Abstract
We examine the dynamics of parasitemia and gametocytemia reflected in the preintervention charts of 221 malaria-naive U.S. neurosyphilis patients infected with the St. Elizabeth strain of Plasmodium vivax, for malariatherapy, focusing on the 109 charts for which 15 or more days of patency preceded intervention and daily records encompassed an average 98% of the duration of each infection. Our approximations of merogony cycles (via “local peaks” in parasitemia) seldom fit patterns that correspond to “textbook” tertian brood structures. Peak parasitemia was higher in trophozoite-induced infections than in sporozoite-induced ones. Relative densities of male and female gametocytes appeared to alternate, though without a discernably regular period. Successful transmission to mosquitoes did not depend on detectable gametocytemia or on absence of fever. When gametocytes were detected, transmission success depended on densities of only male gametocytes. Successful feeds occurred on average 4.7 days later in an infection than did failures. Parasitemia was lower in homologous reinfection, gametocytemia lower or absent.
Plasmodium vivax asexual blood-stage cycles are authoritatively cited as displaying a period of 48 hr (Cox, 1993; Reisberg, 1997). Hence, a single-brood P. vivax infection would be expected to produce peaks of asexual parasitemia (in the form of merozoites) and corresponding fevers on alternate days and a 2-brood infection to produce daily (quotidian) peaks of parasitemia and fever. Whorton et al. (1947) attributed their observations of irregular or steady fevers to irregular parasite segmentation. Kitchen (1949) agreed, noting that the earliest phases of patent P. vivax infections might be marked by “continuous fever caused by more or less constant sporulation,” but argued that “the great majority of the vivax plasmodia are segregated into two pyrogenic broods … whose corresponding developmental stages are separated by approximately twenty-four hours.” Coatney et al. (1971) found P. vivax as often tertian as quotidian and remarked that “if the infection is allowed to run, it is not unusual for multiple broods to get-in-step resulting in a tertian fever pattern; likewise, tertian patterns, sometimes, become quotidian for a time, only to revert to tertian again.” Garnham (1966) reported that “the process of schizogony in P. vivax is never of clockwork regularity.” According to Shute (1958), “the true explanation is that the cycle depends on the patient and not on the parasite,” meaning, in particular, that quotidian behavior marks the initial infection in a malaria-naive patient, whereas tertian behavior typifies relapses and subsequent infections.
A companion paper on trophozoite-induced P. malariae infections addressed some of these phenomena (McKenzie et al., 2001). With the P. vivax charts, they can be addressed in the context of sporozoite- as well as trophozoite-induced infections. Inoculation modes may influence subsequent events: sporozoite-induced infections will show longer prepatency and incubation, of course, and, with P. vivax, only sporozoite-induced infections can produce the hypnozoites from which long-term relapses may arise, but there may be more subtle differences as well. For instance, Coatney et al. (1971) noted that after 7 wk of patency, at roughly the same levels, asexual parasitemia dropped more rapidly in sporozoite-induced than in trophozoite-induced P. vivax infections. Boyd (1938, 1940b) found that peak parasitemia was higher and occurred earlier, that parasitemia at the final fever was higher, and that the mean duration of the febrile phase of infection was shorter in trophozoite-induced infections. He also wrote (Boyd, 1941) that “artificially induced infections in human subjects do not exhibit the chronicity which characterizes the naturally induced infections.” Our previous work on uninterrupted P. falciparum infections indicated that, with all but a non-gametocyte-producing variant of 1 strain, the mean duration of detectable primary parasitemia was greater in the sporozoite- than in the trophozoite-induced infections (Collins and Jeffery, 1999).
The initial pyrogenic density of P. vivax is considerably lower than that of P. falciparum, and parasitemia at the final fever is said to be similar to that at the first fever >104 F (Boyd, 1938, 1944). Plasmodium vivax infections are characterized by relatively low parasitemia and pathogenicity, generally attributed to a restriction to the youngest red blood cells, i.e., its “merozoites can only invade reticulocytes” (Gilles, 1993). The canonical studies of the species drew more cautious conclusions in this regard, however, e.g., stating that P. vivax “merozoites selectively invade reticulocytes” (Shushan et al., 1937) or “show a greater tendency to invade immature red cells than mature erythrocytes” (Kitchen, 1938), and the most recent appraisals show similar reserve (Galinski and Barnwell, 1996; Simpson et al., 1999).
Gametocyte production in P. vivax may start with the first generation of merozoites; gametocytes become infective within 2–3 days and survive perhaps 3 days more (Carter and Graves, 1988). They are said to become patent about 5 days after the asexual forms (Fairley, 1947; Garnham, 1966). Malaria-naive patients often show near-continuous gametocytemia, and near-continuous infectivity, for the first 2 mo of patency, i.e., before, during, and after the period of fevers and peak parasitemia (Boyd, 1949). Gametocyte density is said to peak about 4–6 days after the peak parasitemia and then decline gradually; gametocytemia is usually reported as <400–700 mm−3, only rarely as exceeding 2,000–4,000 mm−3 (Boyd, Stratman-Thomas, and Muench, 1936; Basu, 1947; Garnham, 1966; Sattabongkot et al., 1991). However, “production of gametocytes is not a uniformly continuous affair, but is a process that rhythmically recurs in a ‘shower-like’ manner after completion of a certain fairly definite number of schizogonous divisions … upward trends appear with great regularity at approximately 5-day intervals” (Boyd, Stratman-Thomas, and Muench, 1936). As with most of its congeners, P. vivax gametocyte sex ratios are typically female-biased (Carter and Graves, 1988; Read et al., 1995), with macrogametocyte densities typically about twice those of microgametocytes (Boyd, 1942a). As with P. malariae (McKenzie et al., 2001), the infectivity of P. vivax may vary widely (Boyd et al., 1935; Boyd, 1942a): it seems at most loosely coupled to gametocyte density, and, as one would expect, gametocyte density seems more closely related to mean oocyst density per mosquito than to the proportion of mosquitoes infected (Knowles and Basu, 1944; Gamage-Mendis et al., 1991; Sattabongkot et al., 1991).
The present paper takes advantage of a unique opportunity to address several unresolved questions about relationships between P. vivax parasitemia, fever, gametocytemia, and infectivity by examining the preintervention dynamics of trophozoite-and sporozoite-induced infections in malaria-naive U.S. neurosyphilis patients infected with a well-characterized strain of the parasite.
MATERIALS AND METHODS
Materials and methods are described in detail in a companion paper on P. malariae dynamics (McKenzie et al., 2001), which includes information on malariatherapy treatment procedures, the patient population, and relevant ethical issues. The present study and many others would have been impossible without the participation of hundreds of malariatherapy patients, to whom we are extremely grateful.
The St. Elizabeth strain of P. vivax was isolated in 1937 and was used continuously at the South Carolina USPHS facility until it closed in 1963. Through at least its first 3 yr there, it was characterized as reliable in clinical presentation, fever and gametocyte production, with relapses “decidedly infrequent” (Coatney and Young, 1941). We reviewed the charts of the 221 adult neurosyphilis patients, with no history of previous malaria infection, whose infections with this strain began between September 1942 (patient S-325) and September 1960 (patient S-1335).
Parasitological and clinical data collection
Parasitemia, gametocytemia, and patient rectal temperature were determined during each infection as described previously (McKenzie et al., 2001). We analyzed only those parts of charts that preceded any intervention; such interventions were by drugs for 204 patients (8 after “spontaneous clearance”). Daily records of parasitemia and gametocytemia were available for 88–100% (mean 98%) of the days of each infection, following initial patency; isolated absences or omissions by staff led to occasional blank daily records in charts. Because fever records are available on an hourly (vs. only daily) basis for most of these P. vivax patient charts, their detailed fever dynamics will be the focus of a separate paper.
Data presentation
As with the P. malariae charts (McKenzie et al., 2001), or any truncated time series, the values of some timing-related variables are confounded by dependence on the number of days of preintervention infection observed. This dependence declined much more slowly than in the P. malariae charts, however, and r2 values did not decline monotonically or uniformly across the variables; hence, we found no single clear cutoff point. Linear regression (Sokal and Rohlf, 1981) of the day of peak parasitemia and the day of peak fever on the number of preintervention days observed gave overall r2 values of 0.09 and 0.04, respectively. For the day of peak gametocytemia, r2 values for the 2 male and 1 female categories (see following text) were 0.29, 0.47, and 0.49 overall. The transition point at day 14–15 offered the best combination, with r2 values dropping from 0.49 to 0.40 for the day of peak parasitemia and from 0.28 to 0.22 for the day of peak fever. So we focused our analyses on the 109 patient charts with 15 or more days of patency before intervention (Fig. 1; Tables I, II).
Figure 1.
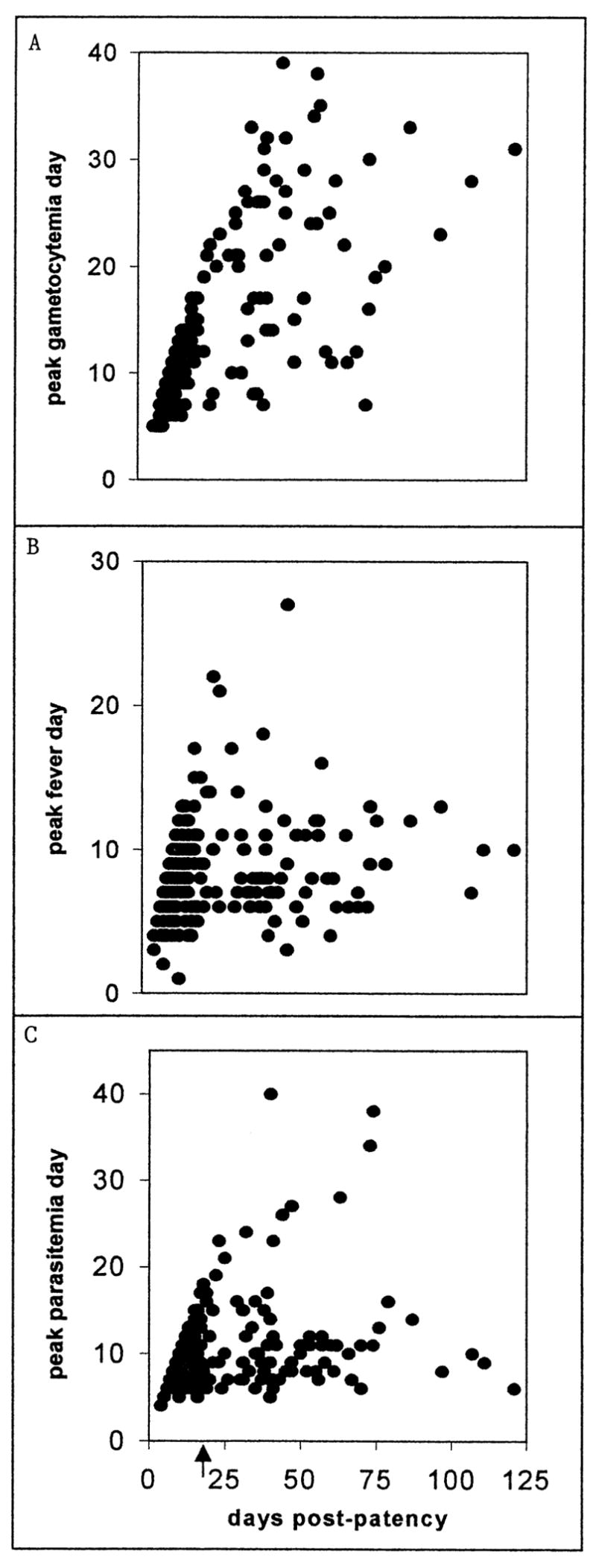
The day of patent infection on which the peak gametocyte density (A), peak fever (B), and peak asexual-form density (C) occurred (vertical axes) plotted against the overall number of days of patent infection before intervention in the corresponding individual chart (horizontal axis). The arrow indicates day 14 of patency (see text).
Table I.
Summary statistics for charts from the 48 sporozoite-induced and 61 trophozoite-induced infections with more than 14 days of observed preintervention patency. Values of magnitude variables are given as absolute numbers, per cubic millimeter densities (mm−3), or degrees Farenheit; values of timing variables (days) are given as relative to the first day of patency (day 1). If 2 or more prospective peaks of equal magnitude occurred in a chart, the timing is considered to correspond to the earliest. Separate sections refer to 66 charts in which gametocytes of both sexes were recorded (and days on which males and females were detected either simultaneously or alone) and 30 in which only male gametocytes were recorded (see Materials and Methods). Abbreviations: minimum, Min; maximum, Max; standard deviation, Std Dev; percent, % recorded, Rec; gametocytes, Gam; male, M; female, F.
| Sporozoite/trophozoite charts | Min | Max | Median | Mean | Std Dev |
|---|---|---|---|---|---|
| Preintervention (days) | 15/15 | 121/97 | 31/38 | 36.8/39.1 | 25.4/21.2 |
| First asexuals (mm−3) | 10/10 | 180/416 | 20/20 | 29.6/45 | 33.7/66.8 |
| First asexual peak (day) | 2/2 | 11/11 | 6/6 | 6/5.8 | 2.4/2.1 |
| Peak asexuals (day) | 5/6 | 34/40 | 10/11 | 11.4/12.3 | 5.9/6.8 |
| Peak asexuals (mm−3) | 693/704 | 20,376/24,668 | 8,131/10,500 | 8,675/11,069 | 4,964/4,718 |
| First fever (day) | 1/1 | 6/6 | 2/2 | 2.5/2 | 1.5/1 |
| First fever (F) | 101/101 | 105.6/105.6 | 103/102.8 | 103/102.9 | 1.3/1.2 |
| Peak fever (day) | 3/4 | 22/27 | 8/9 | 9/9.7 | 4.1/3.9 |
| Peak fever (F) | 101.2/104.6 | 107.2/107.2 | 106/106 | 105.8/106.1 | 0.9/0.5 |
| Gam—both sexes rec | |||||
| Simultaneous M and F rec (days) | 1/1 | 40/29 | 4/7 | 6.7/10.2 | 6.2/8.1 |
| % Days simultaneous M and F rec | 3/2 | 58/74 | 25/24 | 24/27 | 18/19 |
| Only M rec (days) | 1/1 | 6/25 | 1/3.5 | 2.3/7.2 | 2.1/7.6 |
| % Days only M rec | 2/2 | 16/54 | 6/17 | 6/16 | 4/15 |
| Only F rec (days) | 1/1 | 10/19 | 4/17 | 4.3/7 | 2.6/4.8 |
| % Days only F rec | 2/2 | 56/67 | 13/22 | 17/20 | 13/14 |
| First M gametocytes (day) | 7/5 | 27/26 | 12/9 | 13/10.5 | 5.3/4.5 |
| First M gam (mm−3) | 10/10 | 70/140 | 21/16 | 21.9/20.8 | 16.8/22.4 |
| First F gam (day) | 4/5 | 25/27 | 10/9 | 12/10.5 | 6.2/5.9 |
| First F gam (mm−3) | 10/10 | 140/140 | 35/24 | 44.8/33.6 | 37.1/30.8 |
| Peak M gam (day) | 7/7 | 31/43 | 17/16 | 18.1/19.6 | 6.4/9.4 |
| Peak M gam (mm−3) | 10/10 | 350/735 | 40/49 | 71.2/71.7 | 77.9/111.2 |
| Peak F gam (day) | 8/7 | 28/43 | 17/17 | 18/20.5 | 5.6/10.3 |
| Peak F gam (mm−3) | 10/10 | 490/420 | 112/88 | 137.8/113.8 | 122.6/97.3 |
| Gam—only males rec | |||||
| Gam rec (days) | 4/2 | 36/49 | 10/13.5 | 15/20.4 | 9.9/15.8 |
| % Days gam recorded | 10/9 | 74/85 | 53/51 | 44/49 | 22/26 |
| First gam (day) | 6/4 | 31/20 | 10/8 | 10.7/8.9 | 5.9/4 |
| First gam (mm−3) | 30/10 | 175/126 | 70/51 | 71.5/55.5 | 39.5/33.8 |
| Peak gam (day) | 7/7 | 33/35 | 20/15 | 18.9/18.4 | 6.5/8.5 |
| Peak gam (mm−3) | 77/10 | 1,190/1,120 | 222/300 | 357.9/332.6 | 353.2/264.6 |
Table II.
Summary statistics for the 48 sporozoite-induced and 61 trophozoite-induced charts, as in Table I, showing arithmetic means of the asexual-form and gametocyte densities (#/mm−3) and fevers (i.e., ≥101 F) between the first day of patency and the timing points given in Table I. The “mean CV” column gives the arithmetic mean of the coefficient of variation (standard deviation/mean) for each chart, which provides a measure of within-chart variation. Other conventions and abbreviations as in Table I.
| Sporozoite/trophozoite | Min | Max | Median | Mean | Std Dev | Mean CV |
|---|---|---|---|---|---|---|
| Mean number of asexuals per cubic millimeter, to | ||||||
| First asexual peak | 20.0/22.5 | 5,645.0/6,690.3 | 896.0/1,631.7 | 1,426.1/2,019.3 | 1,873.1/2,535.9 | 1.35/1.26 |
| Peak asexuals | 218.0/286.4 | 7,100.0/8,089.0 | 2,494.9/3,850.4 | 2,851.3/4,005.6 | 3,159.4/4,029.2 | 1.14/1.05 |
| First fever | 10.0/10.0 | 1,898.3/1,454.5 | 35.0/54.8 | 162.4/137.8 | 182.7/142.9 | 0.63/0.54 |
| Peak fever | 228.3/131.7 | 7,176.7/9,679.2 | 1,776.7/3,033.8 | 2,138.0/3,313.9 | 2,493.8/3,400.1 | 1.19/1.08 |
| Intervention (if both sexes rec) | 155.8/273.9 | 7,453.5/8,443.1 | 2,139.0/3,585.9 | 2,757.7/3,587.0 | 2,386.9/3,027.8 | 1.03/0.98 |
| First F gam | 255.1/551.3 | 4,575.0/7,487.8 | 2,020.0/2,890.1 | 2,146.4/3,138.7 | 2,483.9/3,235.9 | 1.17/1.10 |
| First M gam | 385.8/601.2 | 5,794.4/7,487.8 | 2,284.0/3,292.2 | 2,758.3/3,573.5 | 2,898.2/3,492.5 | 1.05/1.03 |
| Peak F gam | 385.8/898.2 | 7,310.6/8,165.2 | 3,396.7/3,809.9 | 3,389.4/4,162.5 | 3,008.4/3,408.4 | 0.91/0.85 |
| Peak M gam (if only M rec) | 385.8/680.7 | 7,310.6/8,401.0 | 3,136.8/3,877.3 | 3,305.1/4,139.7 | 2,969.5/3,336.9 | 0.91/0.84 |
| First gam | 623.1/483.7 | 6,387.5/6,672.7 | 2,761.2/2,003.9 | 2,965.5/2,183.6 | 3,004.4/2,355.7 | 1.07/1.27 |
| Peak gam | 682.1/787.3 | 6,720.9/9,350.0 | 3,746.4/4,319.0 | 3,923.9/4,641.6 | 3,066.2/3,727.0 | 0.79/0.89 |
| Gam (if both sexes) | ||||||
| Mean number of F per cubic millimeter to F peak | 10.0/10.0 | 213.5/210.0 | 69.0/47.0 | 70.6/52.7 | 37.0/30.6 | 0.47/0.52 |
| to intervention | 10.0/10.0 | 215.9/128.4 | 70.0/39.2 | 66.6/45.6 | 34.6/28.4 | 0.52/0.55 |
| Mean number of M per cubic millimeter to M peak | 10.0/10.0 | 102.9/292.7 | 28.0/24.5 | 35.7/34.3 | 20.3/21.2 | 0.46/0.54 |
| to intervention | 10.0/10.0 | 98.0/239.2 | 24.5/21.0 | 35.2/28.5 | 18.8/18.5 | 0.46/0.55 |
| Gam (if only M) | ||||||
| Mean number of gam per cubic millimeter to peak | 10.0/10.0 | 324.9/252.9 | 105.7/117.9 | 130.8/126.9 | 92.7/90.0 | 0.56/0.64 |
| to intervention | 10.0/10.0 | 409.0/303.4 | 122.9/112.4 | 134.1/118.9 | 90.9/83.1 | 0.55/0.63 |
These infections were initiated by (1) inoculation of 5 ml of whole blood from a patently infected patient (61 cases); (2) bites of infectious mosquitoes previously fed on a patient (26 cases); or (3) inoculation of glands or sporozoites extracted from infectious mosquitoes (22 cases). Our analyses show no significant differences between routes 2 and 3; so, in line with our earlier work (Collins and Jeffery, 1999), we distinguish “sporozoite-induced” (2 and 3) from “trophozoite-induced” (1) infections. This allows us to compare relatively natural and artificial modes of induction. Information on prepatency was available only for sporozoite-induced infections (mean 15.2, standard deviation 3.7 days). Information on inoculum size was available only for mosquito-initiated infections (2), and then only as an integer, ranging here from 1 to 58, that had been determined by multiplying the number of mosquitoes that fed by their average “intensity” rating (an integer from 1 to 4, indicating whether 1–10, 11–100, 101–1,000, or >1,000 sporozoites were found in their salivary glands at postfeed dissection; Coatney, Cooper, Ruhe et al., 1950). Our analyses of these cases showed no relationships between “inoculum size” and prepatency or any other variable considered here.
Fever records were absent in 2 charts. The presence of gametocytes was reported in 100 patient charts: only females (macrogametocytes) were reported in 4, only males (microgametocytes) in 30, and both sexes in 66. The female-only gametocyte counts totaled only 11 days in the 4 cases, and were excluded from our analyses. The male-only gametocyte counts are problematic. They represent 28 of the 30 gametocytemic patient charts from 1943–1944 and so may reflect standard practice with P. vivax during those years (as with P. malariae throughout). It is generally easier to obtain reliable counts of microgametocytes than of macrogametocytes (De Buck, 1936; E. McKenzie, pers. obs.), and, although most of the relevant technical staff at the South Carolina USPHS facility were exceptionally well-qualified, it may be that less-experienced personnel recorded or attempted to record only microgametocytes. The high densities recorded in these male-only counts further suggest that in some cases, both sexes might have been counted as males. Accordingly, because we are not certain that females would have been recorded as such had they been detected, these counts are reported and analyzed separately. For charts in which both sexes were recorded, we report counts for each sex separately and, where appropriate, for their simultaneous appearance. Mosquitoes were fed on 377 days on 75 patients: 290 feeds on 66 patients before and 87 feeds on 23 patients after subcurative drug administration. As before, we defined as “successful” any instance in which mosquitoes were found to be infected.
We used the same rough approximation of brood structures in these infections as with the P. malariae charts (McKenzie et al., 2001), and, as before, considered only the (85) cases with 4 or more “local peaks” in preintervention parasitemias and thus 3 or more intervals between peaks; the mean interval between these “local peaks” was 4.1 days. We again considered only the “middle” intervals for these “local peaks,” the frequency distributions of which were indistinguishable from those of the full set. Of these 692 intervals between local peaks, 0.006 were 1 day, 0.438 were 2 days, 0.240 were 3 days, and 0.316 were ≥4 days in length. We again calculated 4 × 4 matrices representing the frequencies of transitions between particular intervals.
Statistical procedures
For the patient charts with 15 or more days of preintervention patency we calculated pairwise linear regressions with the variables given in Tables I and II, log-transformed values for the density variables, and patient identification numbers (assigned in order of admission to the facility). We split the full series of 109 charts into trophozoite- and sporozoite-induced groups and into 2 subgroups by patient identification number, ≤S-805 (the first 55, “earlier”) or >S-805 (the last 54, “later”). Note that patient identification numbers are not directly proportional to elapsed time, (e.g., patient S-805 was infected in July 1947) because the size of the patient population dwindled, but are more likely proportional to the number of parasite passages. The “earlier” subgroup consists of 60% sporozoite-induced infections, the “later” subgroup only 28%; among the charts in which gametocytes were recorded, 61% in the earlier subgroup reported only 1 sex (“male”), versus 6% in the later subgroup.
We used the Mann–Whitney test to examine distributional differences for each variable, with P values <0.01 marking significant differences. As with any procedure involving multiple comparisons, the results should be interpreted with caution. To investigate independence within contingency tables for drug response, fevers, and infectivity, we again used G-tests (for 2 × 2 and 2 × N) and log-linear models (for 2 × 2 × 2), again with P values <0.01 marking significant differences (Sokal and Rohlf, 1981). We calculated autocorrelation coefficients and plotted correlograms for the “raw” per cubic millimeter parasitemia data, for the local peaks in parasitemia, and for the data on gametocyte presence or absence, as before (McKenzie et al., 2001).
RESULTS
Tables I and II give summary statistics for the 109 St. Elizabeth P. vivax patient charts with 15 or more days of patency observed before intervention (see Materials and Methods). Figure 2 summarizes daily infection dynamics in these patient charts; Figure 3 depicts the individual charts of 7 infected patients.
Figure 2.
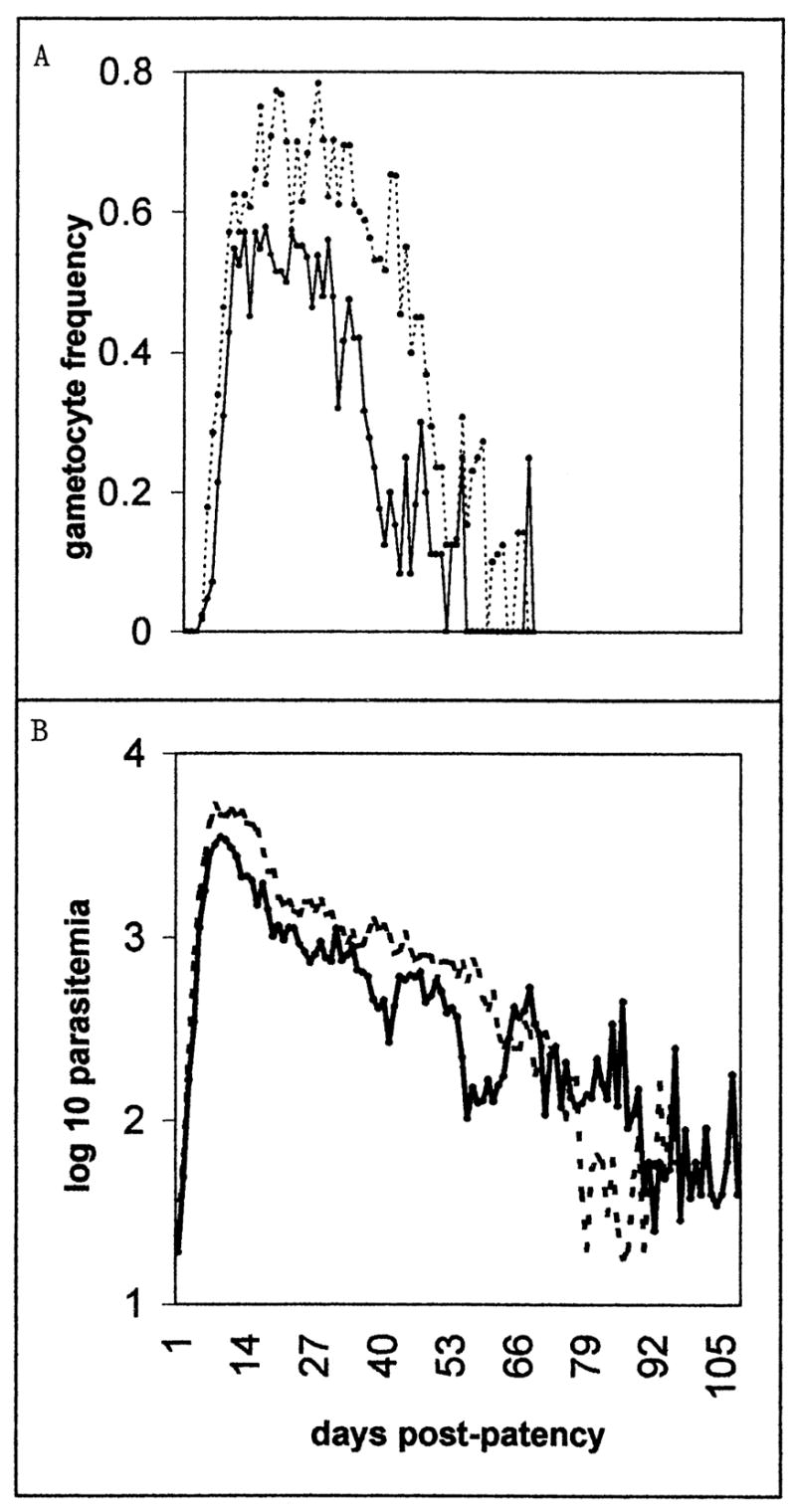
Patent gametocyte frequency (A, binary) and asexual-form density (B, as the mean of the log 10 parasitemia) for each day of patent, preintervention infection in charts with more than 14 days of observed preintervention patency. The solid line represents sporozoite-induced infections, the dotted line trophozoite-induced infections.
Figure 3.
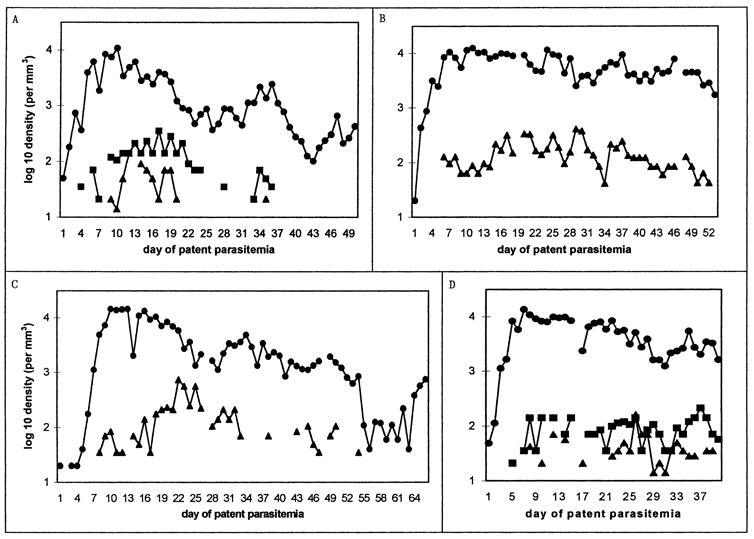
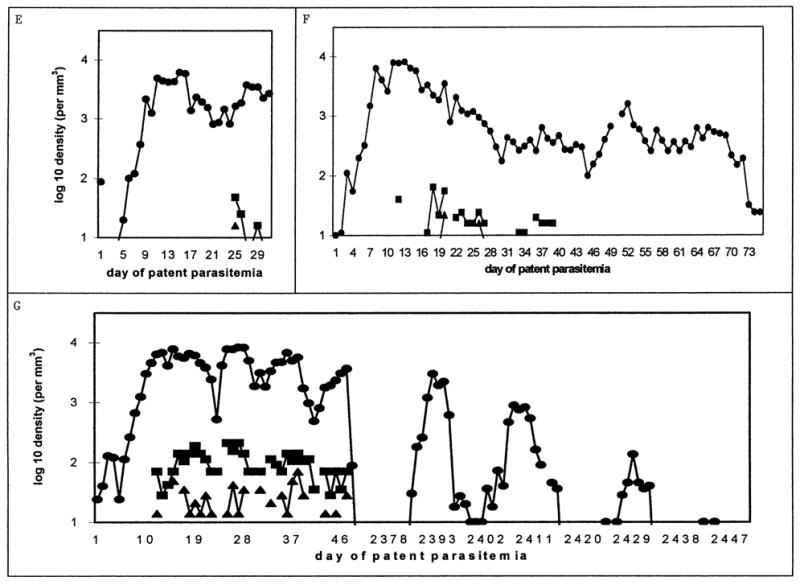
Examples of charts with more than 14 days of preintervention patency. For each chart, the line graph shows log 10 parasitemia (as circles) and gametocytemia (males as triangles, females as squares) per cubic millimeter of blood, plotted from the first day of patent parasitemia to the last day before intervention. Patient identification numbers and days plotted are (A) S-611, 50 days; (B) S-554, 53 days; (C) S-533, 66 days; (D) S-757, 40 days; (E) S-1084, 31 days; (F) S-932, 76 days; (G) S-772, 50 and 60 days (see following text). Single tertian intervals between “local peaks” in parasitemia are most evident in B (days 35–43) and D (days 20–28). Note that only males were reported in B and C (see text). The patient in part G was treated with atabrine (1 g on day 47, 400 mg daily on days 48–53) and reinoculated with St. Elizabeth P. vivax 76 mo later.
The results from linear regression and other statistical procedures on the P. vivax patient charts contrast dramatically with those from the P. malariae patient charts (McKenzie et al., 2001). For the trophozoite-induced infections, r2 values with 122 of the variable pairs exceed 0.33; 60 of these exceed 0.50, and 26 of these 60 exceed 0.67. For the sporozoite-induced infections, r2 values with 239 of the variable pairs exceed 0.33; 118 of these exceed 0.50, and 59 of these 118 exceed 0.67. That is, roughly twice as many pairs exceed a given r2 value for the sporozoite-induced as for the trophozoite-induced infections. Only the pairs with r2 values ≥0.80 are reported in the sections below; no r2 value for a pair involving a fever-related variable exceeds 0.67.
Parasitemia
Parasitemia was <20,000 mm−3 on 99.9% of preintervention days observed and <10,000 mm−3 on 96%; the mean peak parasitemia was roughly 10,000 mm−3. Under the standard curative chloroquine (1,500 mg of base administered over 3 days; 39 cases) and atabrine (300 mg per day for 5 days; 29 cases) regimes, parasites were cleared within a mean 0.7 and 2.1 days, respectively. Prior treatment did not influence rates of clearance.
In charts that showed “spontaneous clearance,” detectable parasitemia lasted, on average, 5 days longer in the 6 trophozoite-induced than in the 6 sporozoite-induced infections. However, in charts that reported at least 7 wk of patency before “spontaneous clearance,” subsequent detectable parasitemia dropped to 0, on average, 6 days more quickly in the 2 trophozoite-induced than in the 2 sporozoite-induced infections.
Peak parasitemia, and the mean parasitemia from first patency to that peak, were higher in trophozoite- than in sporozoite-induced infections, though the peak occurred on approximately the same day of patency. The overall difference derives from a difference in the later (>S-805; mean 11,181 > 6,512 mm−3, and 3,883 > 1,947 mm−3) rather than the earlier (≤S-805) subgroup. In the later subgroup, the mean parasitemia from first patency to the first local peak, the first fever, the first and the peak gametocytemia, and intervention were higher in trophozoite- than in sporozoite-induced infections. In the trophozoite-induced infections, the parasitemia when first detected was higher in the later than in the earlier subgroup (mean 57 > 24). In the sporozoite-induced infections, the mean parasitemia to the peak, the first and peak fevers, the peak male and female gametocytemias, and intervention were higher in the earlier than in the later subgroup.
The correlogram of the “raw” per cubic millimeter parasitemia data shows a smooth tailing-off of positive autocorrelation coefficients, which indicates that these data lack a dominant frequency or period and display only serial day-to-day correlation of densities above or below the mean value. The correlogram for the binary “local peaks” in parasitemia (Fig. 4) indicates positive values only for period 2 and, very slightly, period 9; the other 7 coefficients are slightly negative.
Figure 4.
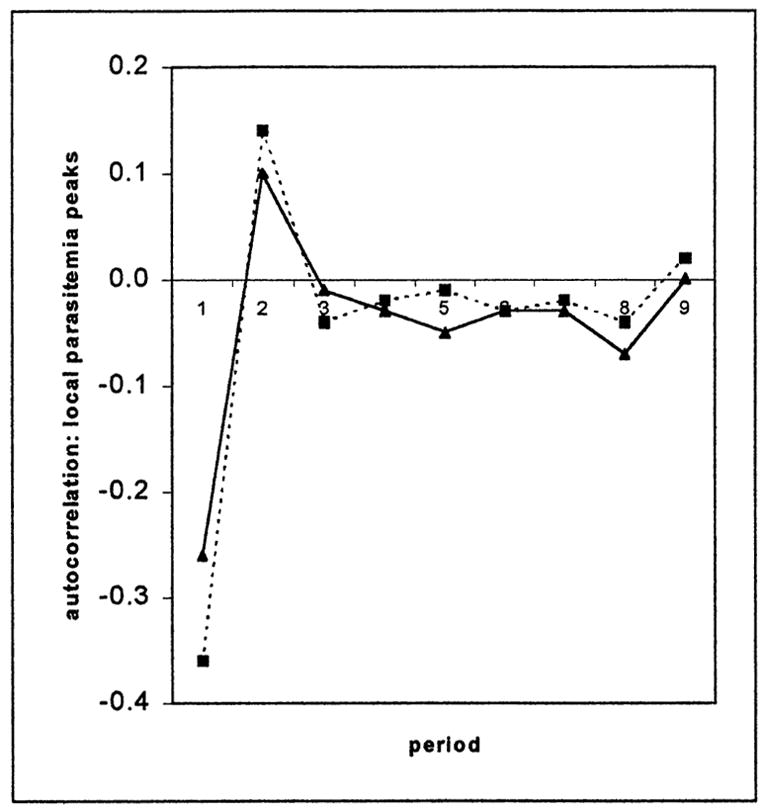
The correlogram of local peaks in parasitemia (binary) in the patient charts with ≥4 local peaks. The solid line represents sporozoite-induced infections, the dotted line trophozoite-induced infections.
Table III gives the transition matrix for the 692 intervals between these local peaks in parasitemia. The 2 “textbook” possibilities (tertian-to-tertian, quotidian-to-quotidian) together account for only 19.2% of the transitions; if the ≥4-day categories are eliminated, for instance, this figure rises to 40%. Even allowing for enormous error in “local peak” approximations, the Table III matrix suggests that changes in brood structure during the course of an infection and differences in brood structure between infections could account for only about 20% of the local-peak transitions, i.e., for strictly tertian brood structures.
Table III.
Transition matrices for the intervals between “local peaks” in parasitemia, in 85 patient charts (see text).
| Transition | To 1 day | To 2 days | To 3 days | To ≥4 days |
|---|---|---|---|---|
| From 1 day | 0.001 | 0.004 | 0.001 | 0.000 |
| From 2 days | 0.000 | 0.191 | 0.097 | 0.140 |
| From 3 days | 0.001 | 0.110 | 0.069 | 0.071 |
| From ≥4 days | 0.001 | 0.118 | 0.082 | 0.114 |
The observed frequencies in the transition matrix are very close to those expected on the basis of the frequency distribution (see Materials and Methods). A priori expectations for longer sequences of intervals can easily be calculated from the frequency distribution. Such calculations (not shown) suggest that whereas consecutive 1-day intervals and 2-day intervals each occurred almost precisely as often as would be expected, overall, this is a consequence of combining results from the earlier sporozoite- and later trophozoite-induced infections, in which these observed frequencies were higher than expected, with those from the later sporozoite- and earlier trophozoite-induced infections, in which they were lower than expected.
Table IV compares transition-matrix entries across different species and induction modes with respect to their conformance to “textbook” brood structures and the degree to which, with respect to “local peaks” in parasitemia, this correspondence might increase or decrease over a sequence of infections or over the course of a single infection. With P. vivax, local peaks in the sporozoite-induced and earlier infections and in later phases of infections fit recognized tertian patterns more often than did those in the trophozoite-induced and later infections and in earlier phases of infections. Considered over all 4 species, and both induction modes, fevers followed patterns that fit recognized brood structures roughly twice as often as did these “local peaks.”
Table IV.
The sums of the transition-matrix entries for local-peak intervals, for different species and induction modes, that conform to familiar, “textbook” brood structures. Correspondence to such brood structures might increase or decrease over a sequence of infections or over the course of a single infection, so the sums for local peaks are given (1) overall, (2) for only those charts in the first half of the corresponding sequence of patient identification numbers, in the “First ID#s” column, and (3) for only the first half of the preintervention days in each infection, in the “first days” column. Transition matrices were calculated from preintervention charts of malaria-naive patients in the USPHS South Carolina facility in which daily records encompassed 92% or more of the duration of each infection and >35 (P. malariae), 21 (P. ovale Donaldson strain; P. falciparum McLendon strain) or 14 (P. vivax) days of patency preceded intervention. Abbreviations: trophozoite, Tropho; Sporozoite, Sporo.
| “Textbook” local peaks
|
||||||
|---|---|---|---|---|---|---|
| Species | Induction mode | Number of charts | Mean number of days per chart | Overall | First ID#s | First days |
| Plasmodium malariae | Tropho | 77 | 91 | 0.15 | 0.14 | 0.14 |
| P. ovale | Tropho | 15 | 67 | 0.31 | 0.33 | 0.38 |
| P. falciparum | Tropho | 49 | 72 | 0.37 | 0.40 | 0.44 |
| P. falciparum | Sporo | 14 | 61 | 0.34 | 0.35 | 0.38 |
| P. vivax | Tropho | 50 | 39 | 0.17 | 0.19 | 0.15 |
| P. vivax | Sporo | 35 | 37 | 0.22 | 0.25 | 0.21 |
Parasitemia and fevers
In all cases, parasitemia was patent on or before the day the first fever was reported. In 71 of the 107 infections with fever records, 1 or more of the fevers exceeded 106 F. There were no apparent differences between the trophozoite- and sporozoite-induced infections, or the earlier and later infections, with respect to any fever-related measures considered here (P > 0.2).
No fevers were detected after day 60. Among charts in which fevers had ceased at least 1 wk before intervention, there was no difference between the (24) trophozoite- and (18) sporozoite-induced infections with respect to the final day on which a fever was reported (mean day 29.5).
Parasitemia at the first fever >104 F was higher than that at the final fever in half of the infections and lower in half, whether trophozoite- or sporozoite-induced; it was higher in two-thirds of the earlier and one-third of the later subgroup. Among charts in which fevers had ceased at least 1 wk before intervention, there was no difference between trophozoite- and sporozoite-induced infections with respect to the parasitemia (mean 1,893 mm−3) or fever (mean 102.7 F) on the final day on which a fever was reported. The mean parasitemia from first patency to the peak fever was higher in trophozoite- than in sporozoite-induced infections, though the peak fever occurred on approximately the same day of patency. The overall difference derives from a difference in the later (mean 3,329 > 1,605 mm−3) rather than the earlier subgroup.
In only 13 of the 107 patient charts with fever records did the peak fever and the peak parasitemia occur on the same day, and in 26 more they occurred within 1 day of each other. In 65 charts, the peak fever preceded and in 29 followed the peak parasitemia. The peak fever and 1 of the next 2 highest parasitemias co-occurred in 29 charts; the peak parasitemia and 1 of the next 2 highest fevers co-occurred in 22 charts. In 34 charts, the peak fever coincided with a local peak in parasitemia; in 72 charts the peak parasitemia coincided with a fever. We found no strong relationships between measures of parasitemia and fever, in timing or magnitude (Tables I, II); we cannot explain such absences of association.
Parasitemia and gametocytemia
Gametocytes were recorded before intervention in 100 patient charts, on a total of 1,533 days. Gametocytes of both sexes were reported in 66 patient charts, with both males and females detected on 581 days, only females on 324 days, and only males on 107 days. Only males were reported in 30 patient charts, on a total of 510 days; recall that 4 patient charts reported only females, on a total of 11 days. In 1 patient, gametocytes were detected only after subcurative drug interference. Gametocytes were frequently detected (Fig. 2) and at densities generally well above the threshold of detection: only 5% of the days recorded 10 gametocytes/mm3 (of one or both sexes), whereas nearly 15% recorded ≥200 mm−3.
Among the infections in which both gametocyte sexes were reported, the mean asexual density from the first day of patency to the peak female density correlates with that to the peak male density (r2 = 0.97 for sporozoite-induced, 0.83 for trophozoite-induced). Among sporozoite-induced infections, the mean asexual density to the day of peak female density correlates with the peak asexual density (r2 = 0.81); the same holds with respect to the day of peak male density, both for infections in which both gametocyte sexes were reported (r2 = 0.80) and those in which only males were reported (r2 = 0.82).
In infections in which both gametocytes sexes were reported, the peak parasitemia and peak female (male) density occurred on the same day in 6 (7) patient charts, and in 6 (9) more occurred within 1 day of each other. In 20 charts, the peak female density and peak male density occurred on the same day and in 16 more occurred within 1 day of each other. Peak parasitemia preceded peak female (male) density in 52 (52) charts, followed it in 8 (11), and coincided with it in 6 (3). In 19 charts, the peak female density preceded, in 26 followed, and in 21 coincided with the peak male density. Peak female (male) density followed peak parasitemia by an average of 7.5 (6.9) days.
In infections in which only males were reported, the peak parasitemia and peak gametocytemia occurred on the same day in 1 chart and in 6 more occurred within 1 day of each other. In 8 charts, the peak gametocytemia preceded, in 19 followed, and in 2 coincided with the peak parasitemia. Peak gametocyte density followed peak parasitemia by an average of 5.2 days. There were no apparent differences between the trophozoite-and sporozoite-induced infections, or the earlier and later infections, with respect to these timing-related measures.
The mean parasitemia from first patency to the day on which females were first detected was higher in trophozoite- than in sporozoite-induced infections; the overall difference derives from a difference in the later (mean 3,321 > 2,169 mm−3) rather than the earlier subgroup. Among the sporozoite-induced infections, females were first detected in the earlier subgroup earlier than in the later subgroup (mean day 8.7 < 16.2).
For all categories of infections, the peak male density correlates with the mean male density from the first day of patency to the day of peak male density (r2 = 0.84 for sporozoite-induced, 0.88 for trophozoite-induced) and to the day of intervention (0.86, 0.89). The last 2 variables are correlated, as are the corresponding variables for females. Among sporozoite-induced infections, the day of peak female density correlates with the day of peak male density (r2 = 0.80), and peak female density correlates with mean female density to the day of peak female density (0.92) and to the day of intervention (0.94); mean female density to the day of intervention is correlated with the mean male density to the day of peak male density (0.81) and to the day of intervention (0.82).
In the later subgroup (>S-805), among infections in which both gametocyte sexes were reported, the proportion of days on which both were detected simultaneously was higher in the trophozoite- than in the sporozoite-induced infections (mean 0.27 > 0.13). In the sporozoite-induced infections in which both gametocyte sexes were reported, the proportion of days on which both were detected simultaneously was higher in the earlier than the in later subgroup (mean 0.32 > 0.13).
The female density when first detected (mean 63 > 25 mm−3), and at their peak (mean 191 > 88 mm−3), and the mean female density to their peak (mean 101 > 38 mm−3) and to intervention (mean 92 > 33 mm−3) were higher in the earlier than in the later subgroup. In the sporozoite-induced infections, the male density when first detected (mean 31 > 11 mm−3), and at their peak (mean 107 > 32 mm−3), and the mean male density to their peak (mean 50 > 17 mm−3) and to intervention (mean 50 > 16 mm−3) were higher in the earlier than in the later subgroup.
As with the per cubic millimeter parasitemia data, the correlograms of gametocyte densities (in the patient charts in which gametocytes were reported before intervention) indicate that these data lack a dominant frequency or period and display only serial day-to-day correlation.
The relative densities of male and female gametocytes appeared to alternate, though not with a discernably regular period. Figure 5 plots the course of male and female gametocytemia relative to parasitemia, and the gametocyte sex ratio, in the infections in which both gametocyte sexes were reported. Notice that the gametocyte densities were higher in sporozoite-than in trophozoite-induced infections, though the day-to-day gametocyte frequencies were lower (Fig. 2A).
Figure 5.
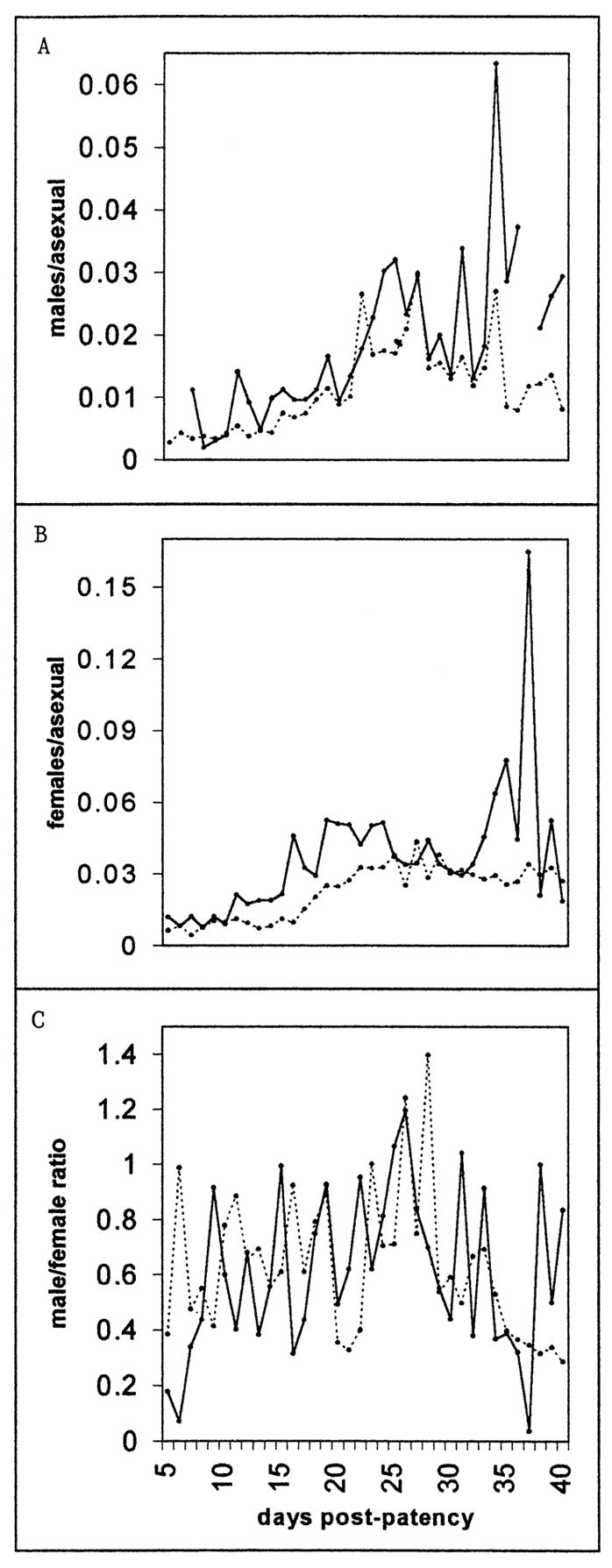
The day-by-day course of the mean male (A) and female (B) gametocytemia per asexual form and the gametocyte sex ratio (C) between days 5 and 40 of patency. Only charts in which each gametocyte sex was reported on at least 1 day were included; charts in which the sex ratio exceeded 10 were excluded (4 charts, on a total of 11 days). If the density of only 1 sex was recorded on a given day, the density of the other on that day was set at 5 mm−3 (the threshold of detection was roughly 10 mm−3). The solid line represents sporozoite-induced infections, the dotted line trophozoite-induced infections.
Gametocytes and infectivity
Feeds on trophozite- and sporozoite-induced infections did not differ with respect to the presence or absence of gametocytes; pre- and postdrug feeds did not either. When gametocytes of both sexes were reported at feeds, the density of females was higher than that of males (mean 113 > 42/mm3, P < 0.001). Among the infections in which both gametocyte sexes were reported, gametocyte densities at feeds on sporozoite-induced infections were higher than those in trophozoite-induced infections (females 217 > 62 mm−3, P < 0.0001; males 73 > 27 mm−3, P < 0.001); among the sporozoite-induced infections, gametocyte densities were higher at postdrug than at predrug feeds (females 328 > 106 mm−3, P < 0.001; males 105 > 41 mm−3, P < 0.01). Parasitemia at feeds was higher in the earlier than in the later subgroup (mean 5,301 > 3,423 mm−3, P < 0.0001) and, in the later subgroup, was higher in trophozoite-than in sporozoite-induced infections (mean 3,807 > 2,341 mm−3, P < 0.01).
Successful transmission occurred in 249 of the 290 mosquito feeds before and 76 of the 87 feeds after subcurative drug administration. One or more of the feeds on each of 71 different patients succeeded; feeds on 4 other patients (total 9 feeds) failed entirely. The earliest successful and failed feeds were each on day 7 of patency, the latest on days 44 and 39. Overall, the success of feeds did not differ between trophozoite- and sporozoite-induced infections or between the earlier and later subgroups; the success of feeds was not affected by the administration of subcurative drugs. Successful feeds occurred later in infections, by a mean of 4.7 days (mean day 25.4 vs. 20.7); the overall difference derives from differences within the pre-drug trophozoite-induced (mean day 25.5 vs. 20.5) and post-drug sporozoite-induced (mean day 29.0 vs. 24.3) infections.
The presence or absence of detected gametocytes did not determine the success or failure of a feed, and levels of parasitemia did not either. Seven of the 8 feeds undertaken at peak parasitemia and 4 of the 5 undertaken at peak fever were successful. When gametocytes were detected, the success or failure of a feed did not depend on whether either or both sexes were detected. Male densities were higher at successful than at failed feeds, both in the infections in which only males were reported and those in which gametocytes of both sexes were reported; among the latter, the difference derives from a difference in the sporozoite-induced infections (mean 77 > 33 mm−3, P < 0.01). Female densities did not differ between successful and failed feeds or between feeds on trophozoite- and sporozoite-induced infections. There was no relationship between the proportion of mosquitoes infected at feeds and the corresponding gametocyte densities in any gametocyte category (r2 < 0.05).
Homologous reinfection
Previously malaria-naive patients were occasionally infected twice in succession with the St. Elizabeth strain of P. vivax in the South Carolina facility; both charts are available for 5 such patients. With 2 of these patients, the first and second infections both occurred in the 1940s, with intervals between the final parasite-positive day of the first and the day of inoculation with the second of 161 and 1,010 days. With 2 others, the first infection occurred in the 1940s and the second in the 1950s, with intervals of 2,115 and 2,302 days. In each pair, with the first patient, the first infection was sporozoite-induced, the second trophozoite-induced, and the order was reversed with the second patient; in each, the first infection lasted 3–7 wk. With the remaining patient, the first and second infections both occurred in the 1950s, with an interval of 172 days; the first infection was trophozoite-induced and lasted 14 wk, and the second was sporozoite-induced.
With all 5 patients, in the second infection the mean fever, peak fever and number of days of fever were lower, and the day of peak fever earlier, than in the first. With 3, the day of the first fever was earlier and with 1 later; with 2, the first fever was lower and with 2 higher. With all 5 patients, the mean asexual density was lower in the second infection than in the first. In 4, the peak asexual density was lower, the day of peak asexual density earlier, and intervention later; in each case 1 of the patients with a short interinfection interval showed the reverse relationship. There were no clear patterns with respect to initial asexual-form density. Gametocytes were detected in all 5 patients in the first infection, but in only 2 in the second infection; in those 2, gametocytemia decreased, by all measures.
DISCUSSION
We have taken advantage of a unique opportunity to examine the preintervention blood-stage dynamics of P. vivax infections in a population of malaria-naive U.S. neurosyphilis patients infected with a well-characterized strain of the parasite, in circumstances nominally identical to those of canonical studies of the species. However, because the dynamics of parasitemia in these patient charts seldom seem to correspond to conventional, “textbook” descriptions of tertian patterns, we join Coatney, Cooper, and Young (1950) in reporting that “the results in our controlled studies serve to reemphasize the variability of vivax malaria.”
Previous work suggests that different P. vivax inoculum sizes may lead to different prepatencies, which may in turn be associated with differences in frequencies of tertian fevers and spontaneous recovery, more strongly so in sporozoite- than in trophozoite-induced infections (Glynn and Bradley, 1995). This may be a complicating factor in our data; e.g., though initial parasitemia did not differ between the trophozoite- and sporozoite-induced infections, variation within each group was high.
Other complicating factors are the skewed distributions of the male-only gametocyte counts and sporozoite-induced infections toward the “earlier” group of infections. Though the notable differences within this data set seem more often related to change over time, i.e., the “earlier” versus the “later” group, than to trophozoite- versus sporozoite-transmission per se, the 2 elements are closely intertwined at many levels; in particular, there was not 1 purely sporozoite- and another purely trophozoite-transmitted line. Furthermore, many of the relevant differences relate to net growth rates, i.e., to the trade-offs between geometric, asexual-form and arithmetic, sexual-form growth we have examined elsewhere (McKenzie and Bossert, 1998a, 1998b); our regression results indicate that the only pairs of variables that yield r2 values ≥0.80 are those that involve gametocytemia or asexual parasitemia in relation to gametocytemia. Parasitemia and gametocytemia in the sporozoite-induced infections were generally higher in the earlier than in the later subgroup. In the trophozoite-induced infections, the only measure of parasitemia that differed between the earlier and later subgroups was the initial density (higher later), and only female gametocytemia declined. Hence, in the later subgroup and overall, parasitemia was higher in the trophozoite- than in the sporozoite-induced infections, whereas gametocytemia per se showed few significant differences (see Figs. 2, 5). The overall success of feeds did not differ between the trophozoite- and sporozoite-induced infections or the earlier and later infections.
Nonetheless, our regression results indicate that pairs of variables show more frequent and stronger correlation in sporozoite- than in trophozoite-induced infections. We speculate that host immune responses contribute to these differences, i.e., that there are relevant differences between host responses to the relatively gradual release of primary merozoites from hepatic schizonts and the relatively instantaneous introduction of a mixture of the various asexual and sexual blood forms of the parasite, spirochaetes, and host antigens. It is also conceivable that a given number of primary merozoites are more closely related than an equivalent number taken at random from the host circulation, in which case one would expect the evolutionary path of the parasite to be biased by the relative frequencies of sporozoite- and trophozoite-based transmission.
Whatever the scenario, whereas the parasites represented in this data seem to have displayed consistencies in line with their continued recognition as a coherent “strain” (see following text), they must have been subject to genetic drift. With respect to possible selective forces, all we can infer is that the regime would consciously have focused only on clinical criteria, thus primarily on whatever phenotypic traits might be associated with fevers. No fever-related variables differed between the trophozoite- and sporozoite-induced infections or the earlier and later infections.
We know of 1 report of dramatic changes in P. vivax with repeated passage, in the form of an oft-cited paper from the Horton Hospital, U.K. (James et al., 1936), which describes an apparent instance of artificial selection, the conversion of a relatively mild strain of P. vivax, “to the production of individuals which represent the species in its perfect form. Between 1925 and 1930 we succeeded in increasing the physical vigour and activity of an endemic strain of P. vivax from Madagascar to the degree in which it caused this severe ‘epidemic type’ of the disease in 80% of our cases. We did so by using a particular procedure in passaging the strain through the human host and insect vector.” The paper gives no details about the “particular procedure” of mosquito and blood inoculations but claims that it led to infections with higher fevers, higher mortality (if untreated), and more consistent transmission to Anopheles, changes “evidenced not only by an increase in the number of asexual parasites but particularly by the earlier appearance and greater number of gametocytes.” We are wary of the discrepancies between this account and reports on the same strain in the same facility during the same period that were published 5 yr earlier (James, 1931) and 15 yr later (Covell and Nicol, 1951), however.
Decades of experience with the common malariatherapy “strains” seemed to confirm the presence and persistence of characteristic differences between primary infections with each of them (Boyd, 1940a; Kaplan et al., 1946), e.g., between the El Limon, McLendon, and Santee-Cooper strains of P. falciparum (Collins and Jeffery, 1999). Several citations in the first paragraph of this paper are generalizations from the authors’ extensive experience, each predominantly with 1 strain of P. vivax: Chesson (Whorton et al., 1947), McCoy (Kitchen, 1949), or Madagascar (Shute, 1958). The quote from Coatney et al. (1971) refers explicitly to Chesson infections and so may not apply to the authors’ experience with St. Elizabeth infections. Our results apply to the St. Elizabeth strain, and we have noted that several contrast with those of Boyd (1938) and Kitchen (1949), for instance, who worked primarily with the McCoy strain. It may be that most or all differences between our results and those of previous authors arise from differences in the circumstances or parasites involved.
Such P. vivax strains may differ dramatically in their pre-patency and relapse intervals (Tiburskaja et al., 1968; Coatney et al., 1971; Shute et al., 1976; Clyde, 1989), in their response to primaquine and other drugs (Collins and Jeffery, 1996), and in their infectivity to various species and strains of Anopheles, e.g., An. culifacies (Collins et al., 1986; Adak et al., 1999). Simultaneous infection with 2 P. vivax strains may interfere with the development of homologous immunity to either (Boyd et al., 1938), allow transmission either of 1 or both strains (Boyd et al., 1941), and produce relapse patterns that combine the characteristics of each (Cooper et al., 1950). Different P. vivax strains are said to produce different numbers of merozoites per schizont, on average, ranging from 12–24 (Boyd, 1941; Pampana, 1963; Garnham, 1966). Perhaps most important with respect to the present paper, however, are reports that such strains differ in periodicity (Garnham, 1966; Coatney et al., 1971). Based on fever-peak-to-fever-peak measurements on patients in the South Carolina USPHS facility, Young (1944) reported periodicities of 43.4 hr for the St. Elizabeth strain, 41.5 hr for a Baltimore strain, and 45.8 hr for a New Hebrides strain; he concluded that “each strain might have a characteristic periodicity” (but see Kitchen and Putnam, 1946).
It is now well-known that different lines cloned from a single P. falciparum isolate may differ in drug susceptibility, antigenic profile, gametocyte production, infectivity, and other phenotypic characteristics (Burkot et al., 1984; Thaitong et al., 1984), but there is no comparable information on P. vivax. There has been remarkably little research on P. vivax diversity, in antigenic (Udagama et al., 1990; Kolakovich, 1996; Putaporntip et al., 1997) or other (Tsuboi et al., 1994; Joshi et al., 1997) terms. Studies of 2 worldwide variants of the P. vivax CSP repeat region, VK210 and VK247, have demonstrated that they may coinfect humans (Kain et al., 1991) and Anopheles (Wirtz et al., 1992) and that they raise distinct antibody responses (Wirtz et al., 1990). It has been reported that VK210 is associated with higher parasitemia and chloroquine resistance (Kain et al., 1993; but see Machado and Povoa, 2000) and that year-to-year changes in the relative prevalence of VK210 and VK247 may be because of immune selection (Burkot et al., 1992). Their relative prevalence may also vary with the season (Suwanabun et al., 1994), and different sympatric species of Anopheles may be more susceptible to each (Gonzalez-Ceron et al., 1999). These contrasting characteristics seem to correspond in part to those of the “strains” discussed in the classic literature, in part to those of “strains” discussed in the recent literature (Gupta et al., 1996), and in part to neither. We hope that more research on within-host dynamics of Plasmodium will address such discrepancies and will clarify the biological nature of strains and of broods.
Our results clearly fit the observation by Kitchen (1949) that per cubic millimeter P. vivax parasitemia “usually remains below 30,000.” The timing of peak parasitemia fell within the range given by Kitchen (1949) but was therefore several days in advance of the range given by Garnham (1966). Peak parasitemia was higher in trophozoite- than in sporozoite-induced infections but, in contrast to Boyd (1938), not earlier. Our results on the duration of trophozoite- versus sporozoite-induced infections differ from the results of Coatney et al. (1971) with a larger sample of St. Elizabeth P. vivax infections, as cited previously, and from our results with P. falciparum (Collins and Jeffery, 1999).
It is not clear whether the magnitudes of the fevers fit the observation by Kitchen (1949) that P. vivax fever “frequently exceeds 106 F.” The timing of the peak fever was several days in advance of the range given by Kitchen (1949), however. In the sporozoite-induced infections, the mean 28.4-day duration of the febrile phase was longer (by 9 days) and the mean maximum fever of 105.8 F higher (by 1.6–3.3 F) than those cited by Coatney et al. (1971) for mosquito-induced infections. It is not clear whether the variation in the relation of parasitemia to the first and final fevers falls within the bounds of the “similarity” remarked on by Boyd (1938, 1944), as cited previously. Neither the duration of the febrile phase of infection nor the parasitemia at the final fever differed between trophozoite- and sporozoite-induced infections, in contrast to Boyd (1938, 1940b).
Taking into consideration the lengths of the initial infections and the interinfection intervals, the data on homologous reinfections appear to conform to those in earlier reports on homologous P. vivax reinfections in malariatherapy (Boyd and Kitchen, 1936; Boyd, Stratman-Thomas, and Kitchen 1936; Boyd, 1942b; Kitchen, 1949; Coatney, Cooper, and Young, 1950; Garnham, 1966; Coatney et al., 1971) and to the corresponding summary hypothesis of that era, i.e., that homologous-strain immunity sufficient to suppress patent parasitemia and prevent clinical attack could be acquired through a P. vivax infection of 6 mo or more that concluded with “spontaneous recovery,” and that, once acquired, such immunity might persist 3–7 yr.
The lag between asexual-form and gametocyte patency was 3–7 days longer than in previous reports (Fairley, 1947; Garnham, 1966); the lag between peak parasitemia and peak gametocytemia was no more than 1–2 days longer, however (Boyd, Stratman-Thomas, and Muench, 1936; Garnham, 1966). Boyd, Stratman-Thomas, and Muench, (1936) calculated that “one-quarter of those previously negative, develop gametocytes in their blood daily”; comparable figures for our data are closer to one-fifth, yet gametocytes were detected on 57% of preintervention days versus the 48% reported by Boyd, Stratman-Thomas, and Muench (1936).
Gametocyte density was usually about 100 mm−3, however, hence well below the previously-reported levels (Boyd, Stratman-Thomas, and Muench, 1936; Basu, 1947; Garnham, 1966; Sattabongkot et al., 1991), as cited previously. We found no evidence of the 5-day intervals between gametocyte “showers” remarked on by Boyd, Stratman-Thomas, and Muench (1936). Fevers did not affect the presence or density of gametocytes or the success of feeds, in contrast to the report of Eyles et al. (1948), who found infectivity lower in asymptomatic than in symptomatic phases of P. vivax infection, and that of Mendis and Carter (1992), who reported a decline in infectivity at the febrile “crisis” stages of infections.
Our results indicate that an absence of detected gametocytes of either sex does not hinder P. vivax transmission to Anopheles and that the presence of abundant gametocytes of either sex does not guarantee it; this is in line with most previous reports (Boyd and Stratman-Thomas, 1932; Boyd et al., 1935; Boyd, 1942a; Watson, 1945). However, our results also support the hypothesis that “the best estimate of the potential infectivity of a patient can be made on the basis of the male gametocyte count” (Eyles et al., 1948). Earlier work reported that the mean oocyst density per mosquito increased with the density of microgametocytes (males) but not macrogametocytes (Boyd and Stratman-Thomas, 1932) and that the mean oocyst density and the proportion of mosquitoes infected tended to vary together (Boyd, 1942a). Boyd and Kitchen (1937) found microgametocyte density critical in the infectivity of P. vivax, but not of P. falciparum, and attributed the difference primarily to a higher overall gametocytemia required for successful P. falciparum transmission.
It is well-known that Duffy-antigen expression on red blood cells is implicated in P. vivax susceptibility (Boyd and Stratman-Thomas, 1933; Bray, 1958; Miller et al., 1976). Recent studies indicate that Duffy-antigen expression is higher on reticulocytes (Woolley et al., 2000), that gametocyte production and infectivity in P. falciparum are higher in reticulocytotic blood, e.g., from sickle-cell anemics (Trager and Gill, 1992; Robert, Tchuinkam et al., 1996; Drakeley et al., 1999; Trager et al., 1999), that P. falciparum infectivity increases with the proportion of male gametocytes (Robert, Read et al., 1996), and that stimulation of erythropoesis, but not reticulocytosis per se, shifts gametocyte sex ratios in P. gallinaceum and P. vinckei toward males (Paul et al., 2000). This new conjunction suggests possible links to the homeostatic mechanisms that boost reticulocyte production in response to red blood cell depletion and potential insights into the within- and between-host dynamics of mixed-species Plasmodium infections (Snewin et al., 1991; McKenzie and Bossert, 1997, 1999; Worku et al., 1997; Mason and McKenzie, 1999). In Aotus monkeys, for instance, prior infection with either P. falciparum or P. vivax increases the infectivity of a subsequent infection with the other species (Collins et al., 1979). Hegner (1938) pointed out a seasonal rise in human reticulocyte counts, coincident with P. vivax relapses and Boyd and Kitchen (1938) a similar seasonal rise in P. vivax infectivity. We hope and expect that research along these lines will develop rapidly.
Acknowledgments
We gratefully acknowledge the support of a NIH National Research Service Award (to Ellis McKenzie) and the contributions of J. Barnwell, W. H. Bossert, D. Curry, L. Elder, P. Griffin, J. Lotz, M. Palmer, 2 anonymous reviewers, and the Countway and Mayr Libraries at Harvard University.
Footnotes
Division of Parasitic Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia 30341.
LITERATURE CITED
- Adak T, Kaur S, Singh OP. Comparative susceptibility of different members of the Anopheles culifacies complex to Plasmodium vivax. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1999;93:573–577. doi: 10.1016/s0035-9203(99)90052-4. [DOI] [PubMed] [Google Scholar]
- Basu BC. The frequency of distribution of gametocytes of the Indian strains of malaria parasites. Indian Journal of Malariology. 1947;1:123–127. [Google Scholar]
- Boyd MF. The threshold of parasite density in relation to clinical activity in primary infections with Plasmodium vivax. American Journal of Tropical Medicine. 1938;18:497–503. [Google Scholar]
- Boyd MF. On strains or races of the malaria parasites. American Journal of Tropical Medicine. 1940a;20:69–80. [Google Scholar]
- Boyd MF. Some characteristics of artificially induced vivax malaria. American Journal of Tropical Medicine. 1940b;20:269–278. [Google Scholar]
- Boyd MF. The infection in the intermediate host: Symptomatology, general considerations. In: Moulton PR, editor. Human malaria. American Association for the Advancement of Science; Washington, District of Columbia: 1941. pp. 163–182. [Google Scholar]
- Boyd MF. On the varying infectiousness of different patients infected with vivax malaria. American Journal of Tropical Medicine. 1942a;22:73–81. [Google Scholar]
- Boyd MF. Criteria of immunity and susceptibility in naturally induced vivax malaria infections. American Journal of Tropical Medicine. 1942b;22:217–226. [Google Scholar]
- Boyd MF. On the parasite density prevailing at certain periods in vivax malaria infections. Journal of the National Malaria Society. 1944;3:159–167. [Google Scholar]
- Boyd MF. Epidemiology of malaria: Factors related to the intermediate host. In: Boyd MF, editor. Malariology. W.B. Saunders; Philadelphia, Pennsylvania: 1949. pp. 551–607. [Google Scholar]
- Boyd MF, Kitchen SF. On the efficiency of the homologous properties of acquired immunity to P. vivax. American Journal of Tropical Medicine. 1936;16:447–452. [Google Scholar]
- Boyd MF, Kitchen SF. On the infectiousness of patients infected with Plasmodium vivax and Plasmodium falciparum. American Journal of Tropical Medicine. 1937;17:253–262. [Google Scholar]
- Boyd MF, Kitchen SF. Demonstrable maturity of gametocytes as a factor in the infection of anophelines with Plasmodium vivax and Plasmodium falciparum. American Journal of Tropical Medicine. 1938;18:515–520. [Google Scholar]
- Boyd MF, Kitchen SF, Matthews CB. On the natural transmission of infection from patients concurrently infected with two strains of Plasmodium vivax. American Journal of Tropical Medicine. 1941;21:645–652. [Google Scholar]
- Boyd MF, Kupfer WH, Matthews CB. A deficient homologous immunity following simultaneous inoculation with two strains of Plasmodium vivax. American Journal of Tropical Medicine. 1938;18:521–524. [Google Scholar]
- Boyd MF, Stratman-Thomas WK. Studies on Plasmodium vivax. 1. The microgametocytes as a factor in the infectiousness of the infected human. American Journal of Hygiene. 1932;16:845–850. [Google Scholar]
- Boyd MF, Stratman-Thomas WK. Studies on benign tertian malaria 4. On the refractoriness of Negroes to inoculation with Plasmodium vivax. American Journal of Hygiene. 1933;18:485–489. [Google Scholar]
- Boyd MF, Stratman-Thomas WK, Kitchen SF. On the relative susceptibility of Anopheles quadrimaculatus to Plasmodium vivax and Plasmodium falciparum. American Journal of Tropical Medicine. 1935;15:485–493. [Google Scholar]
- Boyd MF, Stratman-Thomas WK, Kitchen SF. On the duration of acquired homologous immunity to Plasmodium vivax. American Journal of Tropical Medicine. 1936;16:311–315. [Google Scholar]
- Boyd MF, Stratman-Thomas WK, Muench H. The occurrence of gametocytes of Plasmodium vivax during the primary attack. American Journal of Tropical Medicine. 1936;16:133–138. [Google Scholar]
- Bray RS. The susceptibility of Liberians to the Madagascar strain of Plasmodium vivax. Journal of Parasitology. 1958;44:371–373. [PubMed] [Google Scholar]
- Burkot TR, Williams JL, Schneider I. Infectivity to mosquitoes of Plasmodium falciparum clones grown in vitro from the same isolate. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1984;78:339–341. doi: 10.1016/0035-9203(84)90114-7. [DOI] [PubMed] [Google Scholar]
- Burkot TR, Wirtz RA, Paru R, Garner P, Alpers MP. The population dynamics in mosquitoes and humans of two Plasmodium vivax polymorphs distinguished by different circumsporozoite protein repeat regions. American Journal of Tropical Medicine and Hygiene. 1992;47:778–786. doi: 10.4269/ajtmh.1992.47.778. [DOI] [PubMed] [Google Scholar]
- Carter R, Graves PM. Gametocytes. In: Wernsdorfer WH, McGregor I, editors. Malaria. Churchill Livingstone; Edinburgh, U.K.: 1988. pp. 253–306. [Google Scholar]
- Clyde DF. Epidemiological significance of immunity in vivax malaria. Epidemiologic Reviews. 1989;11:109–125. doi: 10.1093/oxfordjournals.epirev.a036032. [DOI] [PubMed] [Google Scholar]
- Coatney GR, Collins WE, Warren M, Contacos PG. The primate malarias. U.S. Department of Health, Education, and Welfare; Bethesda, Maryland: 1971. [Google Scholar]
- Coatney GR, Cooper WC, Ruhe DS, Young MD, Burgess RW. Studies in human malaria. XVIII. The life pattern of sporozoite-induced St. Elizabeth strain vivax malaria. American Journal of Hygiene. 1950;51:200–215. [Google Scholar]
- Coatney GR, Cooper WC, Young MD. Studies in human malaria. XXX. A summary of 204 sporozoite-induced infections with the Chesson strain of Plasmodium vivax. Journal of the National Malaria Society. 1950;9:381–396. [PubMed] [Google Scholar]
- Coatney GR, Young MD. The taxonomy of the human malaria parasites with notes on the principal American strains. In: Moulton PR, editor. Human malaria. American Association for the Advancement of Science; Washington, District of Columbia: 1941. pp. 19–24. [Google Scholar]
- Collins WE, Jeffery GM. Primaquine resistance in Plasmodium vivax. American Journal of Tropical Medicine and Hygiene. 1996;55:243–249. doi: 10.4269/ajtmh.1996.55.243. [DOI] [PubMed] [Google Scholar]
- Collins WE, Jeffery GM. A retrospective examination of sporozoite-and trophozoite-induced infections with Plasmodium falciparum. American Journal of Tropical Medicine and Hygiene. 1999;61(s1):4–48. doi: 10.4269/tropmed.1999.61-04. [DOI] [PubMed] [Google Scholar]
- Collins WE, Warren M, Huong AY, Skinner JC, Sutton BB, Stanfill PS. Studies of comparative infectivity of fifteen strains of Plasmodium vivax to laboratory-reared anopheline mosquitoes, with special reference to Anopheles culifacies. Journal of Parasitology. 1986;72:521–524. [PubMed] [Google Scholar]
- Collins WE, Warren M, Skinner JC, Richardson BB, Kearse TS. Effect of sequential infection with Plasmoidium vivax and P. falciparum in the Aotus trivirgatus monkey. Journal of Parasitology. 1979;65:605–608. [PubMed] [Google Scholar]
- Cooper WC, Coatney GR, Culwell WB, Eyles DE, Young MD. Studies in human malaria XXVI. Simultaneous infection with the Chesson and the St. Elizabeth strains of Plasmodium vivax. Journal of the National Malaria Society. 1950;9:187–190. [PubMed] [Google Scholar]
- Covell G, Nicol WD. Clinical chemotherapeutic and immunological studies on induced malaria. British Medical Bulletin. 1951;8:51–55. doi: 10.1093/oxfordjournals.bmb.a074054. [DOI] [PubMed] [Google Scholar]
- Cox FEG. Parasitic protozoa. In: Cox FEG, editor. Modern parasitology. Blackwell Scientific; London, U.K.: 1993. pp. 1–23. [Google Scholar]
- De Buck A. Some results of six years’ mosquito infection work. American Journal of Hygiene. 1936;24:1–18. [Google Scholar]
- Drakeley CJ, Secka I, Correa S, Greenwood BM, Targett GA. Host haematological factors influencing the transmission of Plasmodium falciparum gametocytes to Anopheles gambiae s.s. mosquitoes. Tropical Medicine and International Health. 1999;4:131–138. doi: 10.1046/j.1365-3156.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- Eyles DE, Young MD, Burgess RW. Studies on imported malarias. 8. Infectivity to Anopheles quadrimaculatus of asymptomatic Plasmodium vivax parasitemias. Journal of the National Malaria Society. 1948;7:125–137. [PubMed] [Google Scholar]
- Fairley NH. Sidelights on malaria in man obtained by subinoculation experiments. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1947;40:621–676. doi: 10.1016/0035-9203(47)90025-4. [DOI] [PubMed] [Google Scholar]
- Galinski MR, Barnwell JW. Plasmodium vivax: Merozoites, invasion of reticulocytes and considerations for malaria vaccine development. Parasitology Today. 1996;12:20–28. doi: 10.1016/0169-4758(96)80641-7. [DOI] [PubMed] [Google Scholar]
- Gamage-Mendis AC, Rajakaruna J, Carter R, Mendis KN. Infectious reservoir of Plasmodium vivax and Plasmodium falciparum malaria in an endemic region of Sri Lanka. American Journal of Tropical Medicine and Hygiene. 1991;45:479–487. doi: 10.4269/ajtmh.1991.45.479. [DOI] [PubMed] [Google Scholar]
- Garnham PCC. Malaria parasites and other Haemosporidia. Vol. 1. Blackwell Scientific; Oxford, U.K.: 1966. p. 114. [Google Scholar]
- Gilles HM. The malaria parasites. In: Gilles HM, Warrell DA, editors. Bruce-Chwatt’s essential malariology. Edward Arnold; London, U.K.: 1993. pp. 12–34. [Google Scholar]
- Glynn JR, Bradley DJ. Inoculum size, incubation period and severity of malaria. Analysis of data from malaria therapy records. Parasitology. 1995;110:7–19. doi: 10.1017/s0031182000080999. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ceron L, Rodriguez MH, Nettel JC, Villarreal C, Kain KC, Hernandez JE. Differential susceptibilities of Anopheles albimanus and Anopheles pseudopunctipennis to infections with coindigenous Plasmodium vivax variants VK210 and VK247 in Southern Mexico. Infection and Immunity. 1999;67:410–412. doi: 10.1128/iai.67.1.410-412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Maiden MCJ, Feavers IM, Nee S, May RM, Anderson RM. The maintenance of strain structure in populations of recombining infectious agents. Nature Medicine. 1996;2:437–442. doi: 10.1038/nm0496-437. [DOI] [PubMed] [Google Scholar]
- Hegner R. Relative frequency of ring-stage plasmodia in reticulocytes and mature erythrocytes in man and monkey. American Journal of Hygiene. 1938;27:690–719. [Google Scholar]
- James SP. Some general results of a study of induced malaria in England. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1931;24:477–588. [Google Scholar]
- James SP, Nicol WD, Shute PG. Clinical and parasitological observations on induced malaria. Proceedings of the Royal Society of Medicine. 1936;29:879–894. [PMC free article] [PubMed] [Google Scholar]
- Joshi H, Subbarao SK, Adak T, Nanda N, Ghosh SK, Carter R, Sharma VP. Genetic structure of Plasmodium vivax isolates in India. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1997;91:231–235. doi: 10.1016/s0035-9203(97)90235-2. [DOI] [PubMed] [Google Scholar]
- Kain KC, Brown AE, Lanar DE, Ballou WR, Webster HK. Response of Plasmodium vivax variants to chloroquine as determined by microscopy and quantitative polymerase chain reaction. American Journal of Tropical Medicine and Hygiene. 1993;49:478–484. doi: 10.4269/ajtmh.1993.49.478. [DOI] [PubMed] [Google Scholar]
- James SP, Keystone J, Franke ED, Lanar DE. Global distribution of a variant of the circumsporozoite gene of Plasmodium vivax. Journal of Infectious Diseases. 1991;164:208–210. doi: 10.1093/infdis/164.1.208. [DOI] [PubMed] [Google Scholar]
- Kaplan LI, Read HS, Becker FT. Homologous and heterologous strains of Plasmodium vivax: A cross-inoculation study of malaria strain immunity. Journal of Laboratory and Clinical Medicine. 1946;31:400–408. [PubMed] [Google Scholar]
- Kitchen SF. The infection of reticulocytes by Plasmodium vivax. American Journal of Tropical Medicine. 1938;18:347–359. [Google Scholar]
- Kitchen SF. Vivax malaria. In: Boyd MF, editor. Malariology. W.B. Saunders; Philadelphia, Pennsylvania: 1949. pp. 1027–1045. [Google Scholar]
- Kitchen SF, Putnam P. Observations on the character of the paroxysm in vivax malaria. Journal of the National Malaria Society. 1946;5:57–78. [Google Scholar]
- Knowles R, Basu BC. Laboratory studies on the infectivity of Anopheles stephensi. Journal of the Malaria Institute of India. 1944;5:1–29. [Google Scholar]
- Kolakovich KA, Ssengoba A, Wojcik K, Tsuboi T, Al-Yaman F, Alpers M, Adams JH. Plasmodium vivax: Favored gene frequencies of the merozoite surface protein-1 and the multiplicity of infection in a malaria endemic region. Experimental Parasitology. 1996;83:11–18. doi: 10.1006/expr.1996.0044. [DOI] [PubMed] [Google Scholar]
- Machado RLD, Povoa MM. Distribution of Plasmodium vivax variants (VK210, VK247 and P. vivax-like) in three endemic areas of the Amazon region of Brazil and their correlation with chloroquine treatment. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000;94:377–381. doi: 10.1016/s0035-9203(00)90110-x. [DOI] [PubMed] [Google Scholar]
- Mason DP, McKenzie FE. Blood-stage dynamics and clinical implications of mixed Plasmodium vivax–Plasmodium falciparum infections. American Journal of Tropical Medicine and Hygiene. 1999;61:367–374. doi: 10.4269/ajtmh.1999.61.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie FE, Bossert WH. Mixed-species Plasmodium infections of Anopheles (Diptera: Culicidae) Journal of Medical Entomology. 1997;34:417–425. doi: 10.1093/jmedent/34.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie FE, Bossert WH. The optimal production of gametocytes by Plasmodium falciparum. Journal of Theoretical Biology. 1998a;193:419–428. doi: 10.1006/jtbi.1998.0710. [DOI] [PubMed] [Google Scholar]
- McKenzie FE, Bossert WH. A target for intervention in Plasmodium falciparum infections. American Journal of Tropical Medicine and Hygiene. 1998b;58:763–767. doi: 10.4269/ajtmh.1998.58.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie FE, Bossert WH. Multispecies Plasmodium infections of humans. Journal of Parasitology. 1999;85:12–18. [PMC free article] [PubMed] [Google Scholar]
- McKenzie FE, Jeffery GM, Collins WE. Plasmodium malariae blood-stage dynamics. Journal of Parasitology. 2001;87:626–637. doi: 10.1645/0022-3395(2001)087[0626:PMBSD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendis KN, Carter R. The role of cytokines in Plasmodium vivax malaria. Memorias Instituto Oswaldo Cruz. 1992;87(s3):51–55. doi: 10.1590/s0074-02761992000700006. [DOI] [PubMed] [Google Scholar]
- Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in Blacks. The Duffy-blood-group genotype, FyFy. New England Journal of Medicine. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- Pampana E. A textbook of malaria eradication. Oxford; London, U.K.: 1963. p. 508. [Google Scholar]
- Paul REL, Coulson TN, Raibaud A, Brey PT. Sex determination in malaria parasites. Science. 2000;287:128–131. doi: 10.1126/science.287.5450.128. [DOI] [PubMed] [Google Scholar]
- Putaporntip C, Jongwutiwes S, Tanabe K, Thaitong S. Interallelic recombination in the merozoite surface protein 1 (MSP-1) gene of Plasmodium vivax from Thai isolates. Molecular and Biochemical Parasitology. 1997;84:49–56. doi: 10.1016/s0166-6851(96)02786-7. [DOI] [PubMed] [Google Scholar]
- Read AF, Anwar M, Shutler D, Nee S. Sex allocation and population structure in malaria and related parasitic protozoa. Proceedings of the Royal Society of London B. 1995;260:359–363. doi: 10.1098/rspb.1995.0105. [DOI] [PubMed] [Google Scholar]
- Reisberg B. Malaria. In: Schulman ST, Phair JP, Peterson LR, Warren JR, editors. The biologic and clinical basis of infectious diseases. W.B. Saunders; Philadelphia, Pennsylvania: 1997. pp. 454–466. [Google Scholar]
- Robert V, Read AF, Essong J, Tchuinkam T, Mulder B, Verhave JP, Carnevale P. Effect of gametocyte sex ratio on infectivity of Plasmodium falciparum to Anopheles gambiae. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996;90:621–624. doi: 10.1016/s0035-9203(96)90408-3. [DOI] [PubMed] [Google Scholar]
- Robert V, Tchuinkam T, Mulder B, Bodo JM, Verhave JP, Carnevale P, Nagel RL. Effect of the sickle cell trait status on gametocyte carriers of Plasmodium falciparum on infectivity to anopheleines. American Journal of Tropical Medicine and Hygiene. 1996;54:111–113. doi: 10.4269/ajtmh.1996.54.111. [DOI] [PubMed] [Google Scholar]
- Sattabongkot J, Maneechai N, Rosenberg R. Plasmodium vivax: Gametocyte infectivity of naturally infected Thai adults. Parasitology. 1991;102:27–31. doi: 10.1017/s0031182000060303. [DOI] [PubMed] [Google Scholar]
- Shushan M, Blitz O, Adams CC. The role of reticulocytes in malaria. Journal of Laboratory and Clinical Medicine. 1937;22:364–370. [Google Scholar]
- Shute PG. Thirty years of malaria-therapy. Journal of Tropical Medicine and Hygiene. 1958;61:57–61. [PubMed] [Google Scholar]
- Shute PG, Lupascu G, Branzei P, Maryon M, Constantinescu P, Bruce-Chwatt LJ, Draper CC, Killick-Kendrick R, Garnham PCC. A strain of Plasmodium vivax characterized by prolonged incubation: The effect of numbers of sporozoites on the length of the prepatent period. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1976;70:474–481. doi: 10.1016/0035-9203(76)90132-2. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Silamut K, Chotivanich K, Pukrittayakamee S, White NJ. Red cell selectivity in malaria: A study of multiple-infected erythrocytes. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1999;93:165–168. doi: 10.1016/s0035-9203(99)90295-x. [DOI] [PubMed] [Google Scholar]
- Snewin VA, Longacre S, David PH. Plasmodium vivax: Older and wiser? Research in Immunology. 1991;142:631–636. doi: 10.1016/0923-2494(91)90140-e. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. W. H. Freeman; New York, New York: 1981. p. 859. [Google Scholar]
- Suwanabun N, Sattabongkot J, Wirtz RA, Rosenberg R. The epidemiology of Plasmodium vivax circumsporozoite protein polymorphs in Thailand. American Journal of Tropical Medicine and Hygiene. 1994;50:460–464. doi: 10.4269/ajtmh.1994.50.460. [DOI] [PubMed] [Google Scholar]
- Thaitong S, Beale GH, Fenton B, McBride J, Rosario V, Walker A, Walliker D. Clonal diversity in a single isolate of the malaria parasite Plasmodium falciparum. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1984;78:242–245. doi: 10.1016/0035-9203(84)90287-6. [DOI] [PubMed] [Google Scholar]
- Tiburskaja NA, Sergiev PG, Vrublevskaja OS. Dates of onset of relapses and the duration of infection in induced tertian malaria with short and long incubation periods. Bulletin of the World Health Organization. 1968;38:447–457. [PMC free article] [PubMed] [Google Scholar]
- Trager W, Gill GS. Enhanced gametocyte formation in young erythrocytes by Plasmodium falciparum in vitro. Journal of Protozoology. 1992;39:429–432. doi: 10.1111/j.1550-7408.1992.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Trager W, Gill GS, Lawrence C, Nagel RL. Plasmodium falciparum: Enhanced gametocyte formation in vitro in reticulocyte-rich blood. Experimental Parasitology. 1999;91:115–118. doi: 10.1006/expr.1998.4347. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Kappe SHI, Al-Yaman F, Prickett MD, Alpers M, Adams JH. Natural variation within the principal adhesion domain of the Plasmodium vivax Duffy binding protein. Infection and Immunity. 1994;62:5581–5586. doi: 10.1128/iai.62.12.5581-5586.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagama PV, Gamage-Mendis AC, David PH, Peiris JSM, Perera KLRL, Mendis KN, Carter R. Genetic complexity of Plasmodium vivax parasites in individual human infections analyzed with monoclonal antibodies against variant epitopes on a single parasite protein. American Journal of Tropical Medicine and Hygiene. 1990;42:104–110. doi: 10.4269/ajtmh.1990.42.104. [DOI] [PubMed] [Google Scholar]
- Watson BW. On the probability of soldiers with Pacific Plasmodium vivax malaria infecting Anopheles quadrimaculatus. Journal of the National Malaria Society. 1945;4:183–188. [Google Scholar]
- Whorton CM, Yount E, Jr, Jones R, Jr, Alving AS, Pullman TN, Craige B, Jr, Eichelberger L. The Chesson strain of Plasmodium vivax malaria. III. Clinical aspects. Journal of Infectious Diseases. 1947;80:237–249. doi: 10.1093/infdis/80.3.237. [DOI] [PubMed] [Google Scholar]
- Wirtz RA, Rosenberg R, Sattabongkot J, Webster HK. Prevalence of antibody to heterologous circumsporozoite protein of Plasmodium vivax in Thailand. Lancet. 1990;336:593–595. doi: 10.1016/0140-6736(90)93393-4. [DOI] [PubMed] [Google Scholar]
- Wirtz RA, Sattabongkot J, Hall T, Burkot TR, Rosenberg R. Development and evaluation of an enzyme-linked immunosorbent assay for Plasmodium vivax-VK247 sporozoites. Journal of Medical Entomology. 1992;29:854–857. doi: 10.1093/jmedent/29.5.854. [DOI] [PubMed] [Google Scholar]
- Woolley IJ, Hotmire KA, Sramkoski RM, Zimmerman PA, Kazura JW. Differential expression of the Duffy antigen receptor for chemokines according to RBC age and FY genotype. Transfusion. 2000;40:949–953. doi: 10.1046/j.1537-2995.2000.40080949.x. [DOI] [PubMed] [Google Scholar]
- Worku S, Bjorkman A, Troye-Blombergm M, Jemaneh L, Farnert A. Lymphocyte activation and subset redistribution in the peripheral blood in acute malaria illness: Distinct γδ+ T cell patterns in Plasmodium falciparum and P. vivax infections. Clinical and Experimental Immunology. 1997;108:34–41. doi: 10.1046/j.1365-2249.1997.d01-981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MD. Studies on the periodicity of induced Plasmodium vivax. Journal of the National Malaria Society. 1944;3:237–240. [Google Scholar]


