Abstract
Management of acute postoperative pain is challenging, particularly in patients with preexisting narcotic dependency. Ketamine has been used at subanesthetic doses as a N-methyl d-aspartate (NMDA) receptor antagonist to block the processing of nociceptive input in chronic pain syndromes. This prospective randomized study was designed to assess the use of ketamine as an adjunct to acute pain management in narcotic tolerant patients after spinal fusions. Twenty-six patients for 1–2 level posterior lumbar fusions with segmental instrumentation were randomly assigned to receive ketamine or act as a control. Patients in the ketamine group received 0.2 mg/kg on induction of general anesthesia and then 2 mcg kg−1 hour−1 for the next 24 hours. Patients were extubated in the operating room and within 15 minutes of arriving in the Post Anesthesia Care Unit (PACU) were started on intravenous patient-controlled analgesia (PCA) hydromorphone without a basal infusion. Patients were assessed for pain (numerical rating scale [NRS]), narcotic use, level of sedation, delirium, and physical therapy milestones until discharge. The ketamine group had significantly less pain during their first postoperative hour in the PACU (NRS 4.8 vs 8.7) and continued to have less pain during the first postoperative day at rest (3.6 vs 5.5) and with physical therapy (5.6 vs 8.0). Three patients in the control group failed PCA pain management and were converted to intravenous ketamine infusions when their pain scores improved. Patients in the ketamine group required less hydromorphone than the control group, but the differences were not significant. Subanesthetic doses of ketamine reduced postoperative pain in narcotic tolerant patients undergoing posterior spine fusions.
Key words: ketamine, postoperative pain
Introduction
Acute pain management is challenging in patients with preoperative narcotic dependency after spinal fusion surgery. In addition, opioid-induced hyperalgesia [1] and acute opioid tolerance [2] may be partially responsible for acute postoperative pain refractory to traditional doses of narcotics. Both the persistent nociceptive and neuropathic pain, which these patients experience, and opioid-induced hyperalgesia may in part be mediated through N-methyl-d-aspartate (NMDA) receptors.
Ketamine is a phencyclidine derivative used initially as a “dissociative anesthetic”. Ketamine is also a noncompetitive NMDA receptor antagonist [3]. The role of ketamine as a NMDA antagonist in blocking the processing of nociceptive input has led to its use in the treatment of chronic pain syndromes [4]. At subanesthetic doses, ketamine has been found useful in the management of acute pain in some, [5] but not in all studies [6]. This prospective randomized study was designed to assess the use of ketamine as an adjunct to acute pain management in narcotic tolerant patients after spinal fusions.
Material and methods
With Institutional Review Board approval, after written informed consent, 26 patients for 1–2 level posterior lumbar fusions with segmental instrumentation were randomly (computer-generated random numbers table; assigned group concealed in an opaque envelop, which was not opened until the patient signed the consent) assigned to receive ketamine or act as a control. The patients and physical therapists assessing pain and milestones were blinded to the assigned group, but the Post Anesthesia Care Unit (PACU) nurses and physicians were cognitive of the groups. Patients scheduled for elective lumber fusions and who were taking narcotics on a regular schedule daily (> 60 mg equivalent oxycodone) until the time of surgery were included in the study. After written informed consent was obtained, patients received a general endotracheal anesthetic, which consisted of midazolam 5 mg, 70% nitrous oxide, 0.4% isoflurane, fentanyl at 1–2 mcg kg−1 h−1, and propofol at 70–100 mg/h. Spinal preservative free morphine (10 mcg/kg) was administered at instrumentation, one to 2 hours before closure [7]. Patients in the ketamine group received 0.2 mg/kg on induction of general anesthesia and then 2 mcg kg−1 h−1 until discharge from the PACU, goal of 24 hours of treatment. Patients were extubated in the operating room and within 15 minutes of arriving in the PACU they were started on intravenous hydromorphone in a patient-controlled analgesia (PCA) modality, 0.5 mg/ml without a basal infusion (initial settings: 1 ml bolus every 5 minutes). Hydromorphone boluses were administered by the PACU physician assistant every 5 minutes until the NRS pain scale was ≤5; patients who remained at an NRS=10 despite an hour of hydromorphone boluses were started on a ketamine infusion. Any patient who remained at an NRS=10 after 2 hours was excluded from the study and treated with alternative medications.
Postoperative pain was assessed for 48 hours using a verbal Numerical Rating Scale (NRS) at rest and during physical therapy; a NRS of 0 was defined as no pain and a 10 defined as the worst pain ever experienced. On POD 1 and 2, the NRS for pain was recorded by a physical therapist blinded to the study group just before therapy (rest) and during therapy. The volume of intravenous hydromorphone administered was downloaded from the PCA pump. The level of sedation was monitored using a five-point scale, 1 awake and 5 arousable only with vigorous stimulation. The treatment of nausea and vomiting was also recorded for each patient. Delirium was evaluated using the confusion assessment method before discharge from the PACU on POD 1 [8]. Preoperative oral analgesic medications were restarted on postoperative day 1. Patients were assessed for physical therapy milestones (out of bed; walking) by a physical therapist.
Prior experience with narcotic tolerant patients after spinal fusions provided us with a mean first 24-hour hydromorphone requirement of 31 mg with a standard deviation of 16 mg; if one assumed that ketamine therapy would reduce hydromorphone intake by 30%, with a power of 80 and significance of p ≤ 0.05, then we required a total of 20 patients, 10 per group. Statistical analysis of the data was performed using a STAT View Program, with differences between groups analyzed with a t test and box graphs; a probability of ≤0.05 was significant.
Results
Twenty-six patients were enrolled in the study. All of the patients were narcotic tolerant, but utilized different opioid preparations to provide analgesia. Two patients were eliminated from analysis; one patient from the control group who remained intubated and ventilated for 12 hours after surgery and one patient from the ketamine group who remained at a NRS pain scale of 10 for over 2 hours and received alternative analgesics. Of the remaining 24 patients (12 in the control and 12 in the ketamine group) there were no differences in patient demographics, duration of surgery or intraoperative fentanyl administration (Table 1). Patients in the study group received ketamine for a mean of 24 ± 8 hours.
Table 1.
Patient demographics
| Ketamine | Control | |
|---|---|---|
| Patients (n) | 12 | 12 |
| Age (y) | 53 ± 12 | 48 ± 9 |
| Weight (kg) | 80 ± 19 | 78 ± 19 |
| Height (cm) | 168 ± 10 | 165 ± 10 |
| Duration of Surgery (h) | 4.8 ± 1 | 4.5 ± 0.9 |
| ASA Status (1:2:3) | 0:10:2 | 1:10:1 |
| Preop Oral Narcotics (n)a | ||
| Methadone | 1 | 1 |
| OxyContin | 2 | 3 |
| Actiq | 1 | 1 |
| Fentanyl Patch | 2 | 1 |
| MS Contin | 0 | 2 |
| Percocet (10/325) | 8 | 8 |
| Vicodin (5/500) | 3 | 1 |
| Intraop Fentanyl mcg/kg | 13 ± 6 | 12 ± 3 |
aNumber of patients taking each type of narcotic analgesic preoperatively.
Patients in the ketamine group had significantly less pain during their first postoperative hour in the PACU and continued to have less pain during the first postoperative day (POD) at rest and with physical therapy (Table 2). These differences in pain scores were no longer apparent on POD 2. Three patients in the control group failed the initial pain management, defined as remaining at a NRS of 10 for the first 2 hours after surgery despite repeated postoperative intravenous hydromorphone boluses. These three patients were converted to intravenous ketamine infusions and their pain scores were reduced to NRS of 7, 5 and 5, respectively, 1 hour after the initiation of the infusion. Data are presented as intention to treat, therefore those patients that crossed over are still analyzed in the control group.
Table 2.
Postoperative pain management
| Pain Medication | Hours | Hours |
|---|---|---|
| NRS 1 h in PACU | 4.8 ± 3 | 8.7 ± 1* |
| Mean 24 h NRS | 2.6 ± 1.6 | 3.7 ± 1.2* |
| NRS POD 1 Rest | 3.6 ± 2 | 5.5 ± 2* |
| NRS POD1 PT | 5.6 ± 3 | 8.0 ± 2* |
| NRS POD 2 Rest | 2.7 ± 3 | 3.0 ± 2 |
| NRS POD2 PT | 4.7 ± 3 | 5.3 ± 1 |
| Hours on PCA | 60 ± 28 | 66 ± 34 |
| PCA Hydromorphone mg | ||
| 1st 24 h | 18.5 ± 14 | 27 ± 10 |
| 24–48 h | 15.5 ± 8 | 20.5 ± 13 |
Mean hydromorphone PCA, in milligram, infused during the first postoperative 24 hours and 24–48 hours
Mean NRS for the first 24 hours, including periods when the patients were sleeping, was assessed as 0. Mean Hydromorphone PCA in mg infused during the first postoperative 24 hours and 24–48 hours; *p ≤ 0.05.
NRS: numerical rating scale for pain; NRS Rest: in bed before physical therapy; NRS PT, during physical therapy
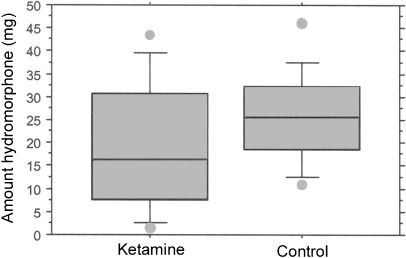
We did not find a significant difference in i.v. PCA hydromorphone use between the two groups (Table 1; Fig. 1). There was, however, considerable variability in hydromorphone usage and even with this variability, the ketamine group used less i.v. hydromorphone than the control group.
Fig. 1.
Box graphs of total dose of intravenous hydromorphone (mg) infused for the first 24 hours of PCA (patient controlled analgesia); line in box at the mean
Two patients randomized to the control group received intraoperative ketamine, but not postoperative ketamine. As both patients had NRS scores similar to the other control patients on POD1 (6 at rest and 8 with PT), their 24-hour hydromorphone consumption was higher than the mean for the ketamine group (20 and 23 mg), and they did not receive postoperative ketamine; the data was analyzed with the control group.
Two patients in the ketamine group and one patient in the control group became confused on POD 1, none with hallucinations, and in all three cases the confusion resolved by POD 2. Eight patients were treated for postoperative nausea and vomiting during the first 48 hours, three in the control group, and five in the ketamine group. The mean sedation score for both groups during the first postoperative 24 hours was 2.2. All of the patients except for three were advanced to solid food by POD 2, one in the ketamine group, and two in the control group. The majority of the patients were out of bed and ambulating by POD 3, but six patients were delayed in their rehabilitation, three in the ketamine group, and three in the control group.
Discussion
Effective management of acute postoperative pain is challenging, particularly in patients with preexisting narcotic dependency. The delivery of large doses of narcotics to these patients does not always provide adequate analgesia and can contribute to the development of severe hyperalgesia [9]. Furthermore, the administration of large doses of narcotics in the perioperative period can have multiple side effects including: ventilatory depression, sedation, nausea and vomiting, pruritus, urinary retention, ileus, and delayed hospital discharge. Even the patient’s satisfaction with the management of their pain does not appear to be addressed with opioids as the sole analgesic intervention (Joint Commission on Accreditation of Heathcare Organizations). It is for these reasons and the evidence for the modulation of pain perception as a complex process involving multiple neural pathways that many clinicians now advocate a multimodal therapeutic approach to pain [10].
There is extensive evidence that NMDA receptors are at least partially involved in this complex pain modulation process [11]. Ketamine is an intravenous anesthetic with analgesic properties, which are partially mediated via its action as a noncompetitive NMDA receptor antagonist [3]. Anesthetic use of ketamine has decreased because of its psychotomimetic side effects, particularly postoperative confusion and hallucinations [12]. However, subanesthetic doses of ketamine have been shown to be efficacious in the treatment of chronic neuropathic pain [4].
In this report, we demonstrated that subanesthetic doses of ketamine reduced postoperative pain in narcotic-tolerant patients undergoing posterior spine fusions. We choose to assess the efficacy of ketamine in postoperative pain management in narcotic tolerant patients because in these patients’ central sensitization mechanisms involving NMDA receptors should have been activated. Furthermore, perioperative pain management in these patients is difficult and often not manageable with narcotics alone. In three control patients, we were unable to reduce their pain to acceptable levels without the administration of ketamine. We were, however, unable to demonstrate a significant reduction in hydromorphone utilization in the ketamine group. Furthermore, none of the other outcome parameters that were selected (nausea, vomiting, sedation, physical therapy milestones) differed between the two groups.
Several previous studies have also reported the use of ketamine as an adjuvant to opioids in the treatment of acute postoperative pain [5, 13]. The Cochrane review of ketamine for postoperative pain reported in 27 of 37 reviewed trials ketamine reduced opioid analgesic requirements or pain intensity, or both [14]. Four of these trials included a preincisional bolus of ketamine (10 mg, 0.15 mg/kg, 0.5 mg/kg) and a continuous infusion of ketamine (10 mg/h and 2 mcg kg−1 h−1) for 24 postoperative hours [2, 14–18]. The four trials included major abdominal surgery. The ketamine group was compared to i.v. PCA morphine alone for pain scores and cumulative morphine administration. All four trials demonstrated that ketamine was morphine sparring. In only one of the trials were the VAS pain scores reduced and only at 1 hour postoperatively.
The Cochrane analysis of 705 patients treated with ketamine and 578 controls, demonstrated a significant reduction in postoperative nausea and vomiting in the ketamine group [14]. However, in the review of postoperative pain management with ketamine, Elia et.al. [19] reported that ketamine did not reduce morphine-related adverse effects. In this report, we were unable to demonstrate a reduction in narcotic related adverse effects with ketamine. Both groups had similar levels of sedation and treated nausea and vomiting. However, the addition of subarachnoid morphine to both groups in our study may have contributed to the increased level of sedation and incidence of nausea and vomiting [20].
The problematic psychotomimetic effects seen with anesthetic doses of ketamine were not apparent in this study or others with subanesthetic doses used for postoperative analgesia [6, 21]. Whereas some investigations have reported memory and cognitive impairment after subdissociative doses of ketamine [22], others have reported improved postoperative mood and function after perioperative administration of ketamine [23]. Furthermore, postoperative perceptual disturbances are common in the absence of ketamine [24]. Our study was not designed or powered to detect these effects of ketamine.
Limitations This study was designed to demonstrate a 30% reduction in hydromorphone use with the addition of ketamine. Although we achieved the reduction, the difference in ketamine utilization between the two groups was not significant because of the large standard deviation within each group. Our second goal was to improve postoperative analgesia in the narcotic tolerant patient; this goal was achieved in the ketamine group. As both groups required large doses of intravenous narcotics, however, we were unable to demonstrate a reduction in the adverse effects of opioids with the addition of ketamine.
Conclusions
Perioperative infusion of subanesthetic ketamine was effective in reducing pain in narcotic-tolerant patients after posterior spinal fusions. It reversed unacceptable levels of pain in patients resistant to conventional narcotic treatment.
Footnotes
This work was funded by the Department of Anesthesia, Hospital for Special Surgery.
This work was completed following IRB approval.
References
- 1.Mao J, Price DD, Mayer DJ (1995) Mechanisms of hyperalgesia and morphine tolerance; a current view of their possible interactions. Pain 62:259–274 [DOI] [PubMed]
- 2.Guignard B, Coste C, Costes H et al (2002) Supplementing desflurane-remifentanil anesthesia with small-dose ketamine reduces perioperative opioid analgesic requirements. Anesth Analg 95:103–108 [DOI] [PubMed]
- 3.Kohrs R, Durieux ME (1998) Ketamine; teaching an old drug new tricks. Anesth Analg 87:1186–1193 [DOI] [PubMed]
- 4.Hocking G, Cousins MJ (2003) Ketamine in chronic pain management: an evidence based review. Anesth Analg 97:1730–1739 [DOI] [PubMed]
- 5.Kock MD, L’homme P, Waterloos H (2001) Balanced analgesia in the perioperative period: is there a place for ketamine? Pain 92:373–380 [DOI] [PubMed]
- 6.Jarvey KB, Ussery TW, Steger HG et al (1996) Comparison of morphine and morphine with ketamine for postoperative analgesia. Can J Anaesth 43:212–215 [DOI] [PubMed]
- 7.Urban MK, Jules-Elysee K, Urquhart B et al (2007) Reduction in postoperative pain after spinal fusion with instrumentation using intrathecal morphine. Spine 27:535–537 [DOI] [PubMed]
- 8.Inuoye SK, Van Dyck CH, Alessi CH et al (1990) Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 113:941–948 [DOI] [PubMed]
- 9.Mao J (2002) Opioid-induced abnormal pain sensitivity; implications in clinical opioid therapy. Pain 100:213–217 [DOI] [PubMed]
- 10.Kehlet H, Werner M, Perkins F (1999) Balanced analgesia: what is it and what are its advantages in postoperative pain? Drugs 58:793–797 [DOI] [PubMed]
- 11.Price DD, Mayer DJ, Mao J et al (2000) NMDA-receptor antagonists and opioid receptor interactions as related to analgesia and tolerance. J Pain Symptom Manage 19(Suppl 1):7–15 [DOI] [PubMed]
- 12.White PF, Way WL, Trevor AJ (1982) Ketamine: its pharmacology and therapeutic uses. Anesthesiology 56:119–136 [DOI] [PubMed]
- 13.Schmid RL, Sandler AN, Katz J (1999) Use and efficacy of low-dose ketamine in the management of acute postoperative pain; a review of current techniques and outcomes. Pain 82:111–125 [DOI] [PubMed]
- 14.Bell RF, Dahl JB, Moore RA et al (2005) Peri-operative ketamine for acute post-operative pain: a quantitative and qualitative systematic review (Cochrane review). Acta Anesthesiol Scand 49:1405–1428 [DOI] [PubMed]
- 15.Stubhaug A, Brievik H, Eide PK et al (1997) Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anesthesiol Scand 41:1124–1132 [DOI] [PubMed]
- 16.Illkjaer S, Nikolajsen L, Hansen TM et al (1998) Effect of i.v. ketamine in combination with epidural bupivacaine and morphine on postoperative pain and wound tenderness after renal surgery. Br J Anaesth 81:707–712 [DOI] [PubMed]
- 17.Guillou N, Tanguy M, Sequin P et al (2003) The effects of small-dose Ketamine on morphine consumption in surgical intensive care unit patients after major abdominal surgery. Anesth Analg 97:843–847 [DOI] [PubMed]
- 18.Argiriadou H, Himmelscher S, Papagiannopoulau P et al (2004) Improvement of pain treatment after major abdominal surgery by intravenous S+-ketamine. Anesth Analg 98:1413–1418 [DOI] [PubMed]
- 19.Elia N, Tramer MR (2005) Ketamine and postoperative pain: a quantitative systematic review of randomized trials. Pain 113:61–70 [DOI] [PubMed]
- 20.Urban MK, Jules-Elysee K, Urquhart B et al (2002) Reduction in postoperative pain after spinal fusion with instrumentation using intrathecal morphine. Spine 27:535–537 [DOI] [PubMed]
- 21.Kudoh A, Takahira Y, Katagai H et al (2002) Small-dose ketamine improves the postoperative state of depressed patients. Anesth Analg 95:114–118 [DOI] [PubMed]
- 22.Wolf K, Winstock AR (2006) Ketamine: from medicine to misuse. CNS Drugs 20:199–218 [DOI] [PubMed]
- 23.Mortero RF, Clark LD, Tolan MM et al (2001) The effects of small dose ketamine on propofol sedation: respiration, postoperative mood, perception, cognition, and pain. Anesth Analg 92:1465–1469 [DOI] [PubMed]
- 24.Reeves M, Lindholm DE, Myles PS et al (2001) Adding ketamine to morphine for patient-controlled analgesia after major abdominal surgery: a double-blinded, randomized controlled trial. Anesth Analg 93:116–120 [DOI] [PubMed]