Abstract
We analyzed records of malariotherapy patients sequentially or simultaneously inoculated with Plasmodium falciparum and Plasmodium malariae. Gametocyte production was enhanced in P. falciparum by prior or concurrent P. malariae infection but diminished or unaffected in P. malariae by P. falciparum. Conversely, asexual-form production was diminished in P. malariae but unaffected in P. falciparum.
INTRODUCTION
The four Plasmodium species that cause human malaria often co-occur in human populations and individuals. In concurrently infected humans, densities of the species’ asexual blood stages typically alternate.1,2 Gametocytes, the sexual, transmissible, nonreplicating blood stages of the parasite, arise from a diversion of asexual-form production; gametocyte density increases by accumulation and represents a complex tradeoff with the compound growth of the asexual cycle.3,4 Asexual forms are associated with pathogenesis; gametocytes are apparently clinically benign. Although gametocyte production has not been studied previously in mixed-species malaria infections, we have speculated that it plays a prominent role in the dynamics of these infections in humans.5,6
Current knowledge of Plasmodium dynamics in infected humans derives mostly from 40 years of work with malaria induced to treat neurosyphilis.7–10 Many fundamental insights are owed to these malariotherapy patients, including the doctrine of species specificity and strain specificity in malaria immunity, inferred from clinical and parasitologic responses to sequential inoculations.
We previously analyzed records of malariotherapy patients inoculated with either the McLendon strain of Plasmodium falciparum or the USPHS strain of Plasmodium malariae.11,12 Here we analyze and compare parasite blood-stage dynamics reflected in the records of patients sequentially or simultaneously inoculated with both. In 14 patients, P. falciparum inoculation followed P. malariae inoculation, and in another 14, the order was reversed. In an additional 2 patients, what were thought to have been single-species inocula proved to include subpatent densities of the other species. We tested these 30 charts against those from 74 P. malariae and 63 P. falciparum single-species infections for differences in gametocyte or asexual-form dynamics.
MATERIALS AND METHODS
Malariotherapy treatment and data collection procedures are described in detail in Collins and Jeffery11 and McKenzie et al12 and references therein; the article by Collins and Jeffery11 contains extensive information about the participation and treatment of the patient population considered here and is accompanied by an explicit, independent analysis of relevant ethical issues.
We examined the records of adult neurosyphilis patients with no known history of previous malaria infection, treated with the McLendon strain of P. falciparum or USPHS strain of P. malariae or both between 1940 and 1958 in the United States Public Health Service (USPHS) facility in Columbia, South Carolina. Occasionally, when medical staff determined that therapeutic objectives had not been met by an initial malaria infection, the patient was reinoculated, with the same or a different Plasmodium species or strain. Here P. falciparum inoculation followed P. malariae inoculation by 36 to 175 days (mean, 67 days), and P. malariae inoculation followed P. falciparum inoculation by 56 to 244 days (mean, 98 days). All P. malariae and most P. falciparum infections were initiated by inoculation of 5 mL of whole blood from a patently infected patient; 17 of the P. falciparum infections analyzed were initiated by inoculation of sporozoites extracted from infectious mosquitoes. The 2 inoculation modes led to a difference in prepatency, as would be expected, but not in any other variable considered here.
We present the first day of patent (detected) parasitemia as Day 1 of an infection, in effect the day on which observed asexual-form parasite density reached 10 per μl of blood. In each infection, only the male (micro-) gametocytes were recorded.11,12 The P. falciparum charts include daily records of parasitemia and gametocytemia for > 99% of the days of each infection after initial patency; the occasional blank daily records in patient charts reflect isolated absences or omissions by staff. The P. malariae charts from 1940–1949 include records of parasitemia and gametocytemia for an average 45% of the days of each infection after initial patency (in an alternate-day pattern but with an additional omission every 9th day); our analysis of P. malariae dynamics includes only charts from this period, rather than the near-complete 1950–1958 charts, because the only post–P. falciparum P. malariae charts that satisfy the subsequent criteria are from 1940–1949.12
Malariotherapy infections generally were allowed to continue as long as possible without intervention. At the discretion of medical staff, subcurative doses of various drugs might be given to provide temporary breaks, or a curative dose might be given to terminate the infection. Our analyses include only the parts of charts that preceded any such intervention. Drug intervention biases the values of timing-related variables (e.g., a natural peak in parasite density on Day 8 of patency cannot be observed in an infection terminated on Day 7). We determined the optimal minimum length for each species infection by regression, as described by McKenzie et al12: only P. malariae infections observed > 35 days and P. falciparum infections observed > 21 days without intervention were included in analyses. Extending these lengths to > 50 days and > 35 days did not alter the results reported here.
Because the observed asexual-form patency before intervention was > 137 days in only 1 post–P. malariae P. falciparum infection and > 191 days in only 1 post–P. falciparum P. malariae infection, we truncated the array of these and the corresponding single-species charts so as to examine intervals of 19 and 27 weeks between the first and final asexual-form patencies, then 18 and 26 weeks, and so forth. Similarly, because gametocyte patency was > 70 days in only 1 post–P. malariae P. falciparum infection and > 75 days in only 1 post–P. falciparum P. malariae infection, we truncated these arrays so as to examine intervals of 10 weeks between the first and final gametocyte patencies, then 9 weeks, and so forth.
We used the Mann-Whitney test to examine distributional differences, with P values < 0.05 taken to mark significance. We used Fisher’s exact test with contingency tables, with a P value < 0.05 marking a significant difference, and simple linear regression.13 The timing of asexual-form and gametocyte peaks was measured relative to the initial patency of asexual forms and gametocytes, as was their mean daily density. Our reliance on 2 untransformed arithmetic mean densities follows in part from the presence of transient daily records of density 0, which in turn follows unavoidably from the presence of a detection threshold (as discussed subsequently). In addition to the 8 variables mentioned subsequently, we compared inoculum size (only for blood-initiated infections), prepatency (the number of days between inoculation and asexual-form patency), initial detected density of asexual forms, and length of time infections were observed before intervention. For each set of charts, we conducted comparisons across all charts and across only the charts in which gametocytes were detected on at least 1 day. We compared all 12 variables in the single-species P. falciparum infections with their pre–P. malariae and post–P. malariae counterparts and in the single-species P. malariae infections with their pre–P. falciparum and post–P. falciparum counterparts and found no differences other than those noted subsequently.
There were no differences between the 25 post–P. malariae and 80 single-species P. falciparum mosquito feeds with respect to the day of asexual patency on which the feed occurred, the detected presence or absence of gametocytes on the day of the feed, gametocyte density on the day of feed (for densities > 0), the proportion of feeds in which at least 1 mosquito was infected, or the proportion of mosquitoes infected at feeds. The detected presence or absence of gametocytes did not affect the proportion of feeds in which at least 1 mosquito was infected, among the feeds either on the post–P. malariae or the single-species P. falciparum infections.
RESULTS
When P. falciparum followed or accompanied P. malariae infection, circulating P. falciparum gametocytes first were detected 4 days earlier, relative to the initial P. falciparum asexual-form patency; their mean daily density was 3.5 times and their peak density 4 times that in P. falciparum single-species infections (Figure 1). Over the first 5 to 10 weeks after their initial detection, the mean daily density of P. falciparum gametocytes was 3 to 5 times that in the single-species infections; the overall peak density of gametocytes, measured relative to their initial detection (see Methods) was delayed by 11 days. There were no differences in any measure of P. falciparum asexual-form production between post–P. malariae and single-species P. falciparum charts.
Figure 1.
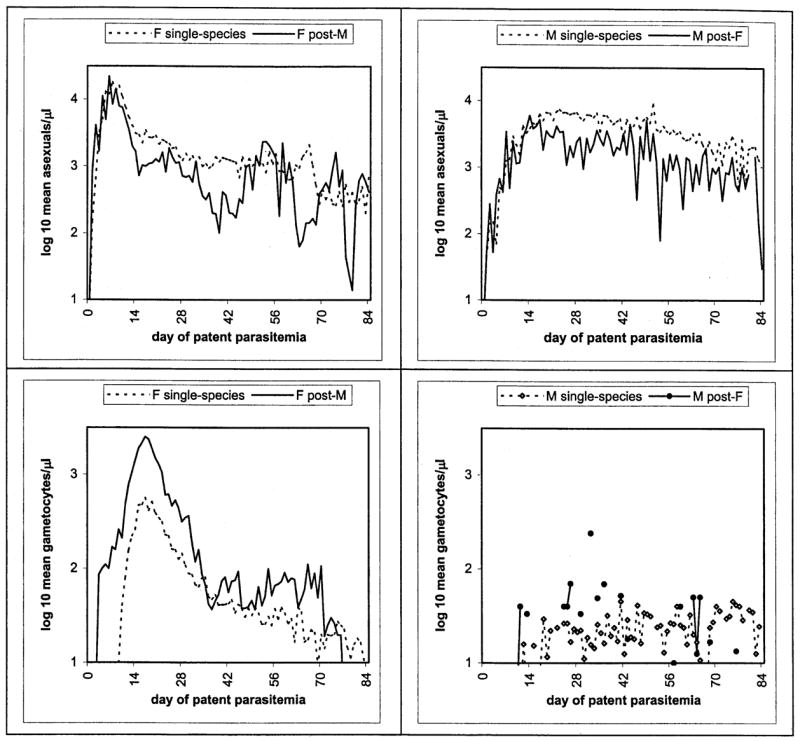
Plots comparing single-species P. falciparum (F, left panels) or P. malariae (M) infections with those that followed or accompanied cross-species infections, in terms of mean daily asexual-form (top panels) and gametocyte densities.
When P. malariae followed or accompanied P. falciparum infection, the peak P. malariae asexual-form density was 40%, and the mean daily density was 55%, less than that in P. malariae single-species infections (Figure 1); the peak density of P. malariae asexual forms occurred 9 days earlier, relative to their initial patency. Considered across all such charts, the initial detected density and mean daily density of P. malariae gametocytes were 45% lower in the post–P. falciparum than the single-species P. malariae charts. An analysis restricted to charts in which gametocytes were detected on at least 1 day showed no differences, however, in any measure of P. malariae gametocyte production; the proportion of such charts did not differ between the post–P. falciparum and single-species P. malariae infections.
No qualitative changes in these results appeared when the 2 simultaneous inoculations or these and the charts with the 2 next shortest interinoculation intervals (36 to 37 days between P. malariae and P. falciparum, 56 to 57 days between P. falciparum and P. malariae) were excluded from analysis, suggesting that, at least within this range of interinfection intervals, our conclusions are independent of the duration of the initial infection. We also asked whether our conclusions with respect to relative densities had been influenced by the duration of patent infection before intervention; we did so by systematically truncating each array of charts so as to examine successively shorter intervals between first and final patencies (see Methods). We found no critical time points with respect to asexual-form densities, but for gametocyte densities, we were unable to distinguish between post–P. malariae and single-species P. falciparum infections when only the 1 to 4 weeks immediately following the initial gametocyte patency were evaluated. That is, at least one of the differences so evident in Figure 1 and in tests over longer intervals disappears if investigated on only a short-term basis.
To prepare for subsequent natural inoculations, cages of mosquitoes sometimes were allowed to feed on malariotherapy patients; on 25 occasions, mosquitoes fed on a patient with a post–P. malariae P. falciparum infection. We compared these with 80 occasions on which mosquitoes fed on a patient with a single-species P. falciparum infection and found no salient differences between them, including, remarkably, density of gametocytes and frequency of transmission (see Methods). Among the feeds on post–P. malariae infections, however, gametocyte density was higher in the 17 in which transmission occurred than in the 8 in which it did not; similarly, these feeds showed a positive relationship between gametocyte density and the proportion of mosquitoes infected (r2 = 0.43 versus r2 = 0.02 among the single-species infections). There were no recorded mosquito feeds on the post–P. falciparum P. malariae infections.
DISCUSSION
The sequence of species inoculation seems to affect the dynamics of malaria infections in humans and specifically the manner in which P. falciparum and P. malariae trade off asexual-form and gametocyte production. Among these patients, P. falciparum gametocytes were patent earlier and at higher daily densities in post–P. malariae infections, despite enormous interindividual variation during the first weeks. Our results are less clear-cut for P. malariae than P. falciparum gametocytes, perhaps because their lower densities overall were affected more strongly by the threshold of detection; although asexual-form densities of P. malariae were also lower, they were typically above the threshold and less variable. Modern methods are also subject to detection thresholds, but they can reveal dynamics inaccessible by microscopy, and we expect that patterns at these subpatent densities would prove at least as intriguing as those analyzed here.14,15 We would like to learn much more about the relatively sustained gameotcytemia and variable asexual-form parasitemia apparent in the post–P. malariae P. falciparum infections, for instance, but no other malaria infections are likely to be monitored so frequently and so long without drug intervention.16
Our results with P. malariae and P. falciparum contrast with anecdotes from fieldwork in Papua New Guinea and studies with Aotus monkeys, which suggest mixed-species and single-species infections with P. vivax and P. falciparum differ in infectivity.17,18 Our results suggest complex relationships between infectivity and gametocyte density, but the question calls for further investigation, particularly given evidence that P. falciparum (and P. vivax) infectivity increases with the proportion of male gametocytes.1–4,19–23 In this context, it is intriguing that although P. falciparum/P. malariae is the mixed-species combination common to Africa and Asia, the frequency of this combination relative to statistical expectation differs dramatically between the 2 continents.5,6
Our mathematical model of mixed P. falciparum/P. malariae infections in its present form assumes that interactions between the parasite species are immune-mediated and depend largely on their relative rates of asexual multiplication; it does not incorporate the influence of gametocyte production on the latter.24 Our results here do not show a straightforward tradeoff between asexual-form and gametocyte production and so indicate a need for further research on this relationship as well. These and other detailed studies of mixed-species malaria infections can provide valuable insights into how parasite dynamics within individual hosts relate to those in populations of hosts and so may bear on intraspecies and interspecies interactions in Plasmodium, with the corresponding implications for interventions.25–29
Acknowledgments
The present study and many others would not have been possible without the participation of hundreds of malariotherapy patients, to whom we are extremely grateful. The authors thank the contributions of W. H. Bossert, K. E. Fischer, S. Katz, D. P. Mason, V. Putney, L. Simonsen, B. C. Sorkin, and an anonymous reviewer.
References
- 1.Boyd MF, editor. Malariology. Philadelphia: WB Saunders; 1949. [Google Scholar]
- 2.Wernsdorfer WH, McGregor I, editors. Malaria. Edinburgh: Churchill Livingstone; 1988. [Google Scholar]
- 3.McKenzie FE, Bossert WH. The dynamics of Plasmodium falciparum blood-stage infection. J Theor Biol. 1997;188:127–140. doi: 10.1006/jtbi.1997.0478. [DOI] [PubMed] [Google Scholar]
- 4.McKenzie FE, Bossert WH. The optimal production of gametocytes by Plasmodium falciparum. J Theor Biol. 1998;193:419–428. doi: 10.1006/jtbi.1998.0710. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie FE, Bossert WH. Mixed-species Plasmodium infections of humans. J Parasitol. 1997;83:593–600. [PMC free article] [PubMed] [Google Scholar]
- 6.McKenzie FE, Bossert WH. Multispecies Plasmodium infections of humans. J Parasitol. 1999;85:12–18. [PMC free article] [PubMed] [Google Scholar]
- 7.Winckel CWF. Are the experimental data of therapeutic malaria applicable to conditions obtaining in nature? Am J Trop Med. 1941;21:789–794. [Google Scholar]
- 8.Covell G, Nicol WD. Clinical, chemotherapeutic and immunological studies on induced malaria. Br Med Bull. 1951;8:51–55. doi: 10.1093/oxfordjournals.bmb.a074054. [DOI] [PubMed] [Google Scholar]
- 9.Jeffery GM. Epidemiological significance of repeated infections with homologous and heterologous strains and species of Plasmodium. Bull WHO. 1966;35:873–882. [PMC free article] [PubMed] [Google Scholar]
- 10.Chernin E. The malariatherapy of neurosyphilis. J Parasitol. 1984;70:611–617. [PubMed] [Google Scholar]
- 11.Collins WE, Jeffery GM. A retrospective examination of sporozoite- and trophozoite-induced infections with Plasmodium falciparum. Am J Trop Med Hyg. 1999;61s:4–48. doi: 10.4269/tropmed.1999.61-04. [DOI] [PubMed] [Google Scholar]
- 12.McKenzie FE, Jeffery GM, Collins WE. Plasmodium malariae blood-stage dynamics. J Parasitol. 2001;87:626–637. doi: 10.1645/0022-3395(2001)087[0626:PMBSD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokal RR, Rohlf FJ. Biometry. New York: WH Freeman; 1981. [Google Scholar]
- 14.Snounou G, Pinheiro L, Goncalves A, Fonseca L, Dias F, Brown KN, do Rosario VE. The importance of sensitive detection of malaria parasites in the human and insect hosts in epidemiological studies, as shown by the analysis of field samples from Guinea Bissau. Trans R Soc Trop Med Hyg. 1993;88:649–653. doi: 10.1016/0035-9203(93)90274-t. [DOI] [PubMed] [Google Scholar]
- 15.Farnert A, Snounou G, Rooth I, Bjorkman A. Daily dynamics of Plasmodium subpopulations in asymptomatic children in a holoendemic area. Am J Trop Med Hyg. 1997;56:538–547. doi: 10.4269/ajtmh.1997.56.538. [DOI] [PubMed] [Google Scholar]
- 16.Delley V, Bouvier P, Breslow N, Doumbo O, Sagara I, Diakite M, Mauris A, Dolo A, Rougemont A. What does a single determination of malaria parasite density mean? A longitudinal survey in Mali. Trop Med Int Health. 2000;5:404–412. doi: 10.1046/j.1365-3156.2000.00566.x. [DOI] [PubMed] [Google Scholar]
- 17.Graves PM, Burkot TR, Carter R, Cattani JA, Lagog M, Parker J, Brabin BJ, Gibson FD, Bradley DJ, Alpers MP. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua New Guinea. Parasitology. 1988;96:251–263. doi: 10.1017/s003118200005825x. [DOI] [PubMed] [Google Scholar]
- 18.Collins WE, Warren McW, Skinner JC, Richardson BB, Kearse TS. Effect of sequential infection with Plasmodium vivax and P. falciparum in the Aotus trivirgatus monkey. J Parasitol. 1979;65:605–608. [PubMed] [Google Scholar]
- 19.Jeffery GM. Infectivity to mosquitoes of Plasmodium vivax and Plasmodium falciparum under various conditions. Am J Trop Med. 1960;9:315–320. doi: 10.4269/ajtmh.1960.9.315. [DOI] [PubMed] [Google Scholar]
- 20.Noden BH, Beadle PS, Vaughan JA, Pumpuni CB, Kent MD, Beier JC. Plasmodium falciparum: The population structure of mature gametocyte cultures has little effect on their innate fertility. Acta Trop. 1994;58:13–19. doi: 10.1016/0001-706x(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 21.Haji H, Smith T, Charlwood JD, Meuwissen JH. Absence of relationships between selected human factors and natural infectivity of Plasmodium falciparum to mosquitoes in an area of high transmission. Parasitology. 1996;113:425–431. doi: 10.1017/s0031182000081488. [DOI] [PubMed] [Google Scholar]
- 22.Robert V, Read AF, Essong J, Tchuinkam T, Mulder B, Verhave JP, Carnevale P. Effect of gametocyte sex ratio on infectivity of Plasmodium falciparum to Anopheles gambiae. Trans R Soc Trop Med Hyg. 1996;90:621–624. doi: 10.1016/s0035-9203(96)90408-3. [DOI] [PubMed] [Google Scholar]
- 23.McKenzie FE, Jeffery GM, Collins WE. Plasmodium vivax blood-stage dynamics. J Parasitol. 2002;88:521–535. doi: 10.1645/0022-3395(2002)088[0521:PVBSD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason DP, McKenzie FE, Bossert WH. The blood-stage dynamics of mixed Plasmodium malariae-Plasmodium falciparum infections. J Theor Biol. 1999;98:549–566. doi: 10.1006/jtbi.1999.0932. [DOI] [PubMed] [Google Scholar]
- 25.Molineaux L, Gramiccia G. The Garki Project. Geneva: World Health Organization; 1980. [Google Scholar]
- 26.McKenzie FE, Bossert WH. Mixed-species Plasmodium infections of Anopheles (Diptera: Culicidae) J Med Entomol. 1997;34:417–425. doi: 10.1093/jmedent/34.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor LH, Walliker D, Read AF. Mixed-genotype infections of the rodent malaria Plasmodium chabaudi are more infectious to mosquitoes than single-genotype infections. Parasitology. 1997;155:121–132. doi: 10.1017/s0031182097001145. [DOI] [PubMed] [Google Scholar]
- 28.McKenzie FE, Bossert WH. A target for intervention in Plasmodium falciparum infections. Am J Trop Med Hyg. 1998;58:763–767. doi: 10.4269/ajtmh.1998.58.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenzie FE, Ferreira MU, Baird JK, Snounou G, Bossert WH. Meiotic recombination, cross-reactivity, and persistence in Plasmodium falciparum. Evolution. 2001;55:1299–1307. doi: 10.1111/j.0014-3820.2001.tb00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


