Abstract
The diagnosis of a postoperative myocardial infarction (PMI) is important in the orthopedic population because these events can be associated with significant cardiac morbidity. Plasma troponin I (cTnI) analysis has markedly increased our ability to detect myocardial damage. Using cTnI analysis for evidence of a PMI, we prospectively assessed all of our patients for (1) the 1-year incidence of PMI, (2) the clinical consequences of a PMI in relation to the level of the cTnI release, and (3) 6-month follow-up for cardiac complications. During a 12-month period, patients at risk for perioperative myocardial ischemia were assessed for a PMI by serum cTnI levels and daily serial ECGs. Patients with cTnI levels above the reference level (≥0.4 ng/ml) were also assessed for new cardiac regional wall motion abnormalities with an echocardiogram and 6-month postdischarge adverse cardiac events. Of the 758 patients who were assessed for a PMI, 49 patients had detectable cTnI levels (≥0.4 ng/ml); the incidence of a PMI was 0.6% of all surgical cases and 6.5% of those patients were at risk for a cardiac event. A PMI was more common after hip arthroplasty than other orthopedic procedures. Twenty-three patients had a cTnI level >3.0 ng/ml, and 74% these patients (17/23) had anginal symptoms and/or ischemic ECG changes. Nine of these patients (9/23) had new postoperative echocardiographic changes, five (5/23) required emergency transfer to a cardiac care unit, and 10 (10/23) had postoperative cardiac complications. In contrast, 15 patients with levels of cTnI <3.0 ng/ml and without ischemic ECG changes and/or anginal symptoms had no postoperative cardiac complications. Fourteen patients (14/47) had cardiac complications 6 months after discharge, including four cardiac deaths, one fatal stroke, and four patients with unstable anginal episodes that required a change in medical management, and six patients required coronary revascularization. Orthopedic surgical patients with cTnI level <3 ng/ml and without symptoms or ECG changes suggestive of myocardial ischemia (15/49) may have different risks than those with higher-level cTn1.
Key words: postoperative myocardial infarction, troponin levels
Introduction
Many orthopedic patients, particularly arthroplasty patients, are elderly and have multiple medical comorbidities. These patients are routinely evaluated for postoperative myocardial damage, “rule-out myocardial infarction” (ROMI). In the past, perioperative myocardial infarctions (PMI) were difficult to diagnose because perioperative ischemic episodes are often clinically “silent,” and objective signs (biochemical markers and ECG changes) do not always provide definitive evidence of a PMI [1, 2]. The diagnosis of a PMI is important, however, because these events can be associated with significant cardiac morbidity and mortality if not treated appropriately [1, 3]. In addition, the decision to initiate postoperative physical therapy and rehabilitation, which is imperative for a favorable outcome in orthopedic patients, or to, instead, undergo invasive cardiac evaluation may depend on the correct diagnosis of a PMI.
The introduction of plasma troponin I (cTnI) analysis has markedly increased our ability to detect myocardial damage because plasma elevations in this protein are highly specific for cardiac injury and cTnI has been shown to be a more specific marker for a PMI than creatine kinase MB isoenzyme (CKMB) after orthopedic surgery [4, 5]. Using cTnI analysis for evidence of postoperative myocardial damage (PMI), we prospectively assessed all of our patients at risk for perioperative myocardial ischemia for (1) the 1-year incidence of a PMI, (2) the clinical consequences of a PMI in relation to the level of the cTnI release, and (3) 6-month follow-up for cardiac complications.
Methods
At our orthopedic institution, during a 12-month period (2002–2003), 758 of ∼7,600 surgical patients with risk factors for perioperative myocardial ischemia, new onset of postoperative arrhythmias, or postoperative symptoms of myocardial ischemia were assessed for a PMI. Preoperative risk factors for perioperative myocardial ischemia included prior MI, prior cardiac revascularization, a stress test positive for myocardial ischemia, anginal symptoms, and treatment by a cardiologist or internist for atherosclerotic cardio-vascular disease. The majority of the 7,600 surgical procedures were elective orthopedic procedures in which the patient was hospitalized for at least 24 h and included 22% total hip arthroplasty (THA), 16% total knee arthroplasty (TKA), 20% posterior lumbar spine fusion/decompression, 11% total shoulder arthroplasty, and 9% open reduction internal fixation of the ankle. Symptoms of postoperative myocardial ischemia are defined as those indicative of congestive heart failure (CHF: acute dyspnea, orthopnea) or anginal equivalents. Serum cTnI levels were assayed every 12 h for 24 h or until the cTnI level fell below 0.4 ng/ml. Daily ECGs were performed for the first three postoperative days or until the cTnI level normalized. A monoclonal antibody analysis was used to measure cTnI by a Baxter Stratus II analyzer (Baxter Healthcare, Round Lake, IL, USA). In those patients with cTnI levels ≥0.4 ng/ml, an echocardiogram (ECHO) was performed within 48 h of the cTnI elevation (postoperative days 3 to 5). All echocardiograms were interpreted by a cardiologist blinded to the level of cTnI release for regional wall motion abnormalities (RWMAs: akinesis or dyskinesis) not present on a preoperative ECHO. For patients without a preoperative history of a myocardial infarction who did not have a preoperative echocardiogram (24/49), RWMAs on the postoperative ECHO were judged to be new ischemic changes. In addition, all patients with a cTn1 ≥0.4 ng/ml were assessed by a cardiologist. All of the patients with elevated cTn1 levels were followed throughout their hospital course for evidence of postoperative cardiac complications, including CHF, new onset of arrhythmias, and hemodynamic instability requiring transfer to a cardiac care unit (CCU).
Six-month postdischarge adverse cardiac events were assessed via two methods: patients were contacted by phone and a questionnaire regarding their cardiac status was faxed to their medical physician. Adverse cardiac events were cardiac death; unstable angina or myocardial infarction requiring a stress test and a change in medical therapy; and coronary revascularization, either angioplasty or a coronary bypass graft procedure (CABG).
Data were analyzed using a STAT View Program Chi-square and student paired t test, p < 0.05 significant. The association between the occurrence of adverse outcomes was examined using a test of their correlation coefficient.
Results
Of the 758 patients who were assessed for a PMI, 49 patients had detectable cTnI levels (≥0.4 ng/ml). Table 1 shows the demographic characteristics of these patients. The patients were elderly (74 ± 11 years). The majority of the patients (∼80%) had undergone elective lower-extremity arthroplasty: total hip arthroplasty (THA) 36/49, 73%, and total knee arthroplasty (TKA) 3/49, 6% of the patients. During the 1-year study period, a THA represented 22% and a TKA 16% of the total surgical cases. All patients who underwent both types of arthroplasty at HSS had similar demographic characteristics. The odds ratio for a PMI for a THA was 9.8; an instrumented posterior spine fusion 4.3 and a TKA 0.3 compared to all the in-patient surgical cases. Many of these patients were treated with β-blockers (39%), but we were unable to demonstrate a protective effect of β-blockers with regard to postoperative cardiac complications. Active ischemic heart disease (IHD), either by a history of anginal symptoms or medical diagnosis, was common (25/49, 51%) among the patients with cTnI elevations. Of the characteristics listed in Table 1, only a preoperative stress test positive for myocardial ischemia (12/49, 24%) correlated positively with the peak level of cTnI release (12.1 vs. 4.5 ng/ml; p = 0.01).
Table 1.
Patients with serum cTnI ≥0.4 ng/ml
| Variable | Value |
|---|---|
| Patients (n) | 49 |
| Age (years) | 74 ± 11 |
| Weight (kg) | 75 ± 15 |
| HTN (%) | 49 |
| DM (%) | 18 |
| IHD (%) | 51 |
| MI (%) | 16 |
| Pos ST (%) | 24 |
| Revasc (%) | 22 |
| β-blocker (%) | 39 |
| Surgery (n) | |
| THA | 36 |
| TKA | 3 |
| LE | 5 |
| PSF | 4 |
| TSR | 1 |
HTN = hypertension; DM = diabetes militias; IHD = ischemic heart disease; MI = previous myocardial infarction; Pos ST = reversible ischemia on a preoperative stress test; Revasc = previous coronary revascularization, angioplasty, or CABG; THA = total hip arthroplasty; TKA = total knee arthroplasty; LE = lower-extremity surgery; PSF = posterior spinal fusion; TSR = total shoulder arthroplasty
Postoperatively, of the patients with elevated cTnI levels, 39% (19/49) had anginal symptoms, 41% (20/49) had ECG changes indicative of myocardial ischemia, and 18% (9/49) had new RWMAs on ECHO (Table 2). Of 758 patients entered into the ROMI protocol, 134 exhibited postoperative anginal symptoms; an odds ratio for symptomatic vs. asymptomatic patients to have a PMI was 2.1. The ischemic period for 79% (15/19) of these symptomatic patients was from postoperative day (POD) 2 to POD-5.
Table 2.
Postoperative characteristics of patients with elevated cTnI
| Patients | % | cTnI Levels | p | ||
|---|---|---|---|---|---|
| + | − | ||||
| ECG changes | 20 | 41 | 9.2 | 4.5 | 0.1 |
| Ischemic symptoms | 19 | 39 | 6.2 | 6.6 | 0.9 |
| New RWMAs | 9 | 18 | 22.4 | 2.8 | 0.001 |
| Postop cardiac comp | 18 | 37 | 9.4 | 4.6 | 0.1 |
| CCU transfer | 6 | 37 | 14.4 | 5.3 | 0.03 |
| Follow-up (n) | 8.6 | 5.5 | 0.34 | ||
| CABG/angioplasty | 6 | 13 | |||
| Unstable angina | 4 | 9 | |||
| Cardiac death | 4 | 9 | |||
ECG changes: new q waves. T-inversions or ST changes not on the preoperative ECG. New regional wall motion abnormalities on ECHO. Postoperative cardiac complications: CHF, arrhythmias, hemodynamically unstable. +/− indicate cTnI levels in patients with (+) or without (−) the indicated postoperative characteristic
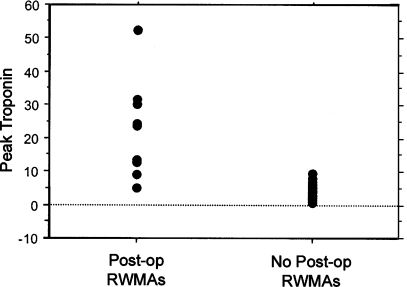
The mean peak cTnI postoperative level for all 49 patients was 6.4 ng/ml (range 0.4–52 ng/ml). Nine patients with new RWMAs (9/49) had significantly higher peak cTnI levels than patients without RWMAs (22.4 vs. 2.8 ng/ml; Fig. 1). There was also a significant correlation between new RWMAs and a major postoperative cardiac event (cty cor coef 7.4, p = 0.007). These nine patients were also more likely to have postoperative anginal symptoms (70 vs. 35%) and ischemic ECG changes (100 vs. 31%) than the patients with only elevated cTnI levels. In four of the nine patients, unstable hemodynamics required transfer to a CCU, and two patients required emergency cardiac catheterization and revascularization. There were no deaths. One patient with elevated cTnI levels (peak 5 ng/ml) and new RWMA on ECHO sustained a cardiac arrest in the operating room after the cementing of a femoral prosthesis during THA. This patient was successfully resuscitated and had ST-T ECG changes that resolved, new RWMAs on day 3, and an uncomplicated postoperative course.
Fig. 1.
Scattergram of peak cTnI levels in patients with and without new regional wall motion abnormalities (RWMAs) on a postoperative echocardiogram
Twenty-three patients had a cTnI level >3.0 ng/ml, and the majority of these patients (74%; 17/23) had postoperative anginal symptoms and/or ischemic ECG changes. All of the patients with new RWMAs had cTnI levels >3 ng/ml. In addition, five of the six patients who required transfer to the CCU had a cTnI level >3 ng/ml. The one patient with a peak cTnI level <3.0 ng/ml (1.1 ng/ml) who required transfer to the CCU for the management of CHF had both severe aortic stenosis and IHD. Of the remaining 26 patients with cTnI levels <3.0 ng/ml, 11 (42%, 11/26) had ischemic ECG changes and/or ischemic symptoms and 8 had postoperative cardiac complications; this is in contrast to the 15 patients with levels of cTnI <3.0 ng/ml (58%, 15/26) without ischemic ECG changes and/or anginal symptoms who had no postoperative cardiac complications.
We were able to contact 47 of the 49 patients with elevated cTnI levels ∼6 months after surgery. Fourteen patients (14/47) had post-hospital-discharge cardiac complications. There were four cardiac deaths, one patient had a fatal stroke, four patients had unstable anginal episodes that required a change in medical management, and six patients required coronary revascularization. Three of these patients had new RWMAs postoperatively and one required transfer to the CCU. There was no significant difference in the troponin levels of patients with or without post-hospital-discharge cardiac complications (8.6 vs. 5.5, p = 0.34) and four patients had postoperative cTnI levels <3 ng/ml.
Discussion
The diagnosis of a myocardial infarction after orthopedic surgery has important clinical implications: initiation of the rehabilitation and physical therapy, which is an integral part of a successful orthopedic procedure, or institution of bed rest; ICU care; and/or cardiac catheterization/revascularization. The diagnosis of a PMI is dependent on “ischemic” symptoms, ECG changes, and the elevation of cardiac-specific enzymes. However, postoperative myocardial ischemia is often clinically silent and the ECG changes are often nonspecific [6]. Furthermore, false elevations of CPKMB have been reported after orthopedic procedures [7, 8]. Elevations in serum troponin are not only more specific than CPK-MB for the diagnosis of a myocardial infarction, but they are also more sensitive in detecting myocardial injury [4, 5, 8, 9].
The recommendation of the Joint European Society of Cardiology/American College of Cardiology Committee is that the detection of troponins in the serum above the upper reference limit is indicative of myocardial injury [10]. Using this guideline, the incidence of myocardial injury as assessed by cTnI serum levels ≥0.4 ng/ml in this orthopedic population for 1 year was 0.6%. If one includes only patients with known IHD, risk factors for IHD, or postoperative ischemic symptoms, then the incidence increases to about 6.5% (49/758). This incidence is consistent with previous published reports for patients undergoing noncardiac surgery and lower than that reported for high-risk vascular surgical patients [11–14]. A 10-year retrospective study of 10,244 primary TKR and THR patients from the Mayo Clinic revealed a 30-day PMI incidence of 0.4% [15]. We previously published a PMI incidence of 3.8% in patients at risk for IHD undergoing TKA [11]. Martinez et al., using cTnI elevation as a marker for PMI, reported an incidence of 11% in noncardiac (mostly vascular patients) at risk for IHD [16]. Barbagallo et al., using cTnI elevations, also reported a 12% PMI incidence after major noncardiac vascular surgery [17].
With the addition of a more sensitive test for myocardial injury, two questions must be addressed: (1) is there a threshold below which an elevated cTn1 would be considered inconsequential and (2) what is the prognostic value of an elevated cTn1 in the postoperative period? In nine of our patients (9/49) with elevated cTn1 levels, we were also able to demonstrate functional cardiac damage on the basis of new RWMAs. These patients had the highest peak Tronponin levels, the majority sustained postoperative cardiac complications, and all had peak cTn1 levels ≥5 ng/ml. If a combination of ECG ischemic changes and/or anginal symptoms and a cTnI peak >3.0 ng/ml is set as the definition of a PMI in this population, then 17 patients (17/49) would be included. These 17 patients would include all of the patients with RWMAs and five of the six patients transferred to a CCU. The incidence of a PMI in this orthopedic population in patients at risk for perioperative myocardial ischemia would then be 2.2% (17/758).
Our in-hospital data support the hypothesis that orthopedic surgical patients with a postoperative troponin I level less than 3 ng/ml and without symptoms or ECG changes suggestive of myocardial ischemia (15/49) may have different risks than those with higher-level cTn1 releases accompanied by ECG changes and symptoms because none of these patients had postoperative cardiac complications and all were discharged without further problems. There is support for this hypothesis in the surgical literature. In the report by Admas et al. [7], a strong concordance was demonstrated between a PMI based on new RWMAs and a cTn1 level above 3 ng/ml. In his study of the 100 patients who did not exhibit postoperative RWMAs, only one had a cTn1 level greater than 3 ng/ml. In the report of Martinez et al. [16], a receiver operator curve was constructed for cTnI and a value of ≥2.6 ng/ml was consistent with a definitive PMI in their noncardiac but mostly vascular population. Postoperative patients with cTnI levels ≥2.6 ng/ml had the highest incidence of ischemic ECG changes and cardiac symptoms. In 229 vascular surgical patients, 42% of the patients had peak postoperative cTn1 levels ≥0.4 ng/ml, but a cTn1 level >1.5 ng/ml (12%) was associated with 27-fold increased risk of a PMI and a cTn1 level >3.0 ng/ml with significantly greater risk of death [12]. Recently, troponin release (>0.4 ng/ml) has been shown to be a common finding of patients who survive severe trauma [18]. Evidence for myocardial damage, however, was only apparent in those patients with troponin levels >2 ng/ml that were sustained for longer than 36 h.
Total hip arthroplasty (THA) patients were significantly overrepresented in the group of patients who sustained a PMI. During this 1-year analysis, patients undergoing a THA were 30× more likely to have a PMI than those patients undergoing TKA. The age distributions for both types of arthroplasty were similar; however, we do not have a comparison of patient comorbidities between procedures. The majority of patients for both procedures received an epidural anesthetic (all in those patients with a troponin ≥0.4 ng/ml), but for THA, the mean arterial pressure was reduced to 50–65 mm Hg during the surgery. This anesthetic technique of controlled hypotension may have increased the risk of perioperative cardiac events in patients with significant IHD, although previous evaluation of this technique has not been implicated in postoperative cardiac complications [19]. An increased incidence of elevated cTnI levels, however, was not seen in our posterior spinal fusion patients who often receive controlled hypotensive general anesthesia. Alternatively, the surgical procedure, THA, which increases the risk of pulmonary complications through emboli, may impose increased physiological stress on the patient leading to an increased incidence of myocardial ischemia in patients at risk [20].
In this study, a significant number of patients (14/49; 30%) with postoperative cTnI elevations had serious cardiac complications within 6 months of discharge from the hospital. There was, however, no significant correlation between follow-up cardiac complications and absolute cTnI levels, postoperative in-hospital cardiac complications, and postoperative RWMAs. Highman et al. [13] reported a significant correlation between postoperative elevated cTn1 levels and cardiac complications to 1 year after vascular and orthopedic surgery. In their study, 12 of 152 surgical patients had elevated cTn1 levels, and 7 of these patients had follow-up cardiac complications. Kim et al. [12] also reported a strong correlation between postoperative elevated troponin levels and 6-month cardiac mortality. In contrast, Godet et al. [21, 22] reported a positive correlation between postoperative cTn1 release and in-hospital cardiac events, but not 1-year cardiac complications. Because we did not follow the 709 ROMI patients without cTn1 elevations, we cannot comment on the risk of discharge cardiac events in ROMI-negative patients.
There are several limitations to this report. Because we did not compile complete demographic data on all 758 patients entered into the ROMI protocol, we cannot assess the association of preoperative risks and postoperative cTn1 levels. It was assumed that patients without a preoperative ECHO, prior MI, or history of cardiac dysfunction would have a normal resting ECHO and that postoperative RWMAs would constitute new changes. However, it is possible that some of these patients may have had silent small MIs in the past that could have affected our interpretation of “new” RMWAs.
We also cannot conclude that an in-hospital cTn1 release increases a patient’s risk for post-hospital-discharge cardiac events because we did not track the discharge course of patients without cTn1 elevations. Although this study and other published reports of cTn1 release after noncardiac surgery suggest that low-level cTn1 release may be relatively benign, more patients are required to substantiate this hypothesis [23].
Conclusions
Our in-hospital data support the hypothesis that orthopedic surgical patients with cTnI level <3 ng/ml and without symptoms or ECG changes suggestive of myocardial ischemia (15/49) may have different risks than those with higher-level cTn1. However, these patients are still at risk for post-hospital-discharge cardiac complications.
Footnotes
This study was funded by the Department of Anesthesiology, Hospital for Special Surgery, New York, NY.
References
- 1.Mangano DT, Hollenberg M, Fegert G et al (1991) Perioperative myocardial ischemia in patients undergoing noncardiac surgery-I. Incidence and severity during the 4 day perioperative period. J Am Coll Cardiol 17:843–850 [DOI] [PubMed]
- 2.Graeber GM (1985) Creatine kinase (CK): its use in the evaluation of perioperative myocardial infarction. Surg Clin North Am 65:539–551 [DOI] [PubMed]
- 3.Mangano DT (1990) Perioperative cardiac morbidity. Anesthesiology 72:153–184 [DOI] [PubMed]
- 4.Adams JE, Bodor GS, Davila-Roman VG et al (1993) Cardiac troponin I: a marker with high specificity for cardiac injury. Circulation 88:101–106 [DOI] [PubMed]
- 5.Jules-Elysee K, Urban MK, Urquhart B et al (2001) Troponin as a diagnostic marker of a perioperative myocardial infarction in the orthopedic population. J Clin Anesth 13:556–560 [DOI] [PubMed]
- 6.Knight AA, Hollenberg M, London MJ et al (1988) Perioperative myocardial ischemia: importance of the preoperative ischemic pattern. Anesthesiology 68:681–688 [DOI] [PubMed]
- 7.Adams JE, Sigard GA, Allen BT (1994) Diagnosis of perioperative myocardial infarction with measurement of cardiac Troponin I. N Engl J Med 330:670–674 [DOI] [PubMed]
- 8.Neil F, Sear JW, French G et al (2000) Increases in serum concentrations of cardiac proteins and the prediction of early postoperative cardiovascular complications in noncardiac surgery patients. Anaesthesia 55:641–647 [DOI] [PubMed]
- 9.Apple FS, Falahati A, Paulsen PR et al (1997) Improved detection of minor ischemic myocardial injury with measurement of serum cardiac troponin I. Clin Chem 43:2047–2051 [PubMed]
- 10.The Joint European Society of Cardiology/American College of Cardiology Committee (2000) Myocardial infarction redefined. J Am Coll Cardiol 36:959–969 [DOI] [PubMed]
- 11.Urban MK, Markowitz SM, Gordon MA et al (2000) Postoperative prophylactic administration of β-adrenergic blockers in patients at risk for myocardial ischemia. Anesth Analg 90:1257–1261 [DOI] [PubMed]
- 12.Kim LJ, Martinez A, Faraday N et al (2002) Cardiac Troponin I predicts short-term mortality in vascular surgical patients. Circulation 106:2366–2371 [DOI] [PubMed]
- 13.Hingham H, Sear JW, Sear YM et al (2004) Peri-operative troponin I concentration as a marker of long-term postoperative adverse cardiac outcomes—A study in high risk surgical patients. Anaesthesia 59:318–323 [DOI] [PubMed]
- 14.Manach YL, Perel A, Coriat P et al (2005) Early and delayed myocardial infarction after abdominal aortic surgery. Anesthesiology 102:885–891 [DOI] [PubMed]
- 15.Mantilla CB, Horlocker TT, Schroeder DR et al (2002) Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology 96:1140–1146 [DOI] [PubMed]
- 16.Martinez EA, Nass CM, Jermyn RM et al (2005) Intermittent cardiac troponin-I screening is an effective means of surveillance for a perioperative myocardial infarction. J Cardiothorac Vasc Anesth 19:577–582 [DOI] [PubMed]
- 17.Barbagallo M, Casati A, Spadini E et al (2006) Early increases in cardiac troponin levels after major vascular surgery is associated with an increased frequency of delayed cardiac complications. J Clin Anesth 18:280–285 [DOI] [PubMed]
- 18.Edouard AR, Felten ML, Herbert JL (2004) Incidence and significance of cardiac troponin I release in severe in severe trauma patients. Anesthesiology 101:1262–1268 [DOI] [PubMed]
- 19.Urban MK, Urquhart B (1993) Is controlled hypotensive anesthesia safe for elderly patients undergoing total hip arthroplasty. Anesthesiology A167
- 20.Urban MK, Sheppard R, Gordon MA et al (1996) Right ventricular function during revision total hip arthroplasty. Anesth Analg 82:1225–1229 [DOI] [PubMed]
- 21.Godet G, Dumerat M, Baillard C et al (2000) Cardiac troponin I is a reliable marker for immediate but not medium-term cardiac complications after abdominal aortic repair. Acta Anaesthesiol Scand 5:592–597 [DOI] [PubMed]
- 22.LeManach YL, Perel A, Coriat P et al (2005) Early and delayed myocardial infarction after abdominal aortic surgery. Anesthesiology 102:885–891 [DOI] [PubMed]
- 23.Agewall S, Lowbeer C (2005) The new definition of myocardial infarction—Can we use it? Clin Cardiol 28:77–80 [DOI] [PMC free article] [PubMed]