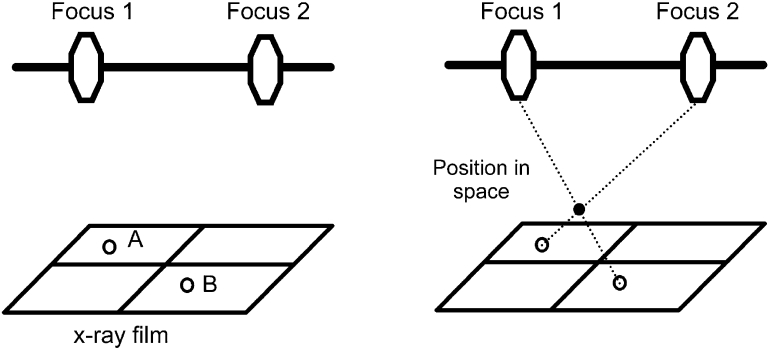
Radiostereometric analysis is an accurate method of determining the migration and wear of orthopaedic implants such as total hip arthroplasties. While the overall concept of RSA is relatively straightforward: determining the precise location of two distinct objects relative to each other in three dimension such as the relative position of the femoral component and the proximal femur, the actual practical application is somewhat more complex. In radiostereometric analysis the position in space of the original object is reconstructed from a two dimensional x-ray film (Fig. 1). In order to reconstruct the position of segments within the human body, each segment is marked with at least three tantalum beads. Movement between segments is then calculated by localizing each segment in a coordinate system.
Fig. 1.
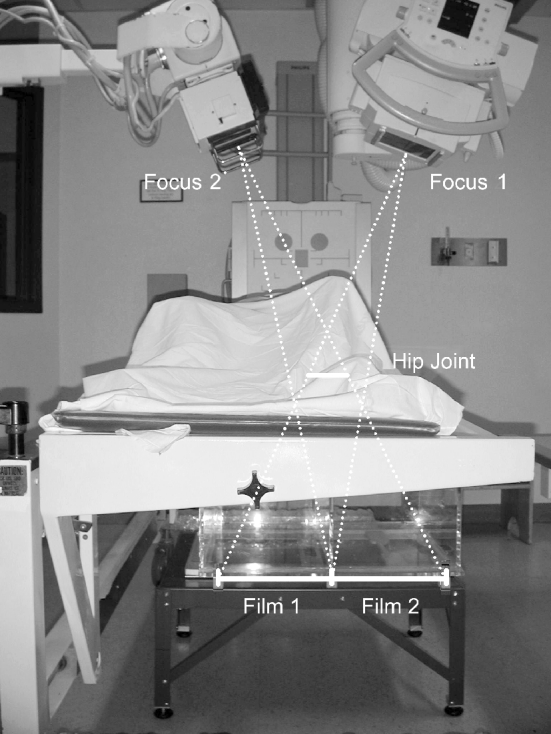
Set up for an RSA x-ray. The hip joint is positioned within the focus of two x-ray machines (Focus 1 and 2). The calibration cage is loaded with two film cassettes (Film 1 and 2)
History of radiostereometric analysis
In 1898 Davidson, a radiologist in London interested in localizing an object in space by means of roentgen beams, fixed an X-ray tube to a horizontal bar and explored the same film from two definite tube positions (stereo method) [1]. He placed two perpendicular wires as coordinate system on the film that would allow him to replace the developed film in exact the same orientation on the table in a so called “localizer”. In the Localizer instead of two x-ray tubes he fixed two threads at the same exact position as the x-ray focus. He then reconstructed the position of the object by stretching a thread between former x-ray focus and the image on the developed film. The location in space, where both threads cross (Fig. 2), determines the exact position of the x-rayed object [2].
Fig. 2.
Once the exact position of focus 1 and 2 and the two image points (A) and (B) are known the location of the object in space can be calculated or visualized using threads (Davidson [1])
The same basic principles apply to modern radiostereometric analysis. Whereas Davidson used an apparatus (“localizer”) with known focus and film position modern radiostereometry uses a cage with fiducial and control marker to calculate a 3-D coordinate system.
Rigid body model
One of the fundamental principals of RSA is the concept of rigid bodies. In simple geometry a rigid body is defined as a system of mass points in which the distance between all pairs of points remain constant throughout motion. Non rigid bodies are called deformable. A rigid body is a mathematical model and is described by a point matrix. Any three non-collinear points in the body matrix form a rigid body that determines the position of the entire body in space. Because the distance between this points remain constant at least 6 parameters are needed to describe the exact position of a rigid body, the so called 6 Degrees of Freedom.
Displacing a rigid body in space so that every point on its matrix has the same movement is called Translation, whereas in Rotation all points on the rotation axis remain constant and all other points move in position to their distances from this axis. The overall movement of a rigid body is the sum of translation of all points within its matrix and rotation about an axis through a point in the matrix or in space. Euler showed that this movement can be described by a transformation vector and a rotation matrix and is visualized by the motion of a body fixed coordinate system relative to a space fixed coordinate system. In radiostereometric analysis the motion of one rigid body (stem of a hip implant) is plotted against another (bone of the proximal femur) in a laboratory coordinate system. To calculate the exact position of each rigid body the coordinates of three points within its matrix are needed. Because determining the exact localization of anatomic bony landmarks of the proximal femur and the acetabulum in a three dimensional coordinate system is almost impossible artificial tantalum markers are used to define the rigid body of interest.
Markers to localize the segment of interest
In order to identify distinct points of measurement for each part of the skeleton and implant involved spherical tantalum markers are inserted into the bone and implant. Tantalum is easily identified on radiographs because of its high anatomic number. Its biocompatibility and resistance to corrosion makes tantalum an ideal implant for the use inside the human body.
Tantalum beads of 0.6 mm, 0.8 mm and 1.0 mm diameter are used for radiostereometric analysis. Although smaller sizes offer the advantage of an increased accuracy their visualization is impaired by the amount of soft tissue coverage. Therefore the use of 0.6 mm tantalum markers has been limited to the knee, ankle, elbow and children. The most commonly used diameter for the hip joint is 0.8 mm. With an increasing soft tissue envelope (pelvis and vertebra) or if tantalum marker are placed within radiodense materials (orthopedic implants) 1.0 mm diameter tantalum markers are useful.
Traditionally tantalum beads have been inserted into orthopedic implants, including polyethylene, however, all studies involving total hip replacements at the Hospital for Special Surgery have avoided implantation of beads into the femoral and acetabular component. This is possible by using the center of the femoral head or acetabular component as reference point. The center of a spherical object (like the head of the femoral component and the center of the acetabular component) allows for the calculation of the exact three dimensional position and therefore help to avoid inserting markers into the implant itself (Fig. 3). While this allows determining translation of the implant by measuring movements of the center of the femoral head or acetabulum in respect to the bone, calculation of implant rotation is not possible.
Fig. 3.

The center of the femoral head is used as the primary reference point for measurement of migration of the femoral component. This allows for measurement of translation, however, additional marker in the implant are needed to measure rotation
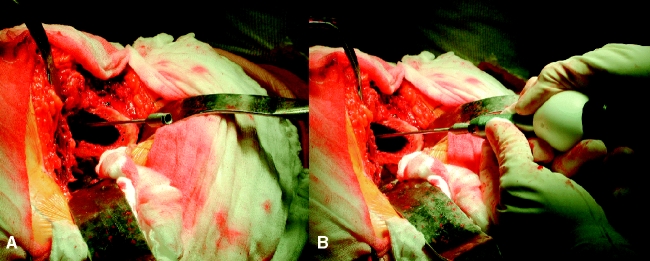
Implantation of markers
Tantalum markers are inserted using a steel canula. An awl or drill bit facilitates the introduction of the needle into cortical or sclerotic bone (Fig. 4). The exact position within the bone can be verified by fluoroscopy, but this is rarely necessary.
Fig. 4.
After drilling a hole into the greater trochanter a trochar is inserted (A) and is loaded with the bead inserter (B)
Although only 3 non-collinear markers in each segment of interest are theoretically necessary for the radiostereometric analysis usually 5–9 tantalum balls are inserted to compensate for loose or invisible markers (Fig. 5). The markers should be randomly distributed over the anatomic segment having a distinctive proximal–distal distance to each other to facilitate their identification. To increase the accuracy of RSA tantalum beads need to be inserted in order to create large rigid bodies [3]. If the marker configuration approaches that of a straight line the condition number (an inverse measure of accuracy) increases and the accuracy of the technique is jeopardized. Therefore at the Hospital for Special Surgery we have excluded patients with condition numbers above 300. It is also important that the markers do not move inside the bone. The amount of movement of tantulum markers is calculated through computer algorithms and is represented by a number known as the mean error. Cases with a mean error exceeding 250 μm are usually excluded (Fig. 6).
Fig. 5.
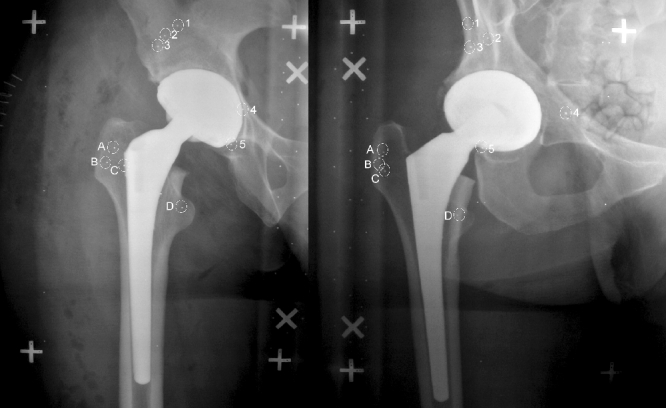
Set of 2 RSA x-rays. The tantalum beads in the pelvic bone are marked with number 1 to 5 and the beads in the proximal femur are marked with letter (A) to (D)
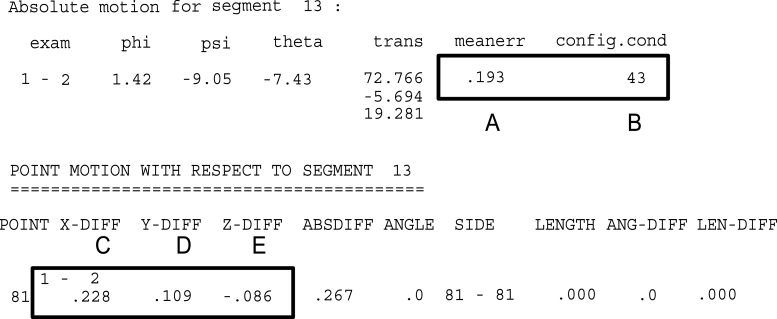
Fig. 6.
Results are reported for each time interval (1–2). Important information for the analysis are mean error (A), condition number (B), translation in x- (C), y- (D) and z (E) direction
Accuracy of RSA and alternative techniques
On a plain radiograph a change of position of approximately 5 mm is needed to prove migration [4]. The addition of markers implanted into the shoulder of the implant and the lesser trochanter improved the accuracy to 3.9 mm [5]. Ein-Bild-Röntgen-Analyse (EBRA) is another tool to measure implant migration. It relies on identification of reference points on plain or scanned radiographs and has an accuracy of 1 to 1.5 mm [6]. Its precision is low with the 95% percentiles differing by 0.74 to 0.87 mm. Compared to the described alternative techniques the accuracy and precision of RSA is very high. In most clinical studies the accuracy has been approximately 0.2 mm [7]. However, in vitro studies show accuracy as low as 0.047mm to 0.121 mm [8]. In general measurements of translation along the longitudinal axis are more accurate than those along the transverse and sagittal axis [8]. Measurement of femoral component migration based on three markers in the implant is about three times more accurate than relying on the calculated center of the femoral head alone. In addition the distribution of the tantalum markers as described by the condition number has an important effect on the accuracy of the technique. A condition number of less than 40 leads to a 3 fold increase of the accuracy compared to a condition number above 143. Although precision depends to a lesser degree on the ideal marker position the best marker configuration for accuracy seems to provide the most precise measurements [8]. In vitro precision has been 0.03 mm for 0.200 mm displacements [8].
RSA in primary total hip replacement
Mjoberg was the first to correlate continous early implant migration of cemented femoral components measured using radiostereometric analysis and late clinical failure [9]. Based on Mjoberg’s findings it was later suggested that RSA might be the best technique to evaluate new implant designs considering that prediction of clinical failure is possible evaluating a smaller number of patients with a shorter follow-up [10]. According to Kärrholm and coworkers subsidence of more than 0.33 mm and a maximal total point migration exceeding 0.85 mm within the first 6 months is an important predictor for subsequent revisions in cemented femoral components [11]. The evaluation of migration of cemented femoral stems and the prediction of late failure is influenced by the implant shape. To evaluate the importance of RSA to predict early failures of cemented femoral components “shape closed” and “force closed” designs need to be distinguished. A “shape closed” design provides immediate stability by match of shapes. An example is a wide collar to be positioned on the calcar [12]. “Force closed” designs obtain stability by the action of forces. Collarless “forced closed” implants initially migrate into the cement mantle to stabilize and are preferably highly polished [13, 14]. While early prediction of long term survival based on RSA migration analysis is possible for “shape closed” designs it is more problematic for “forced closed” designs[11]. In a recent study Stefansdottir et al failed to show a correlation between migration of a polished “forced closed” stems (Exeter) and clinical failure [15].
RSA has also been used to evaluate uncemented femoral components. Although initial implant migration is an important predictor for long term survival of “shape closed” cemented femoral components [11] the analysis of uncemented components is less prognostic. Uncemented components can show up to three times the amount of migration within the first 6 months compared to cemented components without long term failure [7]. There are a number of factors that have an impact on initial implant migration of uncemented femoral components including implant shape, surface coating, amount of press fit, bone quality and activity and weight of the patient. Therefore it is more difficult to predict long term outcome based on short term RSA evaluations. However, RSA does provide valuable information in controlled randomized studies. As an example RSA has been used in the past to show that hydroxyapatite coating improves fixation compared to beaded [16] and grit-blasted porous surfaces [17] as well as plasma sprayed titanium alloy coatings [7].
Most modern uncemented total hip implants provides excellent long term results [18–25]. Although uncemented designs were initially preserved for younger patients with osteoarthritis [26] more recently studies have shown good long term results in older patients [21] and patients with rheumatoid arthritis [27]. Therefore, throughout the US surgeons have widened their indications for uncemented femoral components. Considering that initial fixation is a presupposition for bone in-growth almost all patients in recently published long term studies were mobilized with partial or none weight bearing for 4 to 12 weeks [18–25]. However, immediate weight bearing after surgery has been shown to influence the progress of early rehabilitation, a fact that is especially important for young and active patients with a strong interest to return to work as early as possible. At the Hospital for Special Surgery we have recently finished a randomized prospective trial using RSA to compare initial implant migration in patients mobilized weight bearing as tolerated and toe touch weight bearing respectively [28]. RSA radiographs were taken at 3 days, 6 weeks, and 6 months after surgery to measure migration of the femoral component. The study showed a difference in the vertical (proximal-distal) migration within the first 6 weeks between both groups (0.81 mm versus 0.13 mm) but not thereafter (0.17 mm versus 0.18 mm). There was no loosening in both groups within a two year follow-up. Based on this study initial weight bearing was recommended for patients undergoing uncemented total hip arthroplasty.
Aseptic loosening is the most common reason for failure of acetabular components. In the literature there is some evidence, that cemented acetabular components are more prone to loosening than uncemented press fit cups [29]. Today most surgeons in the US use modular uncemented press fit components for primary total hip arthroplaties. For uncemented components initial stability is essential to allow for adequate bone in-growth into the implant surface. Thanner analyzed 23 pairs of patients with uncemented porous acetabular cups (Harris-Galante, Zimmer Inc.) to investigate the impact of hydroxyapatite and tricalcium phosphate (HA,TCP) coating on implant migration within the first two years after implantation [30]. In their study group HA/TCP improved the fixation for uncemented acetabular components and significantly reduced implant migration (RSA). In another study Thanner evaluated whether screw fixation did improve the initial fixation of HA/TCP coated acetabular components [30]. In a randomized RSA-study of 64 hips the author showed an average translation of 0.2 mm and rotation of 0.2 degrees in both groups without any difference between the groups. The author summarized, that additional screw fixation is not necessary to obtain initial fixation of uncemented acetabular components. Today uncemented press-fit components are the standard in primary total hip arthroplasty. However, over the last decade we have seen an interest in hard-on-hard bearings in an effort to reduce wear in younger and more active patients. The consequences of hard bearings on implant migration and initial stability are unknown. Theoretically ceramic-on-ceramic bearings could increase the load, interfere with initial stability and impair bony in-growth. At the Hospital for Special surgery we are therefore currently evaluating the impact of different bearing materials on the migration of uncemented press fit cups. As part of the ongoing RSA projects migration of acetabular components with metal-on-polyethylene bearings will be compared to patients with ceramic-on-ceramic bearings.
While the quality of the primary implant fixation is determined by the acetabular component geometry and the resulting press fit the surface finishing influences the secondary bone in-growth and long-term stability. Trabecular metal is a new surface coating. It has interconnecting pores and a microstructure and material properties similar to those of cancellous bone. Its modulus of elasticity is similar to cancellous bone (2–3 GPa) and about 50 times lower than that of titanium. Therefore, trabecular metal might reduce stress shielding in the periacetabular bone. In addition trabecular metal has a higher coefficient of friction on cancellous bone than other surface finishings [31, 32]. Trabecular metal also has higher bone interface shear strength [33]. All these properties might improve fixation of uncemented acetabular components. We therefore are currently studying the impact of trabecular metal on implant migration of uncemented acetabular components in a randomized trial.
Use of RSA in the evaluation of modern implant designs
Resurfacing hip replacement was originally described by Charnley in the 1950s [34]. In the US the THARIS resurfacing arthroplasty was introduced in 1975. Soon it became obvious that the device had an unacceptable high failure rate. Amstutz et al reported 72 failures in 584 hip resurfacings (12.3%) and stated that the hip resurfacing arthroplasty clearly did not solve the problem of high failure rates of conventional hip arthroplasties in younger patients [35]. Ritter et al. prospectively evaluated 50 patients with bilateral total hip arthroplasty which underwent conventional hip arthroplasty on one side and resurfacing arthroplasty on the other. At a five year follow-up only 4 percent of the conventional but 26% of the hip resurfacing arthroplasties had failed [36]. More recently there has been a new interest into resurfacing hip arthroplasty. This interest has been mainly driven by the excellent results of the Birmingham hip resurfacing arthroplasty in England [37]. While there is one report in the literature with a minimum of 5 years follow up [37] most studies in the literature only have a short term follow-up of 2 years. Radiostereometric analysis is an ideal tool for the early assessment of new implants, since it can detect continous migration, which is an early warning sign for failure. The failure of hip resurfacing is likely to be aseptic loosening of the femoral and acetabular component. While the acetabular component is similar to standard uncemented press fit sockets the stem design is different and therefore its long term performance is relatively unpredictable. There are now two RSA studies that have evaluated hip resurfacing over a two year follow-up interval. Glyn-Jones and coworker evaluated 24 young patients undergoing hip resurfacing for osteoarthritis [38]. The center of the head showed a total migration of 0.21 mm over a two year period with 62% of the migration occurring within the first year. The distal migration of the device was less than 0.2 mm. Considering that this number is lower than data previously reported for failed cemented components the authors summarized, that the Birmingham hip resurfacing is likely to perform well in the long term. While this study used the outer circumference of the femoral component head and tip of the stem to measure migration a more recent study equipped the implants with tantalum markers and therefore was able to evaluate rotation of the components as well [39]. This study confirmed minimal translational (less than 0.1 mm in all directions) and rotational (less than 1 degree) movements within the first 24 months after implantation. Both studies failed to show evidence for early migration and loosening of the components and therefore support the use of the hip resurfacing implants in the absence of clinical long term data.
Summary
RSA is the most accurate technique available to measure implant migration following total hip arthroplasty. Implant migration is an important predictor of long term fixation of uncemented and cemented femoral and acetabular components. Especially with a more recent interest in uncemented fixation continuous migration after 6 months intervals might be evidence of inadequate bone ingrowths. RSA has the benefit of picking up continous migration long before clinical failure will be evident and is therefore considered to be perfectly suited to evaluate new implant designs and implant coatings. It can also be used to evaluate the impact of hard surface bearings including metal-on-metal and ceramic-on-ceramic on bone in-growth and migration of uncemented components. In the future RSA might be an interesting tool to pick up decreased rates of aseptic loosening with the use of computer navigation within short term follow-ups.
References
- 1.Davidson M (1898) Roentgen rays and localization. An apparatus for exact measurement and localization by means of roentgen rays. Brit Med J 10 [DOI] [PMC free article] [PubMed]
- 2.Selvik G. Roentgen stereophotogrammetry. A method for the study of the kinematics of the skeletal system. Acta Orthop Scand. 1989;232(Suppl):1–51. [PubMed] [Google Scholar]
- 3.Ryd L, Yuan X, Lofgren H. Methods for determining the accuracy of radiostereometric analysis. Acta Orthop Scand. 2000;71:403–408. doi: 10.1080/000164700317393420. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland CJ, Wilde AH, Borden LS, Marks KE. A ten-year follow-up of one hundred consecutive Müller curved-stem total hip replacement arthroplasties. J Bone Jt Surg. 1982;64-A:970–982. [PubMed] [Google Scholar]
- 5.Malchau H, Kärrholm J, Wang YX, Herberts P. Accuracy of migration analysis in hip arthroplasty: digitized and conventional radiography, compared to radiostereometry in 51 patients. Acta Orthop Scand. 1995;66:418–424. doi: 10.3109/17453679508995578. [DOI] [PubMed] [Google Scholar]
- 6.Biedermann R, Krismer M, Stöckl B, Mayrhofer P, Ornstein E, Franzen H. Accuracy of EBRA-FCA in the measurement of migration of femoral components of total hip replacement. J Bone Jt Surg. 1999;81-B:266–272. doi: 10.1302/0301-620X.81B2.8842. [DOI] [PubMed] [Google Scholar]
- 7.Soballe K, Toksvig-Larsen S, Gelineck J, Fruensgaard S, Hansen ES, Ryd L, Lucht U, Bünger C. Migration of hydroxyapatite coated femoral prostheses. A roentgen stereophotogrammetric study. J Bone Jt Surg. 1993;75-B:681–687. doi: 10.1302/0301-620X.75B5.8397213. [DOI] [PubMed] [Google Scholar]
- 8.Önsten I, Berzins A, Shott S, Sumner DR. Accuracy and precision of radiostereometric analysis in the measurement of THR femoral component translations: human and canine in vitro models. J Orthop Res. 2001;19:1162–1167. doi: 10.1016/S0736-0266(01)00039-0. [DOI] [PubMed] [Google Scholar]
- 9.Mjoberg B. Loosening of the cemented hip prosthesis. Theimportance of head injury. Acta Orthop Scand. 1986;221(Suppl): 1–40. [PubMed] [Google Scholar]
- 10.Ryd L. Roentgen stereophotogrammetric analysis of prosthetic fixation in the hip and knee joint. Clin Ortop. 1992;276:56–65. [PubMed] [Google Scholar]
- 11.Kärrholm J, Borssen B, Lowenhielm G, Snorrason F. Does early micromotion of femoral stem prostheses matter: 4–7 year stereoradiographic follow-up of 84 cemented prostheses. J Bone Jt Surg. 1994;76-B:912–917. [PubMed] [Google Scholar]
- 12.Huiskes R, Verdonschot N, Nivbrant B. Migration, stem shape, and surface finish in cemented total hip arthroplasty. Clin Ortop. 1998;355:103–112. doi: 10.1097/00003086-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Dall DM, Learmonth ID, Solomon MI, Miles AW, Davenport JM. Fracture and loosening of charnley femoral stems. J Bone Jt Surg. 1993;75-B:259–265. doi: 10.1302/0301-620X.75B2.8444947. [DOI] [PubMed] [Google Scholar]
- 14.Rockborn P, Olsson SS. Loosening and bone resorption in Exeter hip arthroplasties. Review at a minimum of five years. J Bone Jt Surg. 1993;75-B:865–868. doi: 10.1302/0301-620X.75B6.8245072. [DOI] [PubMed] [Google Scholar]
- 15.Stefansdottir A, Franzen H, Johnsson R, Ornstein E, Sundberg M. Movement pattern of the Exeter femoral stem. A radiostereometric analysis of 22 primary hip arthroplasties followed for 5 years. Acta Orthop Scand. 2004;75:408–414. doi: 10.1080/00016470410001169. [DOI] [PubMed] [Google Scholar]
- 16.Kärrholm J, Malchau H, Snorrason F, Herberts P. Micromotion of femoral stems in total hip arthroplasty. J Bone Jt Surg. 1994;76-A:1692–1705. doi: 10.2106/00004623-199411000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Hamadouche M, Witvoet J, Porcher R, Meunier A, Sedel L, Nizard R. Hydroxyapatite-coated versus grit-blasted femoral stems. A prospective randomized study using EBRA-FCA. J Bone Jt Surg. 2001;83-B:979–987. doi: 10.1302/0301-620X.83B7.11478. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Cimbrelo E, Cruz-Pardos A, Madero R, Ortega-Andreu M. Total hip arthroplasty with use of the cementless Zweymüller Alloclassic system. A ten to thirteen year follow-up study. J Bone Jt Surg. 2003;85-A:296–303. doi: 10.2106/00004623-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Grübl A, Chiari C, Gruber M, Kaider A, Gottsauner-Wolf F. Cementless total hip arthroplasty with a tapered, rectangular titanium stem and a threaded cup. A minimum ten-year follow-up. J Bone Jt Surg. 2002;84-A:425–431. doi: 10.2106/00004623-200203000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Healy WL, Casey DJ, Iorio R, Appleby D. Evaluation of the porous-coated anatomic hip at 12 years. J Arthroplast. 2002;17:856–863. doi: 10.1054/arth.2002.34822. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura H, Dunbar MJ, Murray P, Bourne RB, Rorabeck CH. The porous coated anatomic total hip replacment. A ten to fourteen-year follow-up study of a cementless total hip arthroplasty. J Bone Jt Surg. 2001;83-A:1333–1338. [PubMed] [Google Scholar]
- 22.Keisu KS, Orozco F, Sharkey PF, Hozack WJ, Rothman RH, McGurgan FX. Primary cementless total hip arthroplasty in octogenarians. Two to eleven-year follow-up. J Bone Jt Surg. 2001;83-A:359–363. doi: 10.2106/00004623-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Kim YH. Bilateral cemented and cementless total hip arthroplasty. J Arthroplast. 2002;17:434–440. doi: 10.1054/arth.2002.31073. [DOI] [PubMed] [Google Scholar]
- 24.Nercessian OA, Wu WH, Sarkissian H. Clinical and radiographic results of cementless AML total hip arthroplasty in young patients. J Arthroplast. 2001;16:312–316. doi: 10.1054/arth.2001.21503. [DOI] [PubMed] [Google Scholar]
- 25.Tanzer M, Chan S, Brooks CE, Bobyn JD. Primary cementless total hip arthroplasty using a modular femoral component. A minimum 6-year follow-up. J Arthroplast. 2001;16:64–70. doi: 10.1054/arth.2001.29140. [DOI] [PubMed] [Google Scholar]
- 26.Kim YH, Oh SH, Kim JS. Primary total hip arthroplasty with a second generation cementless total hip prosthesis in patients younger than fifty years of age. J Bone Jt Surg. 2003;85-A:109–114. doi: 10.2106/00004623-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Keisu KS, Orozco F, McCallum JD, Bissett G, Hozack WJ, Sharkey PF, Rothman RH. Cementless femoral fixation in the rheumatoid patient undergoing total hip arthroplasty. Minimum 5-year results. J Arthroplast. 2001;16:415–421. doi: 10.1054/arth.2001.23506. [DOI] [PubMed] [Google Scholar]
- 28.Bottner F, Zawadsky M, Su E, Bostrom M, Palm L, Ryd L, Sculco TP (2005) The impact of the weight bearing protocol on implant migration after cementless total hip arthroplasty. A radistereometric analysis. Clin Ortop (accepted for publication)
- 29.Rorabeck CH, Bourne RB, Mulliken BD, Nayak N, Laupacis A, Tugwell P, Feeney D. The Nicolas Andry Award. Comparative results of cemented and cementless total hip arthroplasty. Clin Ortop. 1996;325:330–344. doi: 10.1097/00003086-199604000-00044. [DOI] [PubMed] [Google Scholar]
- 30.Thanner J. The acetabular component in total hip arthroplasty. Evaluation of different fixation principles. Acta Orthop Scand. 1999;286(Suppl):1–41. [PubMed] [Google Scholar]
- 31.Hacking SA, Bobyn JD, Tanzer M, Krygier JJ. The osseous response to corundum blasted implant surfaces in canine total hip arthroplasty model. Clin Ortop. 1999;364:240–253. doi: 10.1097/00003086-199907000-00031. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Ahn PB, Fitzpatrick DC, Heiner AD, Poggie RA, Brown TD. Interfacial frictional behavior: cancellous bone, cortical bone, and a novel porous tantalum biomaterial. J Musculoskelet Res. 1999;3:245–251. doi: 10.1142/S0218957799000269. [DOI] [Google Scholar]
- 33.Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Jt Surg. 1999;81-B:907–914. doi: 10.1302/0301-620X.81B5.9283. [DOI] [PubMed] [Google Scholar]
- 34.Amstutz HC. Innovations in design and technology: the story of hip arthroplasty. Clin Ortop. 2000;378:23–30. doi: 10.1097/00003086-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Amstutz HC, Dorey F, O’Carroll PF. THARIS resurfacing arthroplasty. Evolution and long-term results. Clin Ortop. 1986;213:92–114. [PubMed] [Google Scholar]
- 36.Ritter MA, Gioe TJ. Conventional versus resurfacing total hip arthroplasty. A long-term prospective study of concomitant bilateral implantation of prostheses. J Bone Jt Surg. 1986;68-A:216–225. [PubMed] [Google Scholar]
- 37.Tracy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Jt Surg. 2005;87-B:167–170. doi: 10.1302/0301-620X.87B2.15030. [DOI] [PubMed] [Google Scholar]
- 38.Glyn-Jones S, Gill HS, McLardy-Smith, Murray DW. Roentgen stereophotogrammetric analysis of the Birmingham hip resurfacing arthroplasty. A two year study. J Bone Jt Surg. 2004;86-B:172–176. doi: 10.1302/0301-620X.86B2.14371. [DOI] [PubMed] [Google Scholar]
- 39.Itayem R, Arndt A, Nistor L, McMinn D, Lundberg A. Stability of the Birmingham hip resurfacing arthroplasty at two years. J Bone Jt Surg. 2005;87-B:158–162. doi: 10.1302/0301-620X.87B2.15394. [DOI] [PubMed] [Google Scholar]