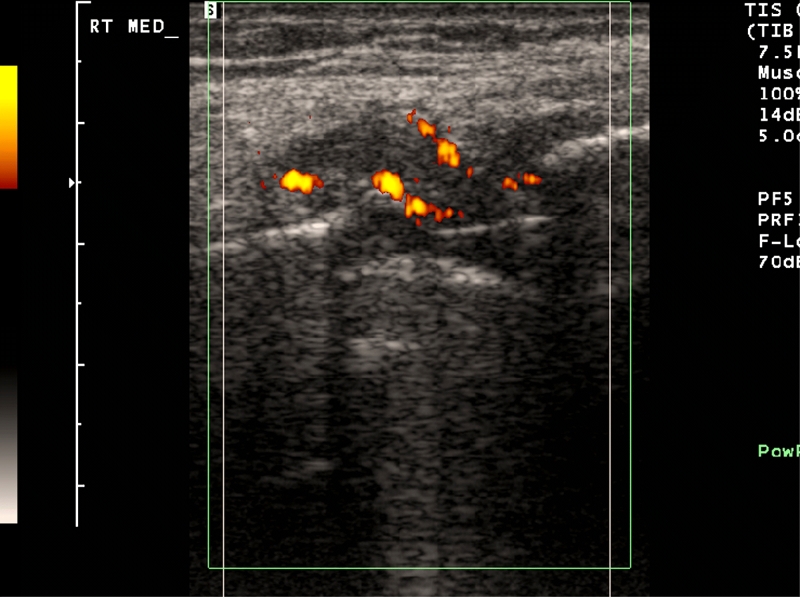
The dynamic capabilities of ultrasound allow for diagnostic or therapeutic interventions to be readily performed and with provocative maneuvers, possible irritation of regional structures such as tendons against indwelling hardware can be seen. The presence of synovitis, possibly indicating particle reaction, can be seen as areas of heterogeneous hypoechogenicity distending the pseudocapsule, often associated with moderately increased power Doppler.
The surrounding ligaments and tendons can be readily evaluated with sonography, as these are relatively superficial structures. The presence of any abnormal fluid collections, either periarticular or within the pseudocapsule, can be identified with sonography and aspirated as needed; the presence of hyperemia can correlate with the potential likelihood of active infection [19]. The application of power Doppler can help to identify areas of active inflammation, and thus identify areas of synovitis or active infection, thus directing areas of potential aspiration or treatment [19] (Fig. 5).
Fig. 5.
Longitudinal ultrasound image along the medial joint line of the knee demonstrating moderate synovitis in a patient with a total knee arthroplasty, with hypoechoic debris and regional increased blood flow with power Doppler indicating inflammation
Sonographic-guided interventions can be performed throughout the musculoskeletal system. Sonography can be used to guide for diagnostic aspirations or injections. By observing needle placement during real-time, accurate delivery of medication at the target of interest or assured placement of the needle in the joint or collection to be aspirated is certain. The surrounding soft tissue neurovascular structures can also be identified and thus avoided. Sonography, specifically, has been demonstrated to be useful in the postoperative setting in evaluating and treating patients with painful hip flexion after total hip arthroplasty due to irritation of the iliopsoas tendon, which can be directly visualized for sonographic guided injection [20].
In the postoperative setting, percutaneous interventions are often intended for diagnostic arthrocenteses. Any joint in the body can be addressed for aspiration using sonography—with technical parameter modifications including change in transducer frequency, depending on how deep or superficial the structure is, as well as choice of the appropriate transducer to best visualize the joint. The needle is visualized during its entire trajectory, confirming accurate needle placement and avoidance of regional neurovascular structures.
Sonography has been traditionally an underutilized modality to evaluate the postoperative orthopedic patient; however, its portability, dynamic capabilities, and absence of ionizing radiation make it an appropriate method for imaging across a broad patient population and a wide variety of postoperative orthopedic conditions.
Nuclear medicine
Nuclear scintigraphy of the musculoskeletal system has traditionally been limited by poor specificity, especially in the postoperative setting. Distinguishing aseptic loosening from infection has been extremely difficult, at best, especially in the perioperative setting. Combined radiotracer agents, usually with an inflammation-specific agent, such as gallium or labeled white blood cells, have shown improved specificity in diagnosing infection, especially in the setting of total knee arthroplasty [21].
More recently, positron emission tomography (PET) has increased its applications in total body imaging to include the postoperative orthopedic patient. With PET scanning, an inflammation-specific agent [usually [18F]fluorodeoxyglucose (18F-FDG)] is used as a radiotracer. One preliminary study has demonstrated potential applications of PET scanning in the setting of total hip and total knee arthroplasty, differentiating between active infection, loosening, and synovitis, based on the degree of uptake (with high 18F-FDG uptake indicating infection, intermediate uptake aseptic loosening, and low uptake a bland synovitis, respectively) [22]. PET scanning for postoperative infection has also been investigated in the spine, also showing promising results, with increased specificity for infection in contrast to routine three-phase bone scan or combination radiotracers [23].
The increasing specificity of nuclear medicine agents continues to broaden nuclear medicine applications in the postoperative musculoskeletal imaging setting.
Conclusion
In conclusion, CT, MRI, ultrasound, and nuclear medicine all have a clinical role in the evaluation of the postoperative orthopedic patient, provided that the modalities are protocoled for the anticipated clinical concern and prescribed by the musculoskeletal radiologist. Parameters and protocols include appropriate MRI coil selection, CT parameter modification, correct ultrasound transducer and frequency selection, and specific nuclear medicine scintigraphic agent selection. The musculoskeletal radiologist is a consultant to the clinician, and open discussion with the orthopedic surgeon benefits the referring doctor, the radiologist, and especially the patient.
References
- 1.Potter HG, Nestor BJ, Sofka CM, Ho ST, Peters LE, Salvati EA. Magnetic resonance imaging after total hip arthroplasty: evaluation of periprosthetic soft tissue. J Bone Jt Surg Am. 2004;86-A(9):1947–1954. doi: 10.2106/00004623-200409000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Borrelli J, Ricci WM, Steger-May K, Totty WG, Goldfarb C. Postoperative radiographic assessment of acetabular fractures: a comparison of plain radiographs and CT scans. J Orthop Trauma. 2005;19:299–304. [PubMed] [Google Scholar]
- 3.White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopaedic patient. Semin Musculoskelet Radiol. 2002;6(1):5–17. doi: 10.1055/s-2002-23160. [DOI] [PubMed] [Google Scholar]
- 4.Larikka MJ, Ahonen AK, Junila JA, Niemela O, Hamalainen MM, Syrjala HP. Extended combined 99mTc-white blood cell and bone imaging improves the diagnostic accuracy in the detection of hip replacement infections. Eur J Nucl Med. 2001;28(3):288–293. doi: 10.1007/s002590000463. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Ryu KN, Hong HP, Park YK, Chun YS, Yoo MC. Focal osteolysis in total hip replacement: CT findings. Skelet Radiol. 2004;33(11):632–640. doi: 10.1007/s00256-004-0812-8. [DOI] [PubMed] [Google Scholar]
- 6.Claus AM, Totterman SM, Sychterz CJ, Tamez-Pena JG, Looney RJ, Engh CA. Computed tomography to assess pelvic lysis after total hip replacement. Clin Ortop. 2004;422:167–174. doi: 10.1097/01.blo.0000129345.22322.8a. [DOI] [PubMed] [Google Scholar]
- 7.Engh CA, Sychterz CJ, Young AM, Pollock DC, Toomey SD, Engh CA. Interobserver and intraobserver variability in radiographic assessment of osteolysis. J Arthroplast. 2002;17(6):752–759. doi: 10.1054/arth.2002.33554. [DOI] [PubMed] [Google Scholar]
- 8.Walde TA, Weiland DE, Leung SB, Kitamura N, Sychterz CJ, Engh CA, Claus AM, Potter HG, Engh CA. Comparison of CT, MRI and radiographs in assessing pelvic osteolysis: a cadaveric study. Clin Orthop Relat Res. 2005;437:138–144. doi: 10.1097/01.blo.0000164028.14504.46. [DOI] [PubMed] [Google Scholar]
- 9.Kitamura N, Naudie DD, Leung SB, Hopper RH, Engh CA. Diagnostic features of pelvic osteolysis on computed tomography: the importance of communication pathways. J Bone Jt Surg Am. 2005;87(7):1542–1550. doi: 10.2106/JBJS.D.02882. [DOI] [PubMed] [Google Scholar]
- 10.Sperling JW, Potter HG, Craig EV, Flatow E, Warren RF. Magnetic resonance imaging of painful shoulder arthroplasty. J Shoulder Elbow Surg. 2002;11(4):315–321. doi: 10.1067/mse.2002.124426. [DOI] [PubMed] [Google Scholar]
- 11.Sofka CM, Potter HG, Figgie M, Laskin R. Magnetic resonance imaging of total knee arthroplasty. Clin Ortop. 2003;406:129–135. doi: 10.1097/01.blo.0000030516.43495.61. [DOI] [PubMed] [Google Scholar]
- 12.Weiland DE, Walde TA, Leung JSJB, Sychterz CJ, Ho S, Engh CA, Potter HG. Magnetic resonance imaging in the evaluation of periprosthetic acetabular osteolysis: a cadaveric study. J Orthop Res. 2005;23(4):713–719. doi: 10.1016/j.orthres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Walde TA, Weiland DE, Leung SB, Kitamura N, Synchterz CJ, Engh CA, Claus AM, Potter HG, Engh CA. Comparison of CT, MRI, and radiographs in assessing pelvic osteolysis: a cadaveric study. Clin Orthop Relat Res. 2005;437:138–144. doi: 10.1097/01.blo.0000164028.14504.46. [DOI] [PubMed] [Google Scholar]
- 14.White LM, Kim JK, Mehta M, Merchant N, Schweitzer ME, Morrison WB, Hutchison CR, Gross AE. Complications of total hip arthroplasty: MR imaging—initial experience. Radiology. 2000;215:254–262. doi: 10.1148/radiology.215.1.r00ap11254. [DOI] [PubMed] [Google Scholar]
- 15.Sofka CM, Potter HG. MR imaging of joint arthroplasty. Semin Musculoskelet Radiol. 2002;6(1):79–85. doi: 10.1055/s-2002-23166. [DOI] [PubMed] [Google Scholar]
- 16.Cook SM, Pellicci PM, Potter HG. Use of magnetic resonance imaging in the diagnosis of an occult fracture of the femoral component after total hip arthroplasty. A case report. J Bone Jt Surg Am. 2004;86-A(1):149–153. doi: 10.2106/00004623-200401000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Sofka CM, Adler RS. Sonographic evaluation of shoulder arthroplasty. Am J Roentgenol. 2003;180(4):1117–1120. doi: 10.2214/ajr.180.4.1801117. [DOI] [PubMed] [Google Scholar]
- 18.Sofka CM, Adler RS, Laskin R. Sonography of polyethylene liners used in total knee arthroplasty. Am J Roentgenol. 2003;180(5):1437–1441. doi: 10.2214/ajr.180.5.1801437. [DOI] [PubMed] [Google Scholar]
- 19.Briedahl WH, Newman JS, Taljanovic MS, Adler RS. Power Doppler sonography in the assessment of musculoskeletal fluid collections. Am J Roentgenol. 1996;166(6):1443–1446. doi: 10.2214/ajr.166.6.8633460. [DOI] [PubMed] [Google Scholar]
- 20.Wank R, Miller TT, Shapiro JF. Sonographically guided injection of anesthetic for iliopsoas tendinopathy after total hip arthroplasty. J Clin Ultrasound. 2004;32(7):354–357. doi: 10.1002/jcu.20043. [DOI] [PubMed] [Google Scholar]
- 21.Schneider R, Soudry M. Radiographic and scintigraphic evaluation of total knee arthroplasty. Clin Ortop. 1986;205:108–120. [PubMed] [Google Scholar]
- 22.Manthey N, Reinhard P, Moog F, Knesewitsch P, Hahn K, Tatsch K. The use of [18F]fluorodeoxyglucose positron emission tomography to differentiate between synovitis, loosening and infection of hip and knee prostheses. Nucl Med Commun. 2002;23(7):645–653. doi: 10.1097/00006231-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 23.DeWinter F, Gemmel F, Wiele C, Poffijn B, Uyttendaele D, Dierckx R. 18-Fluorine fluorodeoxyglucose positron emission tomography for the diagnosis of infection in the postoperative spine. Spine. 2003;28(12):1314–1319. doi: 10.1097/01.BRS.0000065483.07790.34. [DOI] [PubMed] [Google Scholar]