Abstract
Heart failure secondary to myocardial infarction (MI) remains a major source of morbidity and mortality. Long-term outcome after MI can be largely be defined in terms of its impact on the size and shape of the left ventricle (i.e., LV remodeling). Three major mechanisms contribute to LV remodeling: 1) early infarct expansion, 2) subsequent infarct extension into adjacent noninfarcted myocardium, and 3) late hypertrophy in the remote LV. Future developments in preventing post-MI heart failure will depend not only on identifying drugs targeting each of these individual mechanisms, but also on diagnostic techniques capable of assessing efficacy against each mechanism.
Keywords: Myocardial Infarction, Left Ventricular Remodeling, Cardiac Function, Heart Failure
INTRODUCTION
Despite the best efforts of public health and preventative medicine, an estimated 1.2 million Americans will suffer a new or recurrent MI this year, making coronary heart disease the single leading cause of death in the US [1]. Furthermore, it is estimated that 38% of the people who experience an MI in a given year will ultimately die from it [1]. Thus, while improvements in the standard of care for acute MI have led to steady declines in in-hospital mortality (from 11.2% to 9.4% between 1990 and 1999 [1]), heart failure secondary to MI remains a major source of morbidity and mortality. Historically, the treatments offered for heart failure have evolved along with our understanding of the mechanisms responsible for it (as reviewed by Mann [2]). Heart failure was initially viewed as a problem of excessive salt and water retention caused by abnormalities of renal blood flow (the cardiorenal model). This view was gradually replaced by the concept that heart failure was largely a problem of excessive peripheral vasoconstriction and reduced cardiac output (the cardioicirculatory model). Over the past two decades, these models have given way to a “neurohormonal” model, in which heart failure is understood in terms of the elaboration of biologically active molecules that exert deleterious effects on the heart and circulation [3]. While this model has proven valuable in identifying mechanisms and in developing effective new therapies (i.e., angiotensin converting enzyme (ACE) inhibition and β-blockade), it does not completely explain the relentless nature of disease progression. To address the progressive nature of heart failure, a “biomechanical” model was proposed [4] that acknowledges the structural basis of heart failure and postulates that LV remodeling may contribute independently to its progression [2,3,5,6]. For the purposes of this review, LV remodeling can be defined as a change in the size, shape and/or composition of the left ventricular myocardium.
While the etiologies underlying many forms of heart failure are exceedingly complex, the etiology of heart failure secondary to uncomplicated MI can be largely be defined in terms of infarct size, location and its subsequent impact on the function, shape and size of the left ventricle (i.e., LV remodeling [7,8]). For example, a recent animal study showed that infarct size as determined with MRI by late gadolinium enhancement correlated well with both subsequent end-systolic LV volumes and ejection fraction [9]. Similarly, a recent clinical study used infarct imaging to demonstrate a linear relationship between scar size and both LV volumes and ejection fraction [10]. Given that the increase in LV end-systolic volume can be predicted from infarct size, and that LV end-systolic volume is the major determinant of survival after recovery from MI [11], the value of LV remodeling as a surrogate endpoint for use in heart failure trials [12,13] has led to the suggestion that it be considered as a primary target for treatment [14,15].
Considering that LV remodeling is the major determinant of survival after recovery from MI[11], and that it has been strongly associated with clinical outcomes in numerous heart failure trials, the “biomechanical” model of heart failure has gained increasing acceptance during recent years [3]. The importance of maintaining (or regaining) a normal end-systolic volume is further supported by recent reports of “reverse-remodeling” after myocardial revascularization, LV reconstruction, mitral ring annuloplasty and other device-based treatments (e.g., LV assist devices).[16] The clinical data suggesting functional and clinical advantages associated with reverse remodeling have led to the development of innovative mechanical devices such as the Acorn passive cardiac support device [17]. This is a mesh-like, implantable device designed to prevent progressive LV dilatation, increase ejection fraction, lower LV wall stress and attenuate LV chamber sphericity [18].
The current review stresses the “biomechanical” model of heart failure and supports the contention that LV remodeling can be adopted as a primary endpoint for assessing new treatment strategies for heart failure. Furthermore, it proposes that a detailed understanding of the biomechanical mechanisms underlying the efficacy of the various treatment modalities should lead to the rational design of improved drug combinations and treatment windows that may offer increased efficacy. For example, the combination of ACE inhibitors and β-blockers have been shown to inhibit LV remodeling [5,19], but the relative contributions of the various biomechanical mechanisms underlying the efficacy of this combination remain to be fully defined. The three major biomechanical mechanisms contributing to the increase in LV chamber volume over time after MI are: 1) expansion of the infarct in the sub-acute phase [20], 2) subsequent non-ischemic infarct extension into the adjacent noninfarcted region [21,22], and 3) hypertrophy and dilatation of non-infarcted myocardium in the chronic phase [17,23,24] (Figure 1). Because these three biomechanisms operate in different regions of the LV during different time frames after MI, it is unlikely that any single drug will be completely effective in addressing all three mechanisms. The central tenant of this review is that a detailed understanding of the biomolecular progression of LV remodeling and the impact of various drugs and drug combinations on the underlying processes will lead to the development of rational combinations of treatment regimens that can be employed during the optimal time frames necessary to minimize LV remodeling post-MI. Ultimately, these treatment regimens may include invasive surgical- [17] and/or stem cell-based [25] procedures. However, the current standard of care is based on pharmaceutical therapy because it is reliable, cost-effective and straightforward to administer. For the foreseeable future, small molecule drugs will continue to constitute the first-line of defense, at least until such time as more sophisticated and complex therapeutic approaches can demonstrate competitive cost-benefit ratios.
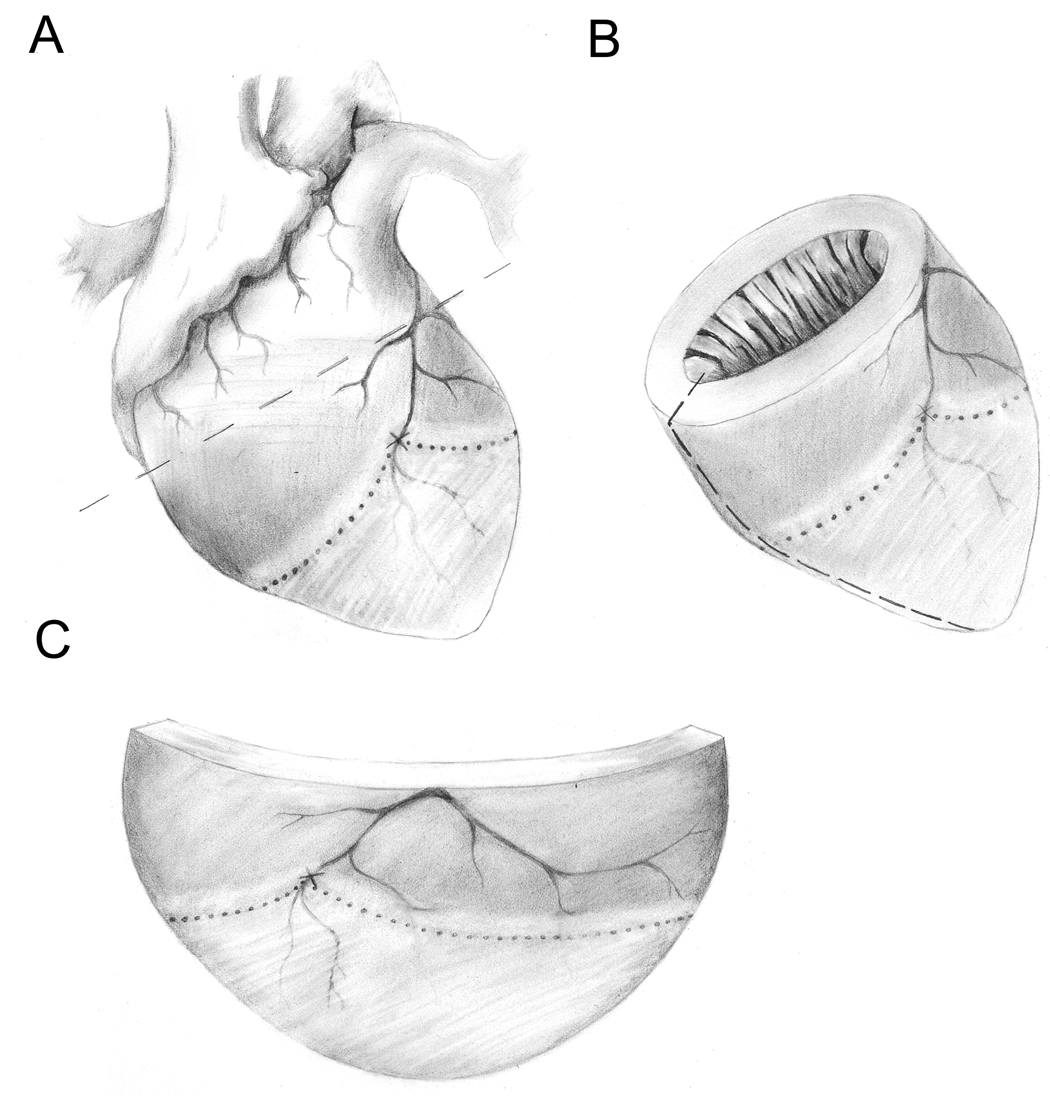
Figure 1.
Location of the infarcted, adjacent and remote regions relative to an occluded LAD. Panel A shows entire heart with atria and major vessels. The occlusion site on the LAD is indicated with an “X”. The infarct is indicted as the lightly shaded region below the dotted line. The adjacent region is indicated as the lightly shaded region above the dotted line and the remote region is indicated as the darkly shaded region above the dotted line. Panel B shows the left ventricle with an arbitrary cut line indicated along the left-hand side as a dashed line. Panel C shows a flattened version of the LV obtained after cutting the LV along the dashed line in Panel B.
A variety of novel therapeutic approaches are being explored to reduce the size of myocardial infarction in patients that present with acute coronary syndromes; however, these lie outside the scope of the current review. However, this review will be restricted to mechanisms that come into play after reperfusion has been achieved and the size of the acute infarct has already been established. Furthermore, this review will assume that reperfusion has been fully and completely achieved, since it is well established that the progression of LV remodeling is very different in reperfused versus non-reperfused infarctions [26]. The beneficial effects of revascularization on LV remodeling are well established, even when revascularization is delayed for a period of days after the onset of MI. Finally, this review will not address the problem of acute pump failure after MI, since the treatment objectives in this class of patients are clearly different from those in patients that stabilize soon after reperfusion.
1. THE INFARCT
Perhaps the most widely recognized and well-understood biomechanism underlying LV remodeling after MI is infarct expansion. Infarct expansion refers to the radial thinning and circumferential increase in the extent of a transmural (or near transmural) infarct that occurs during the days to weeks following an acute MI. In this context, it is important to distinguish between infarct expansion and the wavefront phenomenon by which necrosis proceeds from the endocardium towards the epicardium during the first few hours after reperfusion. Seminal studies conducted in a dog model of reperfused MI characterized the delay between reperfusion and the onset of cell death, but it is important to note that this same study reported infarct size was already two-thirds complete within 3 hours of reperfusion [27]. It should also be noted that the onset of cell death in these studies may have been significantly delayed by the impressive collateral circulation found in dogs. For example, serial contrast-enhanced studies of the evolution of MI indicate that infarct size reaches 95% of its final size within 1 hour of reperfusion in mice [28] (where the collateral circulation is negligible). Finally, one should distinguish between the wavefront phenomenon and non-ischemic infarct extension (as defined below under “Adjacent Noninfarcted Region”). Following ischemia, the wavefront propagation of necrosis is largely complete within the first 6 hours after reperfusion, whereas infarct extension occurs in the weeks to months following reperfusion and is independent of ischemia.
Infarct expansion is perhaps the most important mechanism that comes into play after MI because it is the first mechanism that becomes operative after reperfusion and it has a determining influence on subsequent mechanisms of LV remodeling. The contention that a small, well-healed infarct is not a significant stimulus for LV remodeling is supported by the observation that small infarct scars detected in patients using the cardiac MRI technique of late gadolinium-enhancement are not associated with LV remodeling. Conversely, a large, transmural infarct in the anterior LV wall will be subject to abnormal loading conditions and undergo repeated cycles of systolic bulging with every heartbeat (until such time as mature scar can be formed). It follows that the workload placed on the recovering heart and the number of heartbeats occurring prior to scar maturation are critical factors in the progression of infarct expansion (and ultimately LV remodeling). Indeed, this simple concept is supported by many lines of evidence, from animal models to clinical experience.
A careful examination of the time course of LV remodeling in mice shows that LV end-systolic volume stabilizes within 1 month after reperfused MI [29]. While this is a simple observation, it suggests that the progression of LV remodeling is proportional to heart rate since both heart rate (600 bpm) and the natural progression of LV remodeling (1 month) are approximately 10-fold faster than found in humans. Additional evidence supporting this concept can be found in the clinical literature, since the negative chronotropic effect of beta-blockade may be an important contributing mechanism underlying the efficacy of this class of drugs in clinical trials [30].
Clearly, it is not simply the number of contractions but the wall stress imposed by each contraction that contributes to LV remodeling. Thus the contributions to afterload imposed by increased preload (end-diastolic dimension) as well as peripheral vasoconstriction are also critical, as reflected in the cardiocirculatory model of heart failure. The improvements in systemic hemodynamics brought about by ACE inhibition and the protection this provides against infarct expansion provide convincing arguments for applying ACE inhibition as early as practical in the setting of acute MI [31].
While the effects of LV wall stress are also important in other regions of the heart, and during other phases of LV remodeling (as detailed below), a recent transmural infarct is particularly vulnerable to the effects of wall stress, such that infarct expansion accounts for the majority of the increase in LV chamber volume that occurs during the first hours to weeks after MI. Given LaPlace’s law, these early increases in volume add additional wall stress to other regions of the heart in the months that follow. The fundamental importance of these early events in determining late outcome makes it critical to understand the biological mechanisms governing infarct scar formation and maturation in the post-infarct heart after reperfusion. For while reperfusion is important for myocardial salvage and for initiating the wound-healing process in the infarct, it is not always complete (i.e., due to microvascular obstruction and/or incomplete revascularization), and the re-establishment of blood flow to the infarcted region is relatively short-lived. Indeed, underperfused regions as measured by myocardial contrast echocardiography as early as 6 hours after reperfusion can used to accurately predict infarct size [32].
The complex orchestration of cytokine elaboration, inflammatory cell infiltration, endothelial cell proliferation and myofibroblast activity necessary to create a mature scar out of infarcted myocardium has recently been reviewed by Frangogiannis [33]. The initial inflammatory phase after reperfusion is characterized by cytokine/chemokine signaling and the infiltration of neutrophils that begin to clear the infarct of necrotic cardiomyocytes and cellular debris. This is followed 2–3 days later by the start of the proliferative phase during which monocytes invade the infarct and differentiate into macrophages. During the proliferative phase, lasting 2–7 days post-MI in mice, neovessels are formed to support the proliferation of myofibroblasts in the infarct. The myofibroblasts elaborate extracellular matrix proteins including the collagen necessary for scar formation. As the neutrophils complete the process of clearing necrotic cells from the infarct, they enter apoptosis and are phagocytosed by macrophages. This induces the macrophages to synthesize and release TGF-β which downregulates inflammation and helps promote the transition from the proliferative phase to the maturation phase approximately 7 days post-MI (in mice). During this final phase, fibroblasts undergo apoptosis, neovessels regress from the infarct and the collagen-based matrix matures by condensation into mature scar. It is critical that strong, mature scar be formed as early as possible since the structurally weakened infarct is subject to infarct expansion during the proliferative phase. However, this must be balanced against excessive scar formation at later stages which has the potential to encroach upon the adjacent noninfarcted region through a process that has been referred to as non-ischemic infarct extension [21]. Furthermore, an excessive fibrotic response in the LV has the potential to spill over into the remote regions where it might compromise contractile function. In short, macrophage function, neovessel formation and collagen deposition all are crucial to prevent infarct expansion from contributing to LV remodeling, and yet our understanding of these processes (particularly in humans) is limited. It is clear that LV remodeling proceeds more slowly in larger mammals than in mice, and while some aspects may be driven by heart rate it is also clear that the total mass of the necrotic tissue (i.e., the number of cardiomyocytes involved) will also come into play in determining the rate of LV remodeling in one species versus another.
The formation and regression of neovessels in the infarct after experimental MI in a canine model has also been characterized recently [34]. This study concluded that while neovessel formation is critical in scar formation, the regression of these vessels may be equally important in preventing excessive fibrosis. Others have extensively characterized LV remodeling in dogs [35] and have used it to probe potential targets in the collagen degradation and synthesis pathways after MI [36]. For example, the effects of calcium channel blockers (amlodipine) and ACE inhibition (enalapril) have been examined in this same model. It was found that amlodipine, but not enalapril, preserved collagen in the infarct scar and increased collagen volume fraction in the border region [37]. These studies and others have led to the general consensus that ACE inhibitors and angiotensin receptor blockers (ARBs) have an antifibrotic effect on collagen formation in the infarcted and remote noninfarcted regions. When considered in light of the obvious clinical benefits of ACE inhibitors and ARBs [38], this might suggest that the extent of fibrosis in the infarct of untreated subjects may be excessive, and that a smaller, more compact scar is to be preferred. In accordance with LaPlace’s law, the optimal design criteria for a post-infarct scar would involve minimizing the circumferential and longitudinal extent of the scar, and yet making it as thick (and as strong) as necessary to resist bulging under systolic pressure. Thus the spatial distribution of fibrosis within the infarct is clearly as important as the extent of fibrosis.
While the effects of ACE inhibitors on infarct scar formation have been described, few animal studies have directly examined the effects of β-blockade on infarct scar formation after MI. This stands in contrast to the wealth of clinical experience in which β-blockade has been shown to have favorable effects on the LV remodeling process in addition to those of ACE inhibitors, and where the combination of both drugs yields the greatest benefit in mortality [39]. Thus while much progress has been made, particularly with regard to our understanding of the effect of ACE inhibition on post-MI scar formation, improved methods and more animal studies are needed to characterize the effects of combined ACE inhibition and β-blockade on the progression of wound healing and scar formation in the LV after MI.
Other drugs that show promise in controlling infarct expansion include inhibitors of matrix metalloproteinases (MMPs). Studies conducted in MMP-deficient mice [40] and in animals treated with both non-selective [41] and selective [42] MMP inhibitors suggest that preserving the collagen matrix surrounding the cardiomyocytes in the infarct can help prevent infarct expansion and thus help reduce LV remodeling. It is also conceivable that MMP inhibition could prove useful in inhibiting side-by-side “slippage” of cardiomyocytes in the remote noninfarcted region during the late phase of LV remodeling. However, MMP inhibition is likely to be a two-edged sword since some remodeling of the extracellular matrix will clearly be necessary in order for the post-infarct heart to adjust to the changes in LV wall stress imposed by the non-contractile infarct. Indeed, cardiomyocytes located in the adjacent region situated between the infarct and the remote region are subject to highly abnormal stresses, and their ability to accommodate those new stress patterns is also critical to long-term outcome.
Given that infarct expansion during the sub-acute phase is the primary contributor to the early phase of LV remodeling after MI, it follows that efficient wound healing and scar formation in the infarct are critical in preventing infarct expansion from contributing to LV remodeling. Indeed, recent studies undertaken in an animal model of MI indicate that activated macrophages improve healing, remodeling and function in the post-MI heart [43]. While there has been great interest in the potential of using stem cells for myocardial regeneration, the demonstration of actively-contracting transplanted cells in the infarct has been elusive, and it appears likely that most of the beneficial effects that have been reported to date derive instead from either accelerating scar formation or otherwise enhancing the mechanical properties of the infarct. Indeed, simple mechanical plication of the infarct with purse-string sutures significantly improved long-term end diastolic length, end systolic length and fractional shortening as measured by echocardiography in a rat model of post-MI heart failure [44]. Alternative surgical methods for minimizing infarct expansion by supplementing the mechanical properties of the infarct region include myocardial patches [45] and support devices such as the Acorn passive cardiac support device. This is a mesh-like, implantable device designed to prevent progressive LV dilatation, increase ejection fraction, lower LV wall stress and attenuate LV chamber sphericity [18].
In conclusion, a significant portion of the efficacy attributable to the current standard of care (ACE-inhibition and beta-blockade) may result from the beneficial effects that this drug combination has on infarct expansion during the early phase after MI. This is entirely consistent with current ACC/AHA guidelines recommending the administration of these agents early after reperfusion [31]. While this drug combination has demonstrated efficacy in this setting, the positive results from initial clinical trials undertaken with passive constraint devices or patches to protect the infarct against excessive wall stress suggests that there is further opportunity for improved treatment options targeting infarct expansion. In particular, MMP inhibition has shown promise in limiting infarct expansion in several pre-clinical studies (both in rodents and larger mammals). Small molecule or cell-based strategies aimed at accelerating the formation of mature scar in the infarct would also appear promising. Note, however, that such strategies would need to be carefully timed, since augmenting inflammatory responses too early after reperfusion would aggravate reperfusion injury. Conversely, enhancing the wound-healing response too late during the scar maturation might promote excess fibrosis (not only in the infarct, but potentially in the adjacent and remote regions of the heart as well). The two strategies (MMP inhibition and scar acceleration) could potentially be employed in combination, but the timing of administration will be critical to success. It is therefore likely that such strategies would be implemented in parallel with reliable methods for monitoring wound healing and scar formation in individual patients (either through the use of non-invasive imaging or plasma-based biomarkers). More complex and/or sophisticated approaches entailing surgical or catheter-based intervention (i.e., stem cell therapy or passive restraint devices) also show long-term promise, but it may be some time before such approaches demonstrate a substantial advantage in cost/benefit ratio as compared to the advancing clinical standard of care.
2. THE ADJACENT NONINFARCTED REGION
Of the three regions of myocardium defined by acute MI, the adjacent region of viable tissue immediately bordering the infarct is perhaps the least well characterized. This point notwithstanding, it is nevertheless widely believed to play a critical role in the progression of LV remodeling during the weeks and months that follow acute MI. As introduced above, the recently characterized phenomenon of “non-ischemic infarct extension#x0201D; is likely to emerge as an important biomechanism responsible for the enlargement of chamber volume (and progressive loss in LV function) that continues to operate even after the completion of mature scar formation in the infarct.
Operationally, the adjacent noninfarcted region is defined by its proximity to the infarct (whether it be necrotic tissue early after MI or mature scar late after MI). However, careful studies undertaken with surgically-implanted sonomicrometers have demonstrated the viable myocardium in the adjacent region can progressively be recruited into mature scar, thus increasing the proportion of the LV wall subtended by scar and decreasing the proportion composed of viable, contractile myocardium [22]. Thus the adjacent noninfarcted region does not necessarily remain in a fixed position, even after the formation of mature scar in the infarct, but is instead a moving target whose position is defined by the progressive extension of scar tissue into viable myocardium. While one might suspect that infarct extension is the result of residual ischemia, microsphere-based perfusion studies have shown that it can occur in fully-perfused tissue [22].
From a mechanical point of view, non-ischemic infarct extension can be understood in terms of the highly abnormal stress patterns imposed on cardiomyocytes within the adjacent noninfarcted region due to their juxtaposition between non-contractile infarct scar and the viable, contractile myocardium. While the concept of “tethering” has been used to explain deficits in contractile function observed in the adjacent noninfarcted region, high-resolution myocardial tagging studies conducted with MRI indicate that tethering actually operates in both directions (i.e., not only decreasing strain in the adjacent noninfarcted region [46] but also transferring strain into nearby regions of scar tissue myocardium that lack the contractile elements necessary to generate strain). Thus the abnormal stress patterns resulting from the juxtaposition of contractile and non-contractile tissues are amortized over some distance. Nevertheless, cardiomyocytes lying at the border of the infarct/scar and adjacent noninfarcted regions still experience the greatest stress and due to resultant activation of the renin-angiotensin system [47] undergo myocyte hypertrophy in parallel with loss of function in the adjacent region [23].
From a biological point of view, the mechanical shear stress imposed on cardiomyocytes lining the infarct scar induce oxidative stress and activate pro-inflammatory pathways within these cells. Thus the expression of both TNF-alpha [48] and iNOS protein [49] have been documented in cardiomyocytes bordering the infarct scar (Fig. 2). The combination of oxidative and nitrosative stress ultimately leads to apoptosis in cardiomyocytes adjoining the mature scar, replacement with fibrous tissue and extension of the infarct scar. It is possible that the delayed, yet inevitable progression of heart failure observed clinically in patients surviving large MI is driven, at least in part, by this insidious mechanism of non-ischemic infarct extension.
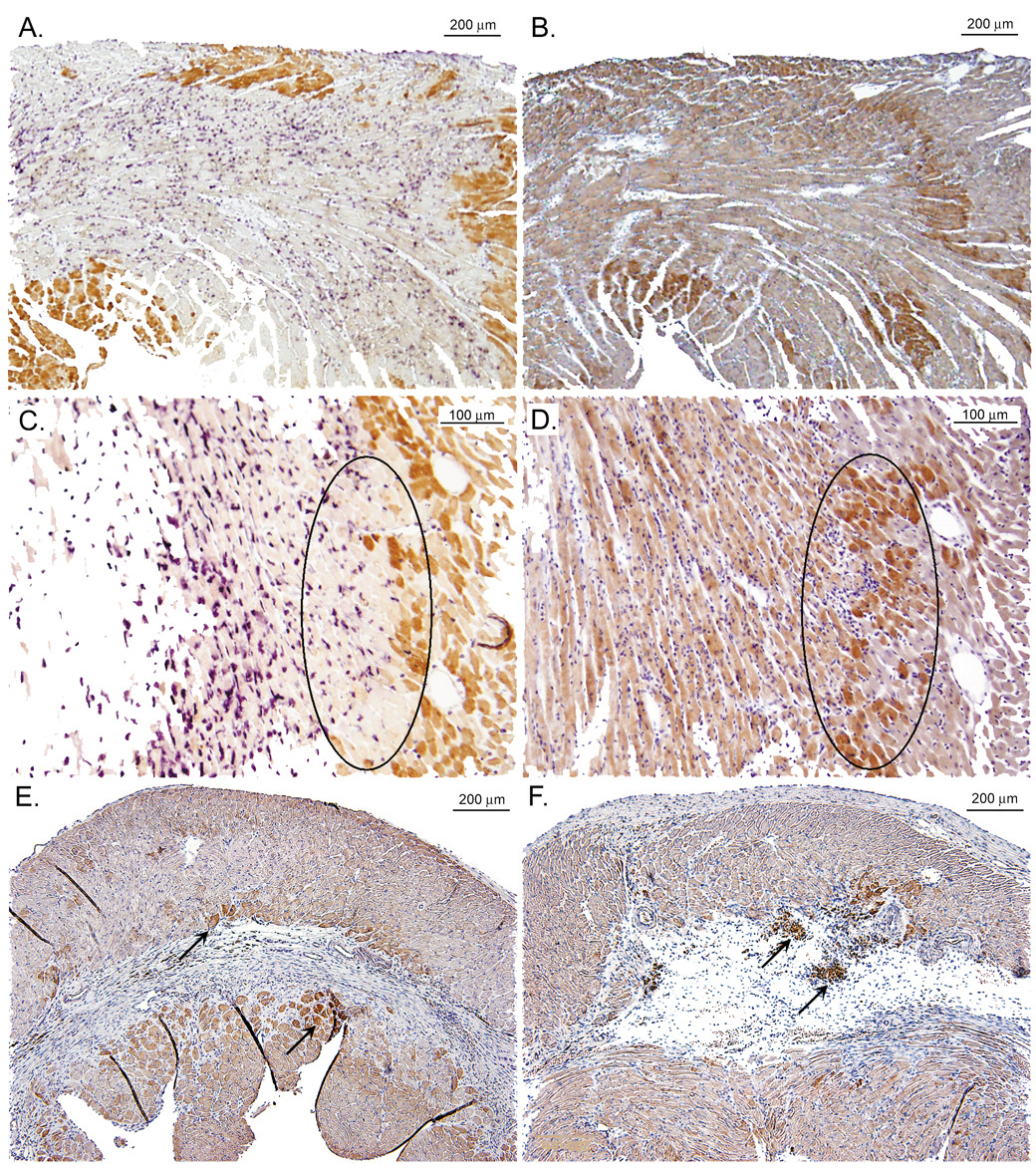
Figure 2.
Immuno of iNOS expression in the adjacent region early and late after MI (from Gilson et al. [49] with permission). Panels A&C show low- and high-power (respectively) magnifications of a wild-type (WT) mouse heart on post-myocardial infarction (MI) day 1 double-immunostained for myoglobin (brown) and neutrophils (purple). Panels B&D show low- and high-power (respectively) serial sections from the same mouse heart immunostained for inducible nitric oxide synthase (iNOS) (brown). The iNOS immunoreactivity in panel D is most abundant in the swath of cardiomyocytes located within the black oval. Comparison with panel C reveals that the same swath of cardiomyocytes contains little myoglobin and is largely nonviable. Panels E&F show sections from WT mouse hearts on post-MI day 28 immunostained for iNOS. Panel E shows iNOS in cardiomyocytes bordering scar tissue (arrows), while Panel F shows iNOS in cardiomyocytes bordering mature scar and in granulation tissue (arrows).
Pre-clinical studies suggest that the current clinical standard of care (ACE inhibition and beta-blockade) may be reasonably effective in controlling non-ischemic infarct extension. For example, one study in a dog model of heart failure secondary to MI found a significantly lower incidence of apoptosis in cardiomyocytes located in LV regions bordering scarred tissue (infarcts) in enalapril-treated dogs as compared with controls [50]. A second study, conducted in an ovine model of myocardial infarction, examined the effects of adding angiotensin II receptor blockade (ARB) to ACE inhibition [51]. This study found that, compared with ACE inhibition alone, the addition of ARB further limits hypertrophy in noninfarcted cardiomyocytes located in the adjacent noninfarcted region. Another study, also conducted in an ovine model of myocardial infarction, found that when added to ACE inhibition after transmural anteroapical MI, beta-blockade improved ejection fraction and sympathetic innervation in the adjacent noninfarcted region, but did not alter LV size [52].
Potential therapeutic approaches for inhibiting the process of non-ischemic infarct extension follow immediately from the biomechanisms that are believed to be operative. ACE inhibition and beta-blockade would both be anticipated to reduce the local stresses experienced by cardiomyocytes located in the adjacent noninfarcted region. Alternative mechanical approaches to reducing wall stress (e.g., myocardial patches and passive restraint devices) may prove beneficial both in the infarct early after MI and in the adjacent noninfarcted region at later time points. Here, as in the remote noninfarcted region, a passive restraint device encircling the entire heart may have logistical advantages over a cardiac “patch” covering a more limited area.
Pro-inflammatory cytokines and proteins have been strongly implicated in the pathogenesis of infarct expansion, and thus anti-inflammatory or anti-cytokine strategies may also prove beneficial. While ACE inhibitors are not generally classified as anti-inflammatory, they certainly counteract the actions of angiotensin II, which include activation of cell adhesion molecules and the release of cytokines/chemokines, which in turn elicit inflammatory responses from leukocytes, endothelial cells and vascular smooth muscle cells. Thus ACE inhibitors may oppose infarct extension through multiple mechanisms, not only through the reduction of wall stress but through anti-inflammatory effects as well. Other anti-inflammatory agents may also prove beneficial against non-ischemic infarct extension. However, it may be desirable to withhold such anti-inflammatory therapy until after the formation of mature scar, since a small patient study of high-dose methylprednisolone administered shortly after MI revealed an increased incidence of arrhythmia and cardiac rupture, which was subsequently attributed to impaired healing in the infarct [53]. From a mechanistic standpoint, more selective anti-cytokine strategies (such as TNF-alpha antagonists) would appear to be attractive. On one hand, there is concern that TNF-alpha antagonists have failed to demonstrate significant efficacy in the setting of chronic heart failure, even after two large randomized, placebo-controlled clinical trials (i.e., RECOVER and RENAISSANCE, [54]). On the other hand, these trials focused on patients with established heart failure, and they were not restricted to a post-MI population. Thus the potential of early intervention with TNF-alpha antagonists in MI patients has yet to be rigorously tested at the clinical level. Based on fundamental mechanisms, anti-apoptosis strategies (such as caspase inhibition [55]) may also help prevent infarct extension, particularly if they can be targeted to the heart or preferably even to the adjacent noninfarcted region. However, apoptosis lies at the distal end of the mechanistic pathway leading to infarct extension, and interventions aimed at the proximal end of that pathway (i.e., reducing wall stress and inflammation) would seem to have greater potential for success.
In summary, the adjacent noninfarcted region is widely believed to play a critical role in the progression of LV remodeling during the weeks and months that follow acute MI. The neurohormonal activation created by increased wall stress that is a result of infarct expansion and cavity dilation leads to myocyte hypertrophy and mechanical dysfunction in the adjacent noninfarcted region. Both ACE inhibition and beta blockade have been shown to intervene in this cascade. Non-ischemic infarct extension is another potential mechanism afflicting the adjacent region that has considerable potential to contribute importantly to the pathophysiology of LV remodeling after MI. However, this mechanism has only recently been described, is technically demanding to characterize, and has yet to be conclusively demonstrated in humans. These points notwithstanding, there is evidence to suggest that at least a portion of the benefits derived from ACE inhibition and beta-blockade can be attributed to their effects on non-ischemic infarct extension. It is possible that further reductions in LV remodeling might be obtained through the well-timed and judicious application of anti-inflammatory, anti-cytokine or anti-apoptosis strategies. However, it remains to be seen whether the additional benefit derived from such interventions will outweigh the additional costs and off-target effects that will be associated with adding them to the contemporary standard of care.
3. THE REMOTE NONINFARCTED REGION
The remote noninfarcted region can operationally be defined as the non-ischemic myocardium lying beyond the adjacent region. In patients with significant atherosclerotic burden, the remote region could possibly be afflicted by ischemia and/or hibernation. However, for the purposes of this review and in most animal models of LV remodeling, the myocardium in the remote region is entirely normal before and shortly after the index event. Nevertheless, the increased workload imposed by large MI can elicit a hypertrophic response in the remote region depending on a number of factors including: size of infarct, type of infarct (subendocardial or transmural), location of infarct (septal vs. anterior, lateral or posterior walls), type of reperfusion (full reperfusion, no reperfusion or reperfused with areas of no-reflow), degree of non-ischemic infarct extension, preload and afterload, health status (obesity, diabetes, hypertension), and other factors (such as state of inflammatory activation). It is reasonable to assume that the onset of hypertrophy after MI will vary depending on the sum total of these and perhaps additional factors. In most patients recovering from MI, reactive hypertrophy in the remote region may not become a concern until sometime after the formation of mature scar. In other patients with larger infarcts, it is conceivable that the threshold needed to activate the gene regulatory pathways responsible for hypertrophy may be reached sooner. The detection of hypertrophy in patients is further complicated by the fact that hypertrophy at the cellular level is a gradual, growth-dependent process, which contributes to the delayed-onset presentation of disease symptoms at the clinical level.
Cardiac hypertrophy plays a central role in the pathophysiology of nearly every form of heart failure (not only those with an ischemic etiology). In animal models, cardiac hypertrophy can be elicited by a wide variety of surgical, chemical, and genetic interventions. It is therefore not surprising that the biomechanisms underlying cardiac hypertrophy are the most widely studied of all the mechanisms that contribute to LV remodeling in response to MI. At the organ level, the hypertrophic response of the heart depends upon the type of load applied, with pressure overload eliciting myocyte thickening to produce concentric hypertrophy and volume overload eliciting myocyte lengthening to produce the eccentric hypertrophy characteristic of a dilated heart. LV remodeling post-MI is primarily a state of volume overload and thus leads primarily to myocyte lengthening and thus eccentric hypertrophy.
The response of the remote region to volume and/or pressure overload is further complicated by the degradation of extracellular collagen (by matrix metalloproteinases (MMPs)) and by the deposition of collagen and other matrix proteins by cardiac fibroblasts. The degradation of matrix components by MMPs and the resulting side-by-side slippage of cardiomyocytes is presumably another mechanism that may contribute to LV wall-thinning in the remote region after MI. Additional mechanisms afflicting the remote region include fibrosis and collagen deposition, which have the potential to increase LV mass at the cost of LV function.
From a cellular point of view, the increased physical, oxidative and nitrosative stresses imposed on cardiomyocytes in the remote region by the changes in cardiac shape, size and function resulting from acute MI lead to the re-induction of a gene expression program reminiscent of that seen during fetal development. While the signal transduction pathways responsible for their activation are different, the transcription factors myocyte enhancer factor-2 (MEF2), GATA4 and myocardin have all been shown to participate importantly, both in prenatal cardiac development and in the hypertrophic response to cardiac stress. MEF2 becomes activated upon its phosphorylation and dissociation from the class II histone deacetylases (HDACS) that serve to repress the cardiac fetal gene expression program. In contrast, GATA4 activity is potentiated by the action of the small GTPase RhoA on rho-associated kinase (ROCK), which then associates with the NFAT transcription factor to activate the hypertrophic growth program. Hypertrophic agonists such as serum and phenylephrine upregulate both the expression and activity of myocardin, which forms stable ternary complexes with serum response factors (SRFs) bound to CArG-box enhancer elements in the DNA, thereby upregulating the expression of fetal genes.
These insights into the transcriptional regulation of cardiac hypertrophy have opened the possibility that hypertrophy itself might serve as a target for novel therapies against heart failure [56]. For example, cardiomyocytes from mice with a mutant form of class II HDACs that lack regulatory phosphorylation sites have been shown to be resistant to serum- or phenylephrine-induced fetal gene expression and hypertrophy [56]. In addition, the calcineurin inhibitors cyclosporin and FK506 have been used to inhibit the calcineurin-dependent activation of NFAT, which in turn represses the fetal gene expression program and the hypertrophic response [57]. Furthermore, the dependence of GATA4 activity on small GTPases can be exploited by using statins (HMG-CoA reductase inhibitors) to block the isoprenylation of small GTPases, thereby blocking membrane targeting and subsequent signal transduction. Indeed, statin treatment has been shown to inhibit both angiotensin II–induced [58] and phenylephrine-induced [59] hypertrophy in isolated cardiomyocytes. With regard to animal studies, simavastatin was recently shown to reduce cardiac hypertrophy in a rat model of pressure overload [60]. The question of whether statin therapy may prove valuable in patients with heart failure has been the subject of recent reviews [61,62] and ongoing clinical trials (e.g., CORONA and GISSI-HF); however, it remains to be seen whether statin treatment offers benefits above and beyond those derived from ACE inhibition and beta-blockade.
In summary, the response of the remote region to the physical, oxidative and nitrosative stresses imposed by MI plays a critical role in determining long-term outcome. The similarities between the fetal program of cardiac gene expression and the hypertrophic program of cardiac gene expression have shed considerable light on the molecular mechanisms underlying reactive hypertrophy. While ACE inhibitors are fairly effective against hypertrophy, they may not be as effective as aldosterone antagonists in inhibiting cardiac fibrosis [63], which might underlie the modest but significant beneficial effect of eplerenone in clinical trials of heart failure.
Summary and Conclusions
The foregoing review of biomechanisms contributing to LV remodeling after myocardial infarction has a number of clinical implications. Most immediately, the determining influence of early events (e.g., infarct expansion) and mid-term events (e.g., non-ischemic infarct extension) on the ultimate outcome after MI provide a mechanistic rationale for implementing drug therapy as soon as possible after the index event. Indeed, one should note that the current ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (STEMI) already list both ACE inhibitors and beta-blockers as Class I drugs for use within the first 24 hours after MI, with ACE-inhibition recommended long-term and beta-blockade recommended during the early phase of convalescence. However, the implementation of these guidelines is occasionally incomplete due to the challenge of achieving efficacious doses of these agents without provoking hypotension in individual patients. The optimal relative dosing of each agent is not well-defined. Dosing varies from patient to patient and often must be found by trial and error.
To best adapt therapy to individual patients, it may be beneficial to identify the patients that would benefit the most from aggressive therapy, for example those with larger infarcts. Towards this end, the current ACC/AHA guidelines also identify the measurement of infarct size as an important element in the overall care of patients with STEMI. While it is usually clear which patients have large vs. small infarcts, the commonly-available methods of infarct size estimation (follow-up EKG, cardiac biomarkers, radionuclide imaging, echocardiography and MRI) are seldom applied in a quantitative fashion, making it difficult to identify which of the patients with intermediate-sized infarcts might benefit the most from more aggressive medical therapy. Fortunately, this situation is steadily improving with increasing implementation of more quantitative techniques for infarct size measurement. With regard to cardiac biomarkers, the area-under-the-curve analysis made possible by serial troponin assays provides for infarct size determinations that correlate moderately well with quantitative cardiac MRI determinations of infarct size by late gadolinium enhancement [64]. Similarly, contemporary methods for the quantitative assessment of infarct size by radionuclide imaging [65] and 3D echocardiography [66] are becoming more common. One recent study used cardiac MRI to show that the risk of LV remodeling increased 2.8-fold with each 10% increase in infarct size [67]. In the same study, infarct size of 24% or more of LV area predicted LV remodeling with high sensitivity (92%), specificity (93%), and accuracy (93%)[67]. Larger confirmatory studies are clearly needed before precise guidelines can be formulated, but from the available data it is clear that more accurate methods of quantitative infarct size determination could improve patient care by facilitating the practice of personalized medicine (i.e., enabling physicians to more precisely adjust the intensity of medical surveillance and dose escalation) as appropriate for the individual patient’s infarct size. Similarly, one can anticipate that the more widespread use of accurate methods for determining LV volumes at end-systole and end-diastole (e.g., cardiac MRI and 3D echocardiography) will heighten awareness of LV remodeling in patients after MI, leading to improved patient monitoring and better outcomes.
While the foregoing discussion involves best-practices using drugs and diagnostics that are already available, future trends in the prevention of heart failure after MI will include the more precise targeting of drugs and more informative diagnostic techniques. As alluded to earlier, the application of targeted drugs (i.e., specifically targeting infarct expansion as opposed to non-ischemic infarct extension or compensatory hypertrophy) will rely greatly on the development of diagnostic techniques capable of monitoring the efficacy of that specific therapy. For example, molecular imaging techniques have already been developed to assess macrophage accumulation [68] and collagen deposition [69] in the heart after MI. However, it may take some time for the necessary contrast agents to gain FDA approval, and it is conceivable that blood-borne biomarkers [70] could provide similar information on the progression of scar formation at lower cost with absolutely no risk to the patient.
In the long term, advances in gene therapy, stem cell therapy and tissue engineering hold promise for transforming infarcted myocardium into viable, contractile tissue. However, even once this is possible, it is important to remember that even novel therapies need to be cost effective in order to gain widespread acceptance in a managed care environment. Until such time as these novel therapies achieve a cost/benefit ratio that rivals conventional therapy, it is likely that they will be reserved for patients with larger infarcts who have more to gain from intervention.
Table 1.
Therapeutic strategies and their targets in specific regions of the heart during different time frames after myocardial infarction
| Region | Time frame | Current Therapies | Developing Therapies |
|---|---|---|---|
| Infarct | Days to weeks | Nitroglycerin [71] | Better scar formation [43] |
| Ace inhibition [72,73] | MMP inhibition [41,57,74] | ||
| Beta blockade [75] | Passive restraint [17,76] | ||
| Stem-cell transplantation | |||
| Adjacent | Weeks to months | Ace inhibition [50,51] | Anti-apoptosis [50,77] |
| Beta blockade [52] | Anti-cytokine [78] | ||
| Passive restraint [17,76] | |||
| Anti-inflammatory | |||
| Remote | Months to years | Ace inhibition [50,51] | Anti-hypertrophy [57] |
| Beta blockade | Anti-cytokine [78] | ||
| Aldosterone inhibition [63] | MMP inhibition | ||
Acknowledgments
This work was supported by NIH grants R01-HL58582 (BAF) and R01-HL69494 (BAF) and R01-HL75792 (CMK). The authors thank Eileen D. French for the contribution of Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflicts of interest.
References
- 1.Rosamond W, et al. Heart Disease and Stroke Statistics--2007 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Mann DL. Mechanisms and models in heart failure: A combinatorial approach. Circulation. 1999;100(9):999–1008. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 3.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111(21):2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 4.Cohn JN. Structural basis for heart failure. Ventricular remodeling and its pharmacological inhibition. Circulation. 1995;91(10):2504–2507. doi: 10.1161/01.cir.91.10.2504. [DOI] [PubMed] [Google Scholar]
- 5.Tiyyagura SR, Pinney SP. Left ventricular remodeling after myocardial infarction: past, present, and future. Mount Sinai Journal of Medicine. 2006;73(6):840–851. [PubMed] [Google Scholar]
- 6.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 7.Lyne JC, Pennell DJ. Cardiovascular magnetic resonance in the quantitative assessment of left ventricular mass, volumes and contractile function. Coronary Artery Disease. 2005;16(6):337–343. doi: 10.1097/00019501-200509000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Judd RM, et al. Technology insight: assessment of myocardial viability by delayed-enhancement magnetic resonance imaging. Nature Clinical Practice Cardiovascular Medicine. 2005;2(3):150–158. doi: 10.1038/ncpcardio0134. [DOI] [PubMed] [Google Scholar]
- 9.Watzinger N, et al. The potential of contrast-enhanced magnetic resonance imaging for predicting left ventricular remodeling. Journal of Magnetic Resonance Imaging. 2002;16(6):633–640. doi: 10.1002/jmri.10206. [DOI] [PubMed] [Google Scholar]
- 10.Orn S, et al. Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. American Journal of Cardiology. 2007;99(8):1109–1114. doi: 10.1016/j.amjcard.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 11.White HD, et al. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76(1):44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 12.Konstam MA. Reliability of ventricular remodeling as a surrogate for use in conjunction with clinical outcomes in heart failure. American Journal of Cardiology. 2005;96(6):867–871. doi: 10.1016/j.amjcard.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Yan RT, et al. Usefulness of temporal changes in neurohormones as markers of ventricular remodeling and prognosis in patients with left ventricular systolic dysfunction and heart failure receiving either candesartan or enalapril or both. American Journal of Cardiology. 2005;96(5):698–704. doi: 10.1016/j.amjcard.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 14.Mann DL. Cardiac remodeling as therapeutic target: treating heart failure with Cardiac Support Devices. Heart Failure Reviews. 2005;10(2):93–94. doi: 10.1007/s10741-005-4635-z. [DOI] [PubMed] [Google Scholar]
- 15.Sharpe N. Pharmacologic effects on cardiac remodeling. Current Heart Failure Reports. 2004;1(1):9–13. doi: 10.1007/s11897-004-0011-x. [DOI] [PubMed] [Google Scholar]
- 16.Mancini D, Burkhoff D. Mechanical device-based methods of managing and treating heart failure. Circulation. 2005;112(3):438–448. doi: 10.1161/CIRCULATIONAHA.104.481259. [DOI] [PubMed] [Google Scholar]
- 17.Cheng A, et al. Passive ventricular constraint prevents transmural shear strain progression in left ventricle remodeling. Circulation. 2006;114(1 Suppl):I79–I86. doi: 10.1161/CIRCULATIONAHA.105.001578. [DOI] [PubMed] [Google Scholar]
- 18.Fundaro P, Fundaro C. Left ventricular remodelling and outcomes after surgery: pathophysiological insights for a modern surgical approach to ischaemic cardiomyopathy. Journal of Cardiovascular Medicine. 2006;7(11):781–784. doi: 10.2459/01.JCM.0000250864.48283.21. [DOI] [PubMed] [Google Scholar]
- 19.Doughty RN, White HD. Carvedilol: use in chronic heart failure. Expert Review of Cardiovascular Therapy. 2007;5(1):21–31. doi: 10.1586/14779072.5.1.21. [DOI] [PubMed] [Google Scholar]
- 20.Kelley ST, et al. Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation. 1999;99(1):135–142. doi: 10.1161/01.cir.99.1.135. [DOI] [PubMed] [Google Scholar]
- 21.Ratcliffe MB. Non-ischemic infarct extension: A new type of infarct enlargement and a potential therapeutic target. Journal of the American College of Cardiology. 2002;40:1168–1171. [Google Scholar]
- 22.Jackson BM, et al. Extension of borderzone myocardium in postinfarction dilated cardiomyopathy. Journal of the American College of Cardiology. 2002;40(6):1160–1167. doi: 10.1016/s0735-1097(02)02121-6. [DOI] [PubMed] [Google Scholar]
- 23.Kramer CM, et al. Regional myocyte hypertrophy parallels regional myocardial dysfunction during post-infarct remodeling. Journal of Molecular & Cellular Cardiology. 1998;30(9):1773–1778. doi: 10.1006/jmcc.1998.0741. [DOI] [PubMed] [Google Scholar]
- 24.Saeed M, et al. Scarred myocardium imposes additional burden on remote viable myocardium despite a reduction in the extent of area with late contrast MR enhancement. European Radiology. 2006;16(4):827–836. doi: 10.1007/s00330-005-0052-x. [DOI] [PubMed] [Google Scholar]
- 25.Wollert KC, Drexler H. Cell-based therapy for heart failure. Current Opinion in Cardiology. 2006;21(3):234–239. doi: 10.1097/01.hco.0000221586.94490.d2. [DOI] [PubMed] [Google Scholar]
- 26.Boyle MP, Weisman HF. Limitation of infarct expansion and ventricular remodeling by late reperfusion. Study of time course and mechanism in a rat model. Circulation. 1993;88(6):2872–2883. doi: 10.1161/01.cir.88.6.2872. [DOI] [PubMed] [Google Scholar]
- 27.Reimer KA, et al. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56(5):786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, et al. Serial contrast-enhanced MRI of myocardial infarction in mice early after reperfusion: Importance of early treatment in strategies to control reperfusion injury. Journal of Cardiovascular Magnetic Resonance. 2003;5(1):3–4. [Google Scholar]
- 29.Ross AJ, et al. Serial MRI evaluation of cardiac structure and function in mice after reperfused myocardial infarction. Magnetic Resonance in Medicine. 2002;47(6):1158–1168. doi: 10.1002/mrm.10166. [DOI] [PubMed] [Google Scholar]
- 30.Sabbah HN. Biologic rationale for the use of beta-blockers in the treatment of heart failure. Heart Failure Reviews. 2004;9(2):91–97. doi: 10.1023/B:HREV.0000046363.59374.23. [DOI] [PubMed] [Google Scholar]
- 31.Antman EM, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction).[see comment][erratum appears in J Am Coll Cardiol. 2005 Apr 19;45(8):1376] Journal of the American College of Cardiology. 2004;44(3):671–719. doi: 10.1016/j.jacc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Micari A, et al. Automated quantification of the spatial extent of perfusion defects and viability on myocardial contrast echocardiography. Journal of the American Society of Echocardiography. 2006;19(4):379–385. doi: 10.1016/j.echo.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Current Medicinal Chemistry. 2006;13(16):1877–1893. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- 34.Dobaczewski M, et al. Vascular mural cells in healing canine myocardial infarcts. Journal of Histochemistry & Cytochemistry. 2004;52(8):1019–1029. doi: 10.1369/jhc.3A6210.2004. [DOI] [PubMed] [Google Scholar]
- 35.Jugdutt BI. The dog model of left ventricular remodeling after myocardial infarction. Journal of Cardiac Failure. 2002;8(6 Suppl):S472–S475. doi: 10.1054/jcaf.2002.129274. [DOI] [PubMed] [Google Scholar]
- 36.Jugdutt BI. Remodeling of the myocardium and potential targets in the collagen degradation and synthesis pathways. Current Drug Targets - Cardiovascular & Haematological Disorders. 2003;3(1):1–30. doi: 10.2174/1568006033337276. [DOI] [PubMed] [Google Scholar]
- 37.Jugdutt BI, et al. Vascular remodeling during healing after myocardial infarction in the dog model: effects of reperfusion, amlodipine and enalapril. Journal of the American College of Cardiology. 2002;39(9):1538–1545. doi: 10.1016/s0735-1097(02)01805-3. [DOI] [PubMed] [Google Scholar]
- 38.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108(11):1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 39.Udelson JE. Ventricular remodeling in heart failure and the effect of beta-blockade. American Journal of Cardiology. 2004;93(9A):43B–48B. doi: 10.1016/j.amjcard.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 40.Hayashidani S, et al. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. American Journal of Physiology - Heart & Circulatory Physiology. 2003;285(3):H1229–H1235. doi: 10.1152/ajpheart.00207.2003. [DOI] [PubMed] [Google Scholar]
- 41.Rohde LE, et al. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;99(23):3063–3070. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 42.Lindsey ML, et al. Selective matrix metalloproteinase inhibition reduces left ventricular remodeling but does not inhibit angiogenesis after myocardial infarction. Circulation. 2002;105(6):753–758. doi: 10.1161/hc0602.103674. [DOI] [PubMed] [Google Scholar]
- 43.Leor J, et al. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation. 2006;114(1 Suppl):I94–I100. doi: 10.1161/CIRCULATIONAHA.105.000331. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz ER, et al. A new model of ventricular plication: a suturing technique to decrease left ventricular dimensions, improve contractility, and attenuate ventricular remodeling after myocardial infarction in the rat heart. Journal of Cardiovascular Pharmacology & Therapeutics. 2000;5(1):41–49. doi: 10.1177/107424840000500106. [DOI] [PubMed] [Google Scholar]
- 45.Leor J, Cohen S. Myocardial tissue engineering: creating a muscle patch for a wounded heart. Annals of the New York Academy of Sciences. 2004;1015:312–319. doi: 10.1196/annals.1302.026. [DOI] [PubMed] [Google Scholar]
- 46.Kramer CM, et al. Regional differences in function within noninfarcted myocardium during left ventricular remodeling. Circulation. 1993;88(3):1279–1288. doi: 10.1161/01.cir.88.3.1279. [DOI] [PubMed] [Google Scholar]
- 47.Malhotra R, et al. Mechanical stretch and angiotensin II differentially upregulate the renin-angiotensin system in cardiac myocytes In vitro. Circulation Research. 1999;85(2):137–146. doi: 10.1161/01.res.85.2.137. [DOI] [PubMed] [Google Scholar]
- 48.Akasaka Y, et al. Myocardial apoptosis associated with the expression of proinflammatory cytokines during the course of myocardial infarction. Modern Pathology. 2006;19(4):588–598. doi: 10.1038/modpathol.3800568. [DOI] [PubMed] [Google Scholar]
- 49.Gilson WD, et al. Borderzone contractile dysfunction is transiently attenuated and left ventricular structural remodeling is markedly reduced following reperfused myocardial infarction in inducible nitric oxide synthase knockout mice. Journal of the American College of Cardiology. 2007;50(18):1799–1807. doi: 10.1016/j.jacc.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 50.Goussev A, et al. Effects of ACE inhibition on cardiomyocyte apoptosis in dogs with heart failure. American Journal of Physiology. 1998;275(2 Pt 2):H626–H631. doi: 10.1152/ajpheart.1998.275.2.H626. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y-K, et al. Adding angiotensin II type 1 receptor blockade to angiotensin-converting enzyme inhibition limits myocyte remodeling after myocardial infarction. Journal of Cardiac Failure. 2003;9(3):238–245. doi: 10.1054/jcaf.2003.32. [DOI] [PubMed] [Google Scholar]
- 52.Kramer CM, et al. Beta-blockade improves adjacent regional sympathetic innervation during postinfarction remodeling. American Journal of Physiology. 1999;277(4 Pt 2):H1429–H1434. doi: 10.1152/ajpheart.1999.277.4.H1429. [DOI] [PubMed] [Google Scholar]
- 53.Kloner RA, et al. Mummification of the infarcted myocardium by high dose corticosteroids. Circulation. 1978;57(1):56–63. doi: 10.1161/01.cir.57.1.56. [DOI] [PubMed] [Google Scholar]
- 54.Mann DL, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109(13):1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 55.Aoki K, et al. Therapeutic effect of caspase inhibitors in the prevention of apoptosis and reversal of chronic cerebral vasospasm. Journal of Clinical Neuroscience. 2002;9(6):672–677. doi: 10.1054/jocn.2002.1088. [DOI] [PubMed] [Google Scholar]
- 56.Frey N, et al. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109(13):1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 57.Yarbrough WM, et al. Selective targeting and timing of matrix metalloproteinase inhibition in post-myocardial infarction remodeling. Circulation. 2003;108(14):1753–1759. doi: 10.1161/01.CIR.0000091087.78630.79. [DOI] [PubMed] [Google Scholar]
- 58.Oi S, et al. Lovastatin prevents angiotensin II-induced cardiac hypertrophy in cultured neonatal rat heart cells. European Journal of Pharmacology. 1999;376(1–2):139–148. doi: 10.1016/s0014-2999(99)00282-4. [DOI] [PubMed] [Google Scholar]
- 59.Luo JD, et al. Simvastatin inhibits noradrenaline-induced hypertrophy of cultured neonatal rat cardiomyocytes. British Journal of Pharmacology. 2001;132(1):159–164. doi: 10.1038/sj.bjp.0703792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo JD, et al. Simvastatin inhibits cardiac hypertrophy and angiotensin-converting enzyme activity in rats with aortic stenosis. Clinical & Experimental Pharmacology & Physiology. 1999;26(11):903–908. doi: 10.1046/j.1440-1681.1999.03165.x. [DOI] [PubMed] [Google Scholar]
- 61.Laufs U, et al. HMG-CoA reductase inhibitors in chronic heart failure: potential mechanisms of benefit and risk. Drugs. 2006;66(2):145–154. doi: 10.2165/00003495-200666020-00002. [DOI] [PubMed] [Google Scholar]
- 62.Liao JK. Statin therapy for cardiac hypertrophy and heart failure. Journal of Investigative Medicine. 2004;52(4):248–253. doi: 10.1136/jim-52-04-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fraccarollo D, et al. Additive improvement of left ventricular remodeling and neurohormonal activation by aldosterone receptor blockade with eplerenone and ACE inhibition in rats with myocardial infarction. Journal of the American College of Cardiology. 2003;42(9):1666–1673. doi: 10.1016/j.jacc.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Selvanayagam JB, et al. Effects of off-pump versus on-pump coronary surgery on reversible and irreversible myocardial injury: a randomized trial using cardiovascular magnetic resonance imaging and biochemical markers. Circulation. 2004;109(3):345–350. doi: 10.1161/01.CIR.0000109489.71945.BD. [DOI] [PubMed] [Google Scholar]
- 65.Hedstrom E, et al. Myocardial SPECT perfusion defect size compared to infarct size by delayed gadolinium-enhanced magnetic resonance imaging in patients with acute or chronic infarction. Clinical Physiology & Functional Imaging. 2004;24(6):380–386. doi: 10.1111/j.1475-097X.2004.00579.x. [DOI] [PubMed] [Google Scholar]
- 66.Jackson BM, et al. Borderzone geometry after acute myocardial infarction: a three-dimensional contrast enhanced echocardiographic study. Annals of Thoracic Surgery. 2005;80(6):2250–2255. doi: 10.1016/j.athoracsur.2005.05.103. [DOI] [PubMed] [Google Scholar]
- 67.Lund GK, et al. Prediction of left ventricular remodeling and analysis of infarct resorption in patients with reperfused myocardial infarcts by using contrast-enhanced MR imaging. Radiology. 2007;245(1):95–102. doi: 10.1148/radiol.2451061219. [DOI] [PubMed] [Google Scholar]
- 68.Sosnovik DE, et al. Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation. 2007;115(11):1384–1391. doi: 10.1161/CIRCULATIONAHA.106.663351. [DOI] [PubMed] [Google Scholar]
- 69.Caravan P, et al. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angewandte Chemie-International Edition. 2007;46(43):8171–8173. doi: 10.1002/anie.200700700. [DOI] [PubMed] [Google Scholar]
- 70.Dhingra R, et al. Cross-sectional correlates of serum heat shock protein 70 in the community. American Journal of Hypertension. 2006;19(2):227–231. doi: 10.1016/j.amjhyper.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Jugdutt BI, Warnica JW. Intravenous nitroglycerin therapy to limit myocardial infarct size, expansion, and complications. Effect of timing, dosage, and infarct location. Circulation. 1988;78(4):906–919. doi: 10.1161/01.cir.78.4.906. [DOI] [PubMed] [Google Scholar]
- 72.Jugdutt BI, Humen DP. Limitation of left ventricular hypertrophy and dysfunction by ACE inhibition after anterior Q-wave myocardial infarction. Cardiology. 1998;89(4):283–290. doi: 10.1159/000006801. [DOI] [PubMed] [Google Scholar]
- 73.Ali SM, et al. Early angiotensin converting enzyme inhibitor therapy after experimental myocardial infarction prevents left ventricular dilation by reducing infarct expansion: a possible mechanism of clinical benefits. Coronary Artery Disease. 1998;9(12):815–821. doi: 10.1097/00019501-199809120-00006. [DOI] [PubMed] [Google Scholar]
- 74.Apple KA, et al. Selective targeting of matrix metalloproteinase inhibition in post-infarction myocardial remodeling. Journal of Cardiovascular Pharmacology. 2006;47(2):228–235. doi: 10.1097/01.fjc.0000200989.23987.b8. [DOI] [PubMed] [Google Scholar]
- 75.Cheng Y, et al. Bradycardic therapy improves left ventricular function and remodeling in dogs with coronary embolization-induced chronic heart failure. Journal of Pharmacology & Experimental Therapeutics. 2007;321(2):469–476. doi: 10.1124/jpet.106.118109. [DOI] [PubMed] [Google Scholar]
- 76.Enomoto Y, et al. Early ventricular restraint after myocardial infarction: extent of the wrap determines the outcome of remodeling. Annals of Thoracic Surgery. 2005;79(3):881–887. doi: 10.1016/j.athoracsur.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 77.Backlund T, et al. Effect of vasopeptidase inhibitor omapatrilat on cardiomyocyte apoptosis and ventricular remodeling in rat myocardial infarction. Cardiovascular Research. 2003;57(3):727–737. doi: 10.1016/s0008-6363(02)00721-6. [DOI] [PubMed] [Google Scholar]
- 78.Mann DL. Targeted anticytokine therapy and the failing heart. American Journal of Cardiology. 2005;95(11A):9C–16C. doi: 10.1016/j.amjcard.2005.03.007. [DOI] [PubMed] [Google Scholar]