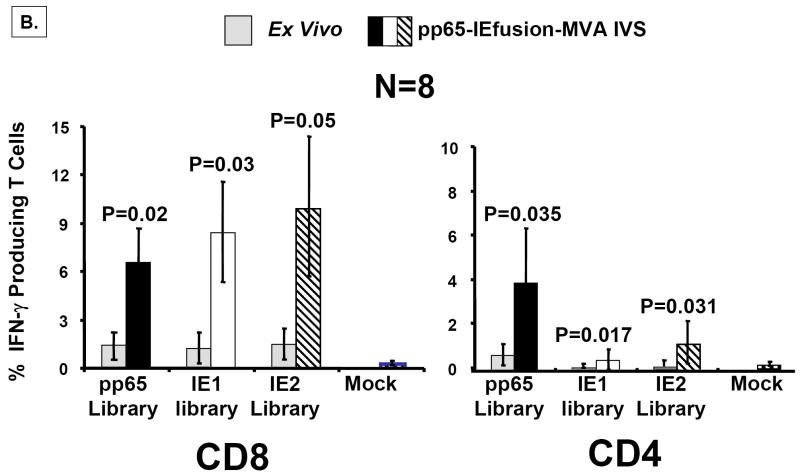
Figure 6. rMVA stimulates CMV-specific T cells in human PBMC.
A) Using the IEfusion MVA, as described in Materials and Methods, APC were infected for 5-6 hours, irradiated, and then co-incubated with unmanipulated PBMC from the autologous individual. The time course and conditions of the IVS are described in Materials and Methods. Four separate evaluations were conducted with each IVS culture as shown in Panel A. After treatment with the peptide library, and ICC was performed, and aliquots of PBMC were either stained with CD4 or CD8 antibodies as described in Figure 5A. Results shown are averages of measurements from three CMV-positive individuals selected randomly from our cohort of blood donors. Not shown is a comparison with a CMV-negative donor who showed no specific recognition of any of the three peptide libraries after IVS with IEfusion- and pp65-IEfusion-MVA. B) Similar to A), but using the pp65-IEfusion-MVA, IVS was carried out using conditions identical as in A) and fully described in Materials and Methods. PBMC from 8 healthy CMV-positive blood donors were evaluated both ex vivo without manipulation and post-IVS following infection with rMVA as described in A). Statistical differences between ex vivo levels of CMV-specific T Cells versus post-IVS were calculated as described in Materials and Methods, and when a P-value is ≤0.05, it is shown above the error bar for each evaluation of individual peptide libraries. All methods for IVS, ICC, and flow cytometry are described in Materials and Methods.