Abstract
Hereditary neuropathy with liability to pressure palsies (HNPP) is an uncommon diagnosis that should be considered in patients with multiple compressive neuropathies. We present the case of a woman who presented with bilateral hand numbness and weakness. Electrodiagnostic testing revealed bilateral carpal tunnel syndrome, bilateral ulnar neuropathy at the elbow, left peroneal neuropathy at the fibular head, and a primarily demyelinating generalized sensorimotor neuropathy. Subsequent genetic testing identified a deletion at chromosome 17p11.2 to confirm the diagnosis of HNPP. Treatment of this largely self-limiting disease is controversial, and this patient suffered minimal disability with treatment including splinting and surgical releases.
Key words: hereditary neuropathy with liability to pressure palsies, electrodiagnostics, compressive neuropathies, carpal tunnel syndrome, genetic testing, tomaculae
Case presentation
The patient is a 38-year-old right-handed woman who presented to her primary medical doctor with complaints of numbness in the first three digits of both hands and weakness of both hands for the previous week since performing modified push-ups at a yoga class. When carpal tunnel wrist splints and anti-inflammatories failed to significantly improve symptoms over the next 2 weeks, she was seen by a hand surgeon whose physical examination revealed several abnormalities. In addition to an atrophied left APB, she demonstrated motor deficits (3/5 L thumb abduction, 4/5 R thumb abduction, and 4+/5 L finger abduction) and sensory deficits (diminished sensation to light touch in B median nerve distributions and poor two-point discrimination in B median and ulnar nerve distributions). She was then referred to a physiatrist for electrodiagnostic testing to evaluate for possible bilateral carpal tunnel and ulnar neuropathies.
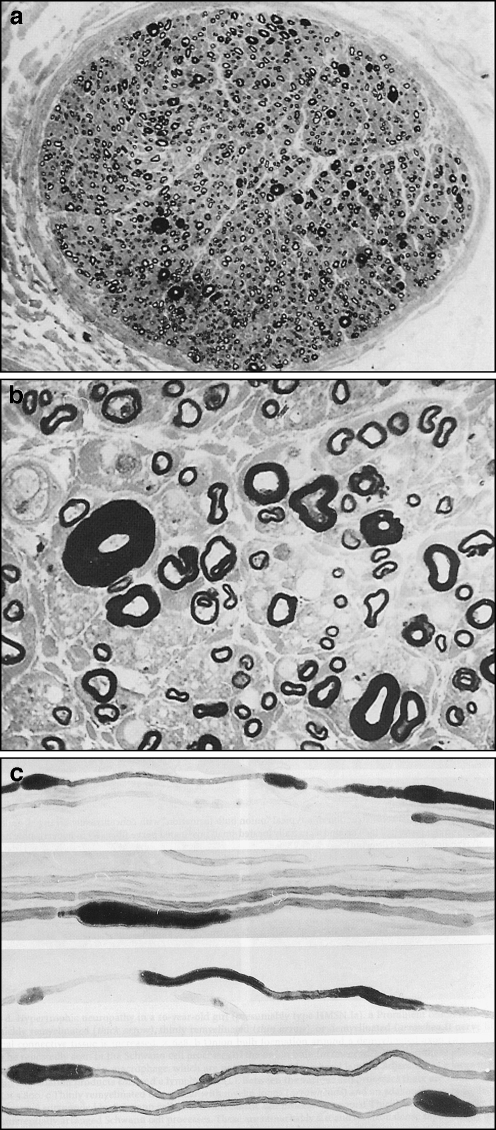
Electrodiagnostic testing performed 3 weeks after initial presentation showed many abnormalities (Tables 1 and 2). To summarize, these abnormalities included bilateral carpal tunnel syndrome, bilateral ulnar neuropathy at the elbow, left peroneal neuropathy at the fibular head, prolonged distal latencies in multiple motor nerves, slowed conduction velocities in multiple sensory nerves, multiple prolonged F-waves, and positive sharp waves in all muscles tested. The overall electrodiagnostic impression was a primarily demyelinating (but also axonal) sensorimotor peripheral polyneuropathy with multiple superimposed compression neuropathies. The patient then saw two neurologists separately for further workup.
Table 3.
EMG Report
| Side | Muscle | Nerve | Root | INS | FIBS | PSW | FAS | AMP | DUR | CONFIGURATION | Pat | REC INTCOMMENT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | APB | Median | C8-T1 | Nml | 0 | 3+ | 0 | Nml | Nml | Di/Tri phasic | Dec | Normal |
| R | APB | Median | C8-T1 | Nml | 0 | 2+ | 0 | Nml | Nml | Di/Tri phasic | Dec | Normal |
| L/R | 1stDorint | Ulnar | C8-T1 | Nml | 0 | 2+ | 0 | Nml | Nml | Di/Tri phasic | Dec | Normal |
| L/R | Nml | 0 | 2+ | 0 | Nml | Nml | Di/Tri phasic | Full | Normal | |||
| L/R | Nml | 0 | 2+ | 0 | Nml | Nml | Di/Tri phasic | Full | Normal | |||
| L/R | Nml | 0 | 2+ | 0 | Nml | Nml | Di/Tri phasic | Full | Normal | |||
| L/R | Nml | 0 | 2+ | 0 | Nml | Nml | Di/Tri phasic | Full | Normal | |||
| L/R | Nml | 0 | 2+ | 0 | Nml | Nml | Di/Tri phasic | Full | Normal |
This F-wave and electromyography data is from an electrodiagnostic study performed three weeks after the patient’s initial presentation. Prolonged latencies, spontaneous activity, and decreased recruitment are present.
Table 1.
Nerve conduction report
| Motor Nerves | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nerve | Site | Onset Lat (ms) | Norm Onset | Amplitude | Norm Amp | Duration (ms) | Seg Name | Distance (cm) | Velocity (m/s) | Norm Vel | |
| L Median | APB | OP (mv) | Full | ||||||||
| 33.0°C | Wrist | 7.03 | <4.2 | 0.82 | >5.0 | 17.86 | Proter-Wrist | 27.00 | 48.8 | >50.0 | |
| Proter | 12.56 | 0.79 | – | ||||||||
| R Median | APB | O-P (mV) | Full | ||||||||
| 32.8°C | Wrist | 5.02 | <4.2 | 6.38 | 5.0 | 12.89 | Proter-Wrist | 29.00 | 55.2 | >50.0 | |
| Proter | 10.27 | 6.26 | 13.55 | ||||||||
| L Peroncal | EDB | O-P (mV) | Full | ||||||||
| 30.8°C | Ankle | 8.86 | <5.5 | 1.90 | >2.5 | 11.25 | AbFbHd-Ankle | 29.00 | 36.2 | >40.0 | |
| AbFbHd | 16.88 | 1.20 | 11.63 | ||||||||
| L Ulnarseg | ADM | O-P (mV) | Full | ||||||||
| 32.7°C | Writst | 4.03 | <4.2 | 2.88 | >3.0 | 22.83 | AbvFCU-Wrist | 21.50 | 50.4 | >53.0 | |
| AbvFCU | 8.30 | 3.18 | – | AbUTGv-AbvFCU | 10.00 | 33.3 | >53.0 | ||||
| AbUTGv | 11.30 | 2.99 | – | ||||||||
| R UlnarSeg | ADM | O-P (mV) | Full | ||||||||
| 31.7°C | Wrist | 3.61 | <4.2 | 3.54 | >3.0 | 22.87 – – | AbvFCU-Wrist | 23.00 | 52.8 | >53.0 | |
| AbvFCU | 7.97 | 3.73 | AbUTGv-AbvFCU | 10.00 | 34.4 | >53.0 | |||||
| AbUTGv | 10.88 | 3.30 | |||||||||
| Sensory Nerves | |||||||||||
| Nerve | Site | Onst lat (ms) | Norm Onstet | Peak Lat (ms) | Amplitude | Norm Amp | Duration (ms) | Seg Name | Distance (cm) | Velocity (m/s) | Norm Vel |
| L med Palm | 2ndDig | O-P (μV) | Neg | ||||||||
| 31.4°C | MidPlm | 2.19 | 3.03 | 21.57 | – | MidPlm-2ndDig | 7.00 | 32.0 | >45.0 | ||
| Wrist | NR | NR | |||||||||
| R Med Palm | 2ndDig | O-P (μV) | Neg | ||||||||
| 33.7°C | MidPlm | 1.97 | 2.91 | 12.18 | 1.91 | MidPlm-2ndDig | 7.00 | 35.6 | >45.0 | ||
| Wrist | NR | NR | |||||||||
| L medMixed | Wrist | O-P (μV) | Neg | ||||||||
| 32.9°C | Palm | NR | NR | ||||||||
| L SpRadial | FWS | O-P (μV) | |||||||||
| 32.9°C | Forarm | 2.41 | 3.22 | 34.80 | – | Forarm-FWS | 10.00 | 41.6 | |||
| R SpRadial | FWS | O-P (μV) | Neg | ||||||||
| 32.4°C | Forarm | 2.34 | 3.03 | 18.65 | 1.31 | Forarm-FWS | 10.00 | 42.7 | |||
| L Sural | LatMal | O-P (μV) | Neg | ||||||||
| 31.1°C | Calf | 2.75 | 3.84 | 7.52 | – | Calf-LatMal | 10.00 | 36.4 | |||
| L Uln Palm | 5thDig | O-P (μV) | Neg | ||||||||
| 32.2°C | Palm | 2.88 | 3.53 | 9.88 | 1.28 | Palm-5thDig | 6.50 | 22.6 | |||
| Wrist | 4.8 | <3.4 | 5.34 | 15.27 | 2.91 | Wrist-palm | 6.00 | 40.0 | |||
| R Uln Palm | 5thDig | O-P (μV) | Neg | ||||||||
| 33.0°C | Palm | 1.56 | 2.13 | 8.76 | 1.19 | Palm-5thDig | 5.50 | 35.2 | |||
| Wrist | NR | NR | |||||||||
This nerve conduction data is from an electrodiagnostic study performed 3 weeks after the patient’s initial presentation. Multiple prolonged latencies, slowed conduction velocities, and diminished amplitudes are present.
Table 2.
F/H report
This F-wave and electromyography data is from an electrodiagnostic study performed 3 weeks after the patient’s initial presentation. Prolonged latencies, spontaneous activity, and decreased recruitment are present.
These two neurologists examined the patient approximately 5 weeks after initial presentation. (In the interim, the patient had a left carpal tunnel steroid injection performed by the hand surgeon.) They discovered a history of a weak grip, a history of clumsiness with ambulation (especially during the past year), and a mother with high-arched feet and hammertoes. Physical exam revealed tandem gait difficulty, bilateral finger abduction weakness (left worse than right), diminished ankle reflexes, and high-arched feet with hammertoes. Subsequently, genetic testing showed an abnormal PMP-22 allele caused by a deletion mutation which confirmed the diagnosis of hereditary neuropathy with liability to pressure palsies (HNPP).
After the diagnosis of HNPP, the patient continued to develop symptoms as a result of compressive neuropathies. Seven weeks after initially presenting, she developed clawing of her left fourth and fifth digits as well as a positive Froment’s sign suggesting worsening left ulnar neuropathy. At 10 weeks, she underwent left carpal tunnel and Guyon’s canal releases, which yielded significant improvement in symptoms within 1 week. Over the next few years, she experienced waxing and waning of symptoms caused by right carpal tunnel syndrome, right ulnar neuropathy, bilateral superficial radial neuropathy, peripheral neuropathy (affecting her toes bilaterally), and right suprascapular neuropathy. Currently, more than 2 years after her initial presentation, she complains of some permanent residual deficits caused by left carpal tunnel syndrome, although a recent electrodiagnostic study revealed significant axonal recovery of the left median motor nerve.
Hereditary neuropathy with liability to pressure palsies
The discovery of HNPP is credited to Dutch neuropsychiatrist J.G.Y. de Jong in 1947 after his work with a family afflicted by numerous compressive neuropathies [1]. The disease has also been called potato-grubbing disease and tulip-bulb diggers palsy because of the compressive neuropathies resulting from these activities in people with HNPP [1]. The incidence is estimated at 16/100,000, although there may be more cases that are not diagnosed [2]. The hallmark of the disease is recurrent focal compression neuropathies, especially of the median, ulnar, and peroneal nerves, which tend to improve in hours to months [3]. Usually, onset of symptoms is between the ages of 10 and 30 [4]. Interestingly, muscle wasting is sometimes observed in the absence of previous nerve palsy [5]. Also, cases of brachial plexus involvement have been documented [6]. Subtle findings which may be present on physical exam include a mild peripheral neuropathy, absent ankle reflexes (in 50–80%), and pes cavus foot deformities (in 20%) [7].
Patients suffering from possible entrapment neuropathies typically undergo electrodiagnostic testing. In HNPP patients, this testing will often reveal decreased sensory and motor conduction velocities across many of the aforementioned entrapment sites. In addition, prolonged distal motor nerve conduction latencies with slowed distal sensory nerve conduction velocities will demonstrate a peripheral neuropathy. There are presently no universally accepted electrodiagnostic criteria for the diagnosis of HNPP. Studies by Gouider et al. [8] in 1995 and Mouton et al. [4] in 1999 demonstrated that diagnostic criteria of bilateral slowing of sensory and motor nerve conduction at the carpal tunnel with at least one abnormal parameter for motor conduction in one peroneal nerve is sufficient for diagnosis in patients with a family history. In 2001, Infante et al. [9] preferred sural nerve slowing and any two entrapments as criteria for diagnosis.
HNPP’s electrodiagnostic profile is unique. Andersson et al. [10] in 2000 compared electrodiagnostic features of HNPP with diabetic polyneuropathy and chronic inflammatory neuropathy (CIDP), two diseases which have similar clinical presentations. He found HNPP patients to have more distal sensory nerve conduction velocity slowing and distal motor latency prolongation than patients with diabetic polyneuropathy or CIDP. Li et al. [11] in 2002 found HNPP distal sensory nerve conduction slowing to be a more diffuse process than the distal motor latency prolongation which has a predilection for certain nerves. Finally, whereas HNPP should be considered in patients with multiple entrapment neuropathies, an electrodiagnostic study by Sander et al. [12] in 2005 of patients with multiple surgically treated entrapment neuropathies demonstrated the low prevalence of the disease.
While electrodiagnostic examination is an easy and reliable method for determining which patients may have HNPP, molecular genetic testing is the most reliable tool for diagnosis [4, 13]. The disease is an autosomal dominant condition caused by a defect at chromosome 17p11.2 that results in abnormal peripheral myelin protein 22. In approximately 80% of cases, the defect is a 1.5 Mb deletion, whereas the other 20% has a point mutation. Both the deletion and point mutation are detectable by a variety of sophisticated genetic tests [7]. Interestingly, a 1.5-Mb duplication at the same locus as the deletion produces Charcot-Marie-Tooth 1A which shares some clinical and histologic features with HNPP. The two diseases are thought to be reciprocal products of the same unequal crossover [2]. Studies have reported that the deletion and point mutation percentages may vary considerably by ethnicity [14].
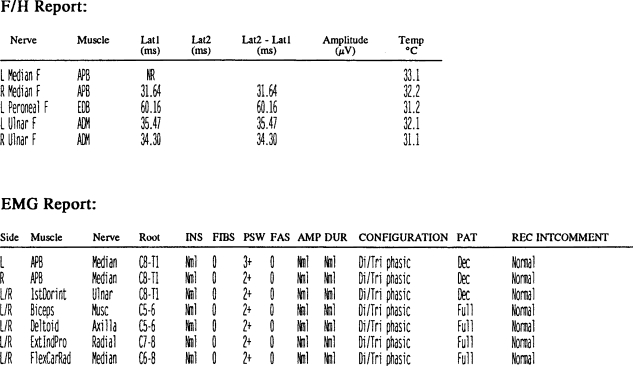
HNPP nerves have characteristic histopathologic features. It is understood that peripheral myelin protein 22 is responsible for producing compact and stable myelin sheets around large and small myelinated nerve fibers. However, the abnormal peripheral myelin protein 22 present in HNPP results in paradoxically thickened myelin sheaths in the large myelinated fibers [15]. These thickenings are focal, amounting to segmental hypermyelination and appear in teased fiber preparations as sausage-like expansions of myelinated fibers called tomaculae (Fig. 1). The term “tomaculous neuropathy” describes this condition which is present in diseases including HNPP, CMT, CIDP, IgM paraproteinemia, HNA, and Dejerine–Sottas [8].
Fig. 1.
a This low-power magnification photomicrograph of an axial section of a nerve shows scattered large myelinated fibers that have thickened myelin sheaths representing tomaculae (reprinted from Pathology of Peripheral Nerves, 2001, page 116, Chap 7, J.M. Schroeder, Figure 101a, copyright Springer, Heidelberg, with kind permission of Springer Science and Business Media). b This high-power magnification photomicrograph of the same nerve in a shows a detailed view of a tomaculous fiber with marked thickening of the myelin sheath and narrowing of the axonal cross-sectional area (×600 magnification, reprinted from Pathology of Peripheral Nerves, 2001, page 116, Chap 7, J.M. Schroeder, Figure 101b, copyright Springer, Heidelberg, with kind permission of Springer Science and Business Media). c These low-power magnification photomicrographs of teased fiber preparations of isolated nerve fibers show discontinuous tomaculous distentions mainly localized at paranodal regions (×140 magnification, reprinted from Pathology of Peripheral Nerves, 2001, page 116, Chap 7, J.M. Schroeder, Figure 101c–f, copyright Springer, Heidelberg, with kind permission of Springer Science and Business Media)
The abnormal peripheral myelin in HNPP nerves also increases susceptibility to injury by compression, resulting in the reduction of conduction velocities associated with compression neuropathies [16, 17]. The effects of trauma may also induce degeneration of the myelin sheaths with subsequent remyelination, resulting in hypertrophic nerves with the classic onion-bulb features of demyelinating diseases. In many cases, the effects of the compression are short-lived and relatively reversible. In some chronic cases, the muscles innervated by damaged nerves (in this case, those of the left hand from chronic carpal tunnel syndrome) are likely to show evidence of denervation and atrophy. Historically, HNPP tomaculae and onion-bulbing have been observed with sural nerve biopsy, but biopsies are now rarely performed because of the utility of genetic testing [18].
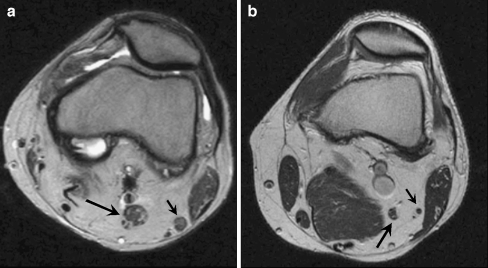
The imaging of HNPP has been poorly described in the literature, although there is an ultrasound case report describing nerve hypertrophy in a patient with HNPP [19]. Magnetic resonance imaging (MRI) is currently useful for demonstrating hypertrophic nerves in diseases such as CMT (Fig. 2). Because the pathology of HNPP, like CMT, leads to hypertrophic nerves, MRI can be expected to detect the nerve enlargement in HNPP patients. Conversely, HNPP should be considered in the differential diagnosis of patients who present with hypertrophic nerves by imaging. Interestingly, MRI has also shown subclinical central nervous system demyelination associated with HNPP [20, 21; http://www.emedicine.com/neuro/topic718.htm].
Fig. 2.
a This axial knee MRI of a patient with Charcot-Marie-Tooth disease demonstrates diffuse enlargement of the tibial nerve (long arrow) and common peroneal nerve (short arrow). b This axial knee MRI demonstrates a normal tibial nerve (long arrow) and common peroneal nerve (short arrow)
Prevention of compression neuropathies such as carpal tunnel syndrome, ulnar neuropathy, and peroneal neuropathy includes activity modification and protective padding. Activity modification such as avoiding repetitive hand movements and minimizing time spent leaning on elbows, crossing legs, and kneeling can be combined with protective elbow and knee pads [7].
When compressive symptoms develop, orthoses such as wrist splints and ankle–foot orthoses may be utilized transiently for relief of carpel tunnel symptoms and foot drop, respectively. Full recovery in hours to months occurs in the majority of episodes, whereas incomplete recovery is somewhat common but rarely debilitating. Incomplete recovery correlates with a history of prolonged focal compression. Controversy exists as to the benefit of surgical decompression. Typically, carpal tunnel release is of little benefit, and ulnar nerve transposition at the elbow may worsen symptoms. However, in cases with severe symptoms, debility, and/or deformity, surgical intervention should be considered on an individual basis and in this patient appears to have been beneficial.
References
- 1.Koehler PJ (2003) Hereditary neuropathy with liability to pressure palsies: the first publication (1947). Neurology 60(7):1211–1213 [DOI] [PubMed]
- 2.Mertogja P, Silander K, Kalimo H et al (1997) Epidemiology of hereditary neuropathy with liability to pressure palsies in south western Finland. Neuromuscul Disord 7(8):529–532 [DOI] [PubMed]
- 3.Koc F, Guzel R, Benliday IC et al (2006) A rare genetic disorder in the differential diagnosis of the entrapment neuropathies: hereditary neuropathy with liability to pressure palsies. J Clin Rheumatol 12(2):78–82 [DOI] [PubMed]
- 4.Mouton P, Tardieu S, Gouider R et al (1999) Spectrum of clinical and electrophysiologic features in HNPP patients with the 17p11.2 deletion. Neurology 52(7):1440–1446 [DOI] [PubMed]
- 5.Dubourg O, Mouton P, Brice A, et al (2000) Guidelines for diagnosis of hereditary neuropathy with liability to pressure palsies. Neuromuscul Disord 10:206–208 [DOI] [PubMed]
- 6.Crum BA, Sorenson EJ, Abad GA, et al (2000) Fulminant case of hereditary neuropathy with liability to pressure palsy. Muscle Nerve 23:970–983 [DOI] [PubMed]
- 7.Bird TD (2005) Hereditary neuropathy with liability to pressure palsies. Gene reviews: http://www.geneclinics.org/profiles/hnpp/details.html
- 8.Gouider R, LeGuern, E, Gugenheim M, et al (1995) Clinical, electrophysiologic, and molecular correlations in 13 families with hereditary neuropathy with liability to pressure palsies and a chromosome 17p11.2 deletion. Neurology 45(11):2018–2023 [DOI] [PubMed]
- 9.Infante J, Garcia A, Combarros O et al (2001) Diagnostic strategy for familial and sporadic cases of neuropathy associated with 17p11.2 deletion. Muscle Nerve 24(9):1149–1155 [DOI] [PubMed]
- 10.Andersson PB, Yuen E, Parko K et al (2000) Electrodiagnostic features of hereditary neuropathy with liability to pressure palsies. Neurology 54(1):40 [DOI] [PubMed]
- 11.Li J, Krajewski K, Shy ME et al (2002) Hereditary neuropathy with liability to pressure palsy: the electrophysiology fits the name. Neurology 58(12):1769–1773 [DOI] [PubMed]
- 12.Sander MD, Abbasi D, Ferguson AL (2005) The prevalence of hereditary neuropathy with liability to pressure palsies in patients with multiple surgically treated entrapment neuropathies. J Hand Surg (Am) 30(6):1236–1241 [DOI] [PubMed]
- 13.Lane JE, Foulkes GD, Hope TD et al (2001) Hereditary neuropathy with liability to pressure palsies mimicking multifocal compression neuropathy. J Hand Surg 26A:670–674 [DOI] [PubMed]
- 14.Bissar-Tadmouri, N et al (2000) Mutational analysis and genotype/phenotype correlation in Turkish Charcot-Marie-Tooth Type 1 and HNPP patients. Clin Genet 58(5):396–402 [DOI] [PubMed]
- 15.Li J, Krajewski K, Lewis RA et al (2004) Loss-of-function phenotype of hereditary neuropathy with liability to pressure palsies. Muscle Nerve 29:205–210 [DOI] [PubMed]
- 16.Rizzuto N, Moretto G, Galiazzo Rizzuto S (1993) Clinical spectrum of the tomaculous neuropathies. Report of 60 cases and review of the literature. Ital J Neurol Sci 14(9):609–617 [DOI] [PubMed]
- 17.Pellissier JF, Pouget J, de Victor B et al (1987) Tomaculous neuropathy. A histopathological study and electroclinical correlates in 10 cases. Rev Neurol (Paris) 143(4):263–278 [PubMed]
- 18.Stogbauer F, Young P, Kuhlenbaumer G, et al (2000) Hereditary recurrent focal neuropathies: clinical and molecular features. Neurology 54(3):546 [DOI] [PubMed]
- 19.Beekman R, Visser LH (2002) Sonographic detection of diffuse peripheral nerve enlargement in hereditary neuropathy with liability to pressure palsies. J Clin Ultrasound 30:433–436 [DOI] [PubMed]
- 20.Sanahuja J, Franco E, Rojas-Garcia R et al (2005) Central nervous system involvement in hereditary neuropathy with liability to pressure palsies: description of a large family with this association. Arch Neurol 62(12):1911–1914 [DOI] [PubMed]
- 21.Amato AA, Barohn RJ (1996) Muscle Nerve 19(6):770–773 [DOI] [PubMed]