Abstract
A high-precision pressure probe is described which allows non-invasive online-monitoring of the water relations of intact leaves. Real-time recording of the leaf water status occurred by data transfer to an Internet server. The leaf patch clamp pressure probe measures the attenuated pressure, Pp, of a leaf patch in response to a constant clamp pressure, Pclamp. Pp is sensed by a miniaturized silicone pressure sensor integrated into the device. The magnitude of Pp is dictated by the transfer function of the leaf, Tf, which is a function of leaf patch volume and ultimately of cell turgor pressure, Pc, as shown theoretically. The power function Tf=f(Pc) theoretically derived was experimentally confirmed by concomitant Pp and Pc measurements on intact leaflets of the liana Tetrastigma voinierianum under greenhouse conditions. Simultaneous Pp recordings on leaflets up to 10 m height above ground demonstrated that changes in Tf induced by Pc changes due to changes of microclimate and/or of the irrigation regime were sensitively reflected in corresponding changes of Pp. Analysis of the data show that transpirational water loss during the morning hours was associated with a transient rise in turgor pressure gradients within the leaflets. Subsequent recovery of turgescence during the afternoon was much faster than the preceding transpiration-induced water loss if the plants were well irrigated. Our data show the enormous potential of the leaf patch clamp pressure probe for leaf water studies including unravelling of the hydraulic communication between neighbouring leaves and over long distances within tall plants (trees).
Keywords: Irrigation, leaf patch clamp pressure, liana, Tetrastigma voinierianum, transfer function, turgor pressure, turgor pressure probe
Introduction
Optimum water supply, particularly at peak needs of the plants, is an important issue for greenhouse and field vegetable production. In a greenhouse on a sunny day, evaporation and transpiration (so-called evapotranspiration) can occur so rapidly that water loss can cause plant damage before wilting symptoms are visible. Even at lower temperatures the restricted rooting in greenhouses leads frequently to plant water deficiency. Thus, no matter how slight, drought stress will result in a significant reduction in growth and, in turn, of harvest yield. On the other hand, over-irrigation must be avoided and the appropriate method of watering must be selected because wet soils and permanent moisture on plant organs promote the incidence of root mould, grey mould (Botrytis blight), formation of leaf oedema, and other plant diseases (Sherf and MacNab, 1986). Conservation of water is another important issue in semi-arid and arid regions where water is scarce. The advent of (subsurface) drip irrigation has considerably reduced water consumption of agricultural, horticultural, and greenhouse crops, but has also highlighted the need for sensors monitoring water deficiency of plants directly and online (Jones, 2004). In plant physiology and agriculture the pressure bomb technique pioneered by Scholander et al. (1965) is a widely accepted reference technique for measuring leaf water status. However, the method is massively invasive, slow, labour-intensive (and therefore expensive), unsuitable for automation, and gives only spot measurements. Furthermore, interpretation of the data is still a matter of debate (Zimmermann U et al., 2004; Zimmermann D et al., 2007). Leaf thickness is also sometimes used as an indicator for water stress (Burquez, 1987; McBurney, 1992; Malone, 1993). Leaf thickness monitoring devices are commercially available. They are non-invasive and suitable for online measurements, but have the disadvantage that changes in water status are frequently not reflected sensitively in changes of leaf thickness. The pressure probe technique pioneered by Zimmermann, Tomos, and others (Zimmermann et al., 1969, 2004; Tomos, 1988; Balling and Zimmermann, 1990), on the other hand, is a well established technology for measuring turgor and xylem pressure on the levels of individual cells and xylem vessels, respectively. This technique is not suitable for automation. Moreover, the probe technique requires rather sophisticated equipment and a high level of technical skill. These disadvantages are also shared by the ball tonometry, a method which allows non-invasive monitoring of cell turgor pressure by application of an external pressure (Lintilhac et al., 2000; Geitmann, 2006). Other water stress monitoring devices are described in the literature, but all of them have various disadvantages which prevented their implementation for leaf water status measurements in greenhouses or in the field (see, for example, the review article of Jones, 2004, and also Grant et al., 2007).
In this communication a novel, non-invasive, online-monitoring plant-based probe is described that meets the demands of controlling horticultural and agricultural water applications. The probe is characterized by high precision, operating convenience, minimum costs, and by automation suitability. Data can be transferred wireless to a personal computer or to an Internet server via a mobile phone network for real-time evaluation and for the automatic regulation of irrigation. The technology includes a miniaturized silicone pressure sensor integrated into a spring clamp that is clamped to a patch of an intact plant leaf. The patch clamp pressure probe measures the attenuated pressure response of the leaf patch upon the application of a constant, clamped pressure. The attenuation of the applied pressure depends on the transfer function of the leaf. The magnitude of the transfer function depends on two terms, a turgor pressure-independent term (related to the compression of the cuticle, cell walls, and other structural elements) and a turgor pressure-dependent term. Theory shows that the turgor pressure-dependent part of the transfer function Tf is a power function of the cell turgor pressure, Pc, and predicts that Tf assumes values close to zero if Pc is high and, vice versa, values close to unity if Pc is low. This could be verified by combined turgor pressure probe and leaf patch clamp pressure probe measurements on leaflets of the liana Tetrastigma voinierianum. The 10-m tall liana growing in a tropical greenhouse was selected for the first studies because a comprehensive data set about diurnal changes in xylem and turgor pressure gradients under various environmental conditions existed for this greenhouse plant (Benkert et al., 1995; Thürmer et al., 1999; see also Zimmermann et al., 2004). Measurements of diurnal changes of the patch clamp pressure performed here yielded results which were consistent with the previous pressure probe work on the liana. The data also demonstrated that changes in the irrigation regime and in microclimate can be sensed by this novel probe very sensitively and that use of several probes allowed the study of the diurnal water transport at multiple scales from single leaves to the whole organism (Meinzer et al., 2001). This can be done without knowledge of the turgor pressure because the transfer function is a direct measure of leaf water status.
Materials and methods
Plant material
Experiments were performed on two specimens of the liana Tetrastigma voinierianum, growing in the c. 12 m tall tropical greenhouse of the University of Salzburg, Austria. The ground ascended by about 5 m towards the backstage of the greenhouse. The height of the plant in this area was about 4.5 m, whereas the height of the plant in the front area reached 10 m. However, the stem and the branches of the two plants were considerably longer, because the plants had grown vertically upwards and then part of the way downwards. First measurements were performed during the last week of May, 2007. This week was very sunny and warm. Experiments were repeated during the last week of August and the first week of September, 2007. At this time of the year the weather conditions were quite poor. The weeks were rainy, sunshine occurred only occasionally. On average, the sky was very cloudy. Due to the variable weather conditions the ambient temperature, T, in the greenhouse usually varied between 19 °C and 27 °C. By contrast, during the hot week in May temperatures at the top of the plants could reach up to 44 °C. The greenhouse was illuminated between 07.30 h and 19.00 h (CET=Central European Time). During the day-time, the artificial illumination was switched off automatically once the natural irradiance exceeded about 45 μmol photons m−2 s−1 at ground level. At full sunshine, the light intensity was limited by automatically operating blinds. The relative humidity (r.h.) of the air was regulated by overhead misters. Despite attempts to keep the r.h. above 60–70% large axial r.h. gradients along the stem of the liana were observed during sunny days (see below).
The ambient temperature and relative humidity at the measuring sites were determined by using thermistors (Tinytag; RS Components GmbH, Mörfelden-Walldorf, Germany).
Leaf patch clamp pressure probe and data acquisition
The pressure sensor chip allows pressure measurements up to 100 kPa. The sensor consists of a miniature piezoresistive Wheatstone bridge. The sensors used in this work were purchased from the company ‘Raumedic’ (Helmbrechts, Germany) and from the company ‘Keller’ (AG, Druckmesstechnik; Winterthur, Switzerland). However, it has to be pointed out that any other miniaturized pressure or force sensor could be used. The sensor chip was integrated into one of the planar circular pads of a spring clamp shown schematically in Fig. 1. The spring clamp consisted of two curved arms bridged by a spring in the middle (Wolfcraft GmbH; microfix S (B3630Fz60), Kempenich, Germany). The clamp pressure exerted by the spring on the leaf, Pclamp, could be varied by a stiff strap (made of synthetic material) spanned between the arms of the spring clip at the handhold site (Fig. 1). The length of the strap and thus the spring load acting on the leaflet between the two pads could be varied by regularly punched holes (as in a belt).
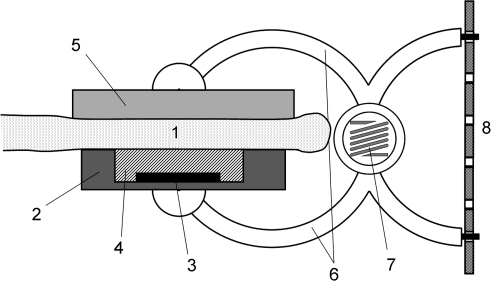
Fig. 1.
Schematic diagram of the leaf patch clamp pressure probe. A leaf patch (1) was clamped between two planar circular pads. One pad (2) was made of steel (diameter=6 mm; height=1.5 mm) and contained a receptacle (area=4×2.5 mm2; height=0.8–1.2 mm) for integration of the sensor chip (3) which is covered with a thin silicone membrane (4). The pressure-sensitive area of the chip used was either 1.3×1.3 mm2 or 1.9×1.2 mm2. The counter pad (5) was made of aluminium (diameter=8 mm; height=1 mm). The pads were attached to two curved arms (6) bridged by a spring (7) in the middle (Wolfcraft GmbH; microfix S, B3630Fz60; Kempenich, Germany). The clip was made of synthetic material. Optimum adjustment of the clamp pressure, Pclamp, to leaf thickness and stiffness was accomplished by a stiff strap (8), made of neoprene (rubber type CR-50, 50° Shore), containing regularly punched holes spanned between the arms of the spring clip at the handhold site.
Calibration of the sensor chips was performed by pressurization in a pressure chamber equipped with an integrated manometer (LEO 1, Keller AG, Winterthur, Switzerland). The readings of all sensors used in this study increased linearly with pressure in the range between 0 kPa and 100 kPa. The pressure sensitivity of the various sensors was found to be almost identical for all probes. Before use the probes were also tested for temperature-sensitivity in an accessible climate chamber because it is well-known that silicone-embedded sensors tend to become temperature-sensitive during storage. Probes were subject to temperature regimes ranging from 10 °C to 35 °C. Probes were not used if the temperature treatment induced a pressure change larger than 0.5 kPa.
The signals of the leaf patch clamp pressure probe were transmitted by a telemetry system (teleBITcom gmbh, Teltow, Germany). The operating distance between the battery-powered wireless telemetric transmitter and the receiver base station was up to 400 m. Each transmitter read, amplified, and converted the analogue signals of the pressure sensor into digitalized signals. The data were sent together with the transmitter ID-code every 90 s via the ISM band of 433 MHz to the receiver base station which logged and transferred the data to a personal computer or to a GPRS modem linked to an Internet server (NTBB Systemtechnik GmbH, Zeuthen, Germany) for analysis and archival storage. For sensor calibration and data recording the SENBIT software (teleBITcom gmbh, Teltow, Germany) was used. The software allowed communication with up to 32 sensors at regular short intervals (90 s to 5 min). In this study, up to 10 sensor/transmitter units were installed at different heights on the two lianas. Data transfer to the Internet server operated without any problem during the entire experimental period. However, for safety reasons hard-wired conventional data acquisition was also performed using dataloggers (Raumedic, Helmbrechts, Germany). The dataloggers were controlled by a computer program that provided functions for data storage and sensor calibration. Data were transferred regularly from the dataloggers to a personal computer.
Cell turgor pressure probe
Turgor pressure measurements were performed on leaflets of the 10-m tall liana at c. 0.2 m height above the roots. The construction and function of the cell turgor pressure probe has been described elsewhere (Zimmermann et al., 1969, 2004). Briefly, the microcapillary was filled with oil up to the very tip and was then inserted into the upper leaflet surface between the main vein and the leaflet edge. Insertion was made under a small overpressure (about 20 kPa) achieved by appropriate displacement of the metal rod in the probe in order to avoid tip clogging. Penetration was stopped upon reaching the mesophyll cell layer and formation of a stable oil/sap meniscus within the tip region of the microcapillary of the probe. During the measurements it was regularly proved whether the tip was clogged or not by appropriate displacement of the metal rod.
Theoretical background
The input pressure, Pin, experienced by the cells in a leaf patch is equal to the clamp pressure, Pclamp, only if the pressure signal is transferred lossless to the cells in the leaves. However, losses usually occur due to the compressibility and the deformability of the silicone of the pressure sensor as well as of the compressibility of the cuticle and other structural elements of the leaf. Therefore, theory shows that only a fraction of Pclamp may arrive at the cells, i.e. that the attenuation factor, Fa=Pin/Pclamp, is smaller than unity. Fa depends on the individual leaf properties. Fa can be assumed to be constant (and leaf thickness changes are negligible) if the structural elements are completely pre-compressed by application of an appropriate Pclamp. If Pclamp is kept constant during the following experimental period, Pin is constant and the output pressure, Pp, is only determined by the cell transfer function, Tf(V), where V is the leaf patch volume. In other words, Tf(V) determines the fraction of Pin sensed by the probe. It is dimensionless and assumes values between zero and unity:
| (1) |
Tf depends on the volume of the leaf patch, V, at constant ambient temperature, T:
| (2) |
δV depends on changes in cell turgor pressure, δPc, as follows:
| (3) |
where op is the average volumetric elastic modulus of the clamped tissue (Philip, 1958). op is a very complex parameter and will be dictated by the turgor pressure in the turgescent state. For a first approximation it is assumed that op increases linearly with Pc (support for this assumption is given by Zimmermann and Steudle, 1978; Zimmermann and Hüsken, 1980; Wendler et al., 1983):
| (4) |
where a and b are constants for individual leaf properties. Because of the viscoelastic properties of the cell wall, the magnitude of the constants depends on the duration of the external pressure application (Zimmermann and Hüsken, 1980). The constants are relatively large if rapid turgor pressure changes are induced (e.g. by using the cell turgor pressure probe), whereas slow turgor pressure changes (e.g. under transpirational conditions) result in small values.
Combining equations 2–4 leads to equation 5.
| (5) |
Equation 5 can be integrated by assuming for a first approximation that at Pc=0 Tf=1 and that the internal osmotic pressure of the cells remained constant. After appropriate re-arrangements equation 6 is obtained:
| (6) |
and, respectively, if the denominator is replaced by equation 4:
| (7) |
Introducing equation 6 into equation 1 yields the wanted relationship between the parameters Pp and Pin:
| (8) |
Equation 8 can be verified experimentally. Inspection of the equation shows that the patch clamp pressure, Pp, is a power function of the turgor pressure, Pc. The exponent of the function is equal to or smaller than unity. If a=1 and b <<Pc, equation 8 turns into Pp=b/Pc, i.e. both parameters are directly reciprocally coupled with each other. Thus Tf assumes low values if Pc is high and, vice versa, a value close to unity if Pc is close to zero. Using appropriate values for a and b for a given leaf (see below) it can be shown that below Pc=100 kPa, Pp increases dramatically. This means that the transfer function responds very sensitively upon loss of complete turgor pressure.
In the derivation of equation 8 it was assumed that op is temperature-independent. However, temperature effects on cell elasticity are well-known, although they are not very large in the temperature range investigated here (see, for example, Petersen et al., 1982; Niklas, 1992; Hogan and Niklas, 2004; Edelmann et al., 2005). Temperature effects on op cannot be excluded if different values for the Pclamp, and, in turn, for the input pressure, Pin, are selected, as well as if significantly different values for the constants a and b are assumed for optimum fitting of the Pp=f(Pc) curves. Therefore, in the case of large temperature gradients as observed here (see Figs 2 and 3) only the relative, but not the absolute changes in Pp measured at the different heights can be compared with each other. However, Pp values measured at the same height are still comparable and give information about the turgescence, i.e. about the water status of the leaf cells.
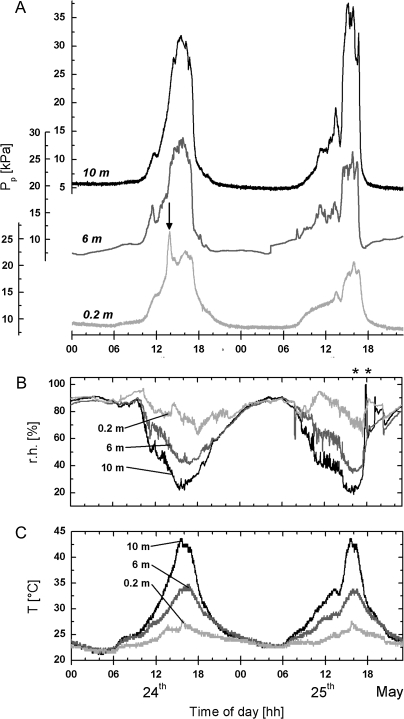
Fig. 2.
Diurnal changes of the leaf patch clamp pressure (Pp) recorded online on leaflets (average area=176 cm2) of the liana Tetrastigma voinierianum at 0.2 m, 6 m, and 10 m height (A). (B, C) The corresponding diurnal changes in relative humidity (r.h.) and ambient temperature (T) measured in the neighbourhood of the patch clamp pressure probes. Measurements were taken from 1 week recordings performed in the last week of May 2007 in the greenhouse of the University of Salzburg, Austria. Days were very sunny and hot. The plant was well irrigated before and during the experiments. Sensor-containing pads of the probes were clamped to the abaxial side of the leaflets at the periphery. Note that the diurnal changes in r.h. and T, respectively, were very often reflected in corresponding changes of the Pp values at the three measuring sites, but that there were also Pp changes (arrow) which could not be correlated with appropriate changes in the microclimate. Asterisks in (B) indicate the failure of r.h. measurements during the night, presumably because of water droplets on the r.h. sensor. For further details, see text.
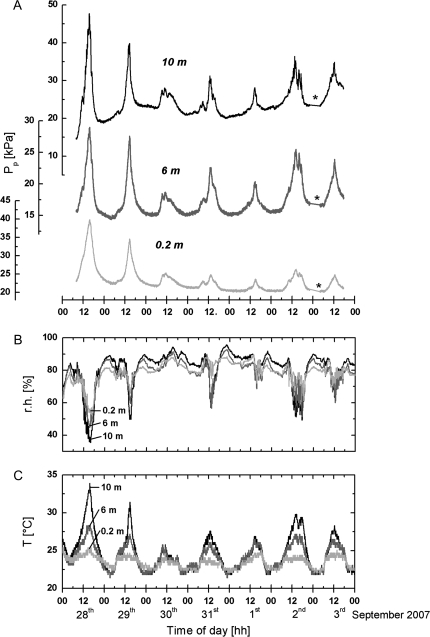
Fig. 3.
1 week online leaf patch clamp pressure (Pp) recordings (A) together with the corresponding r.h. (B) and ambient T (C) diurnal changes performed in the late summer of 2007. The plant was watered only sporadically in the weeks before the measurements. The soil was extremely dry. From 10.00 h on 28 August onwards the plant was watered continuously (c. 400 l d−1) up to the end of the experiments on 3 September. Weather conditions: 28 August sunny, cloudy sky and rain throughout 29 August to 1 September except a few hours of sunshine on 31 August. Light irradiance was, on average, below 45 μmol photons m−2 s−1 at ground level. 2 and 3 September were partly sunny. Note that peaking of the Pp values on 29 August was much higher (particularly at 0.2 m height) than on 2 and 3 September despite comparable diurnal changes of T and r.h.. This was most probably due to the efficient watering of the plant. Asterisks in (A) show the short-time interruption of data transmission for unknown reasons. For further details, see text.
Results
The leaf patch clamp pressure probe was clamped about 2 cm away from the edge of a leaflet of the compound leaves. Leaflets of similar size (176±58 cm2, n=12) were used. Inspection of the leaf patches after removal of the probes under the microscope revealed no changes in leaflet appearance beneath the pads, even after several weeks. Lesions on the leaves were never found. Only occasionally were very slight impressions of the pads on the leaflet surface observed.
Clamping of the leaves could be performed at any time of the day. Clamp pressures, Pclamp, applied to the leaves were of the order of 80–200 kPa. The optimum value for Pclamp of a given leaf has to be found empirically. Experience shows that Pclamp values could be considered as optimal if the output pressure Pp assumed values between 10 kPa and 25 kPa upon clamping in the early morning (when turgor pressure was high) or between 50 kPa and 70 kPa upon clamping around noon or towards early afternoon (when turgor pressure was usually low). The magnitude of Pp depended on the compressibility and deformability properties of the individual leaves which may vary considerably due to age, morphology, leaf thickness, abiotic factors etc. The magnitude of Pclamp had no effect on the diurnal profiles of Pp, in response to changes in microclimate and/or changes in the irrigation regime. Probes clamped on the same leaflet or nearby leaflets of leaves responded almost identically upon temporary changes in transpiration and/or water supply even if the Pclamp values were different due to different leaf thickness as well as local compressibility and deformability properties (data not shown). This was found over the entire height of the plants.
Screening experiments proved further that a high surface roughness prevented a uniform contact between the pads of the probe and the leaf. Non-uniform contact resulted in a reduced pressure response of the probe (data not shown). Thus, in order to obtain maximum resolution of microclimate-induced and/or plant-based effects on the Pp values, it was crucial that the probe was placed on relatively smooth areas, i.e. preferentially between the veins rather than on the veins in order to guarantee a uniform pressure transmission. It also turned out that areas with lesions or covered with dust should be avoided. Probably due to the absence of dust, high resolution results were obtained when the sensor-containing pad faced the abaxial side, but not the adaxial side of the leaf.
Diurnal changes in the patch clamp pressure
Figure 2A represents Pp recordings conducted on the 10 m tall liana on 24 May and 25 May, 2007. The plant was well-watered before the beginning of the measurements (22 May) and was also watered regularly during the experimental period. The sufficient supply of the leaves with water was indicated by high turgor pressure values of c. 500 kPa at predawn and guttation up to a height of 6 m around sunrise (see also Thürmer et al., 1999). Diurnal changes of the Pp values were recorded simultaneously at 0.2 m, 6 m, and 10 m height.
The corresponding diurnal changes in the ambient temperature, T, and relative humidity, r.h., measured close to the sites of the patch clamp pressure probes, are given in Fig. 2B and C. It was very sunny on 24 May resulting in a rapid heating-up of the greenhouse, particularly in the upper part. This led to the development of large vertical gradients in T and r.h. along the stem of the liana (Fig. 2B, C). Because sunlight was dimmed by operation of the automatic blinds at noon (see Materials and methods) the gradients did not reach maximum values before the afternoon. Between 15.30 h and 16.30 h an ambient temperature of 44 °C and a relative humidity of 20% were recorded at the top of the liana, whereas at 0.2 m height T and r.h. were 27 °C and 70%, respectively. The gradients in T and r.h. disappeared at c. 22.00 h. Due to the dense foliage of the plant above c. 7 m and due to light dimming by the blinds, leaves closer to the ground only became exposed to direct sunshine towards the early afternoon. In contrast to 24 May, the following day was cloudy until noon. Thus, leaves in the upper part of the liana became fully exposed to bright sunshine first around 14.00 h as indicated by the rapid increase in T and the corresponding decrease of r.h. above a height of 6 m (Fig. 2B, C). Despite the strongly delayed exposure of the leaves to sunshine, comparable maximum values for T and r.h. at the three measuring sites, as well as vertical gradients in T and r.h. of comparable size, were measured between 15.30 h and 16.30 h as on the day before.
Inspection of Fig. 2 shows that the diurnal changes of T and r.h. at the three measuring sites are reflected in corresponding changes of Pp. During the nights the Pp values were low. After sunrise at 04.24 h the Pp values remained low for further 4–5 h, except at 6 m height where a slight and continuous increase in the Pp values was very often recorded. After about 09.00 h the Pp values increased over the entire height of the liana. Peaking of the Pp values occurred between 15.30 h and 16.30 h at all heights when the T and r.h. gradients reached maximum values. Towards the evening the Pp values decreased again in order to reach the original low values at c. 22.00 h. Until peaking in the afternoon, more or less pronounced fluctuations of the Pp values were seen. A part of these fluctuations seemed to be correlated with changes in the local ambient temperature, relative humidity and/or with a temporary exposure of the clamped leaflet to sunshine (compare Fig. 2A with Fig. 2B, C). However, there was also a considerable number of fluctuations which could not be traced back to changes in the microclimate. An example can be found in Fig. 2A (arrow). The peaking of the Pp value measured at a height of 0.2 m around 14.00 h is obviously not induced by changes in the local temperature and relative humidity or by exposure to sunshine. Similar diurnal changes in Pp, T, and r.h. were also found for the 4.5 m tall liana in the backstage of the greenhouse (data not shown).
Figure 3 shows long-term Pp, T, and r.h. measurements performed at 0.2 m, 6 m, and 10 m height in late summer 2007. In contrast to the experimental conditions in late spring (Fig. 2) the liana had been watered only sporadically during the weeks before the measurements. The soil was extremely dry and no guttation was observed at predawn, even at 0.2 m height. Turgor pressure measurements performed at 0.2 m height yielded values below 250 kPa at predawn. Continuous irrigation was started at 10.00 h on 28 August (c. 400 l d−1). Guttation occurred at 0.2 m height, but not at 6 m height on 1 September. At sunrise (05.25 h), turgor pressures of c. 500 kPa were recorded at the base of the liana. Guttation at 6 m height was still not observed on 3 September indicating that the hydration state of the plant was still less than in the last week of May.
The T and r.h. profiles during the end of summer were also different to those in late spring (compare Fig. 2B, C with Fig. 3B, C). On 28 August when irrigation started it was sunny. Similarly to May, peak values of T and r.h. were reached at c. 15.30 h. However, temperatures at the top of the plant did not exceed 35 °C and r.h. did not drop below 40%. Accordingly, the vertical T and r.h. gradients were smaller than those measured at the end of May. On 29 August, when it became very rainy and light irradiance dropped dramatically, gradients in T and r.h. of slightly decreased size were still recorded. Peaking of T and r.h. occurred at c. 14.30 h. T and r.h. gradients did not develop in the following three days which were very cloudy and rainy. An almost constant temperature of c. 25 °C and a relative humidity of 80% were measured on the ground and at the top of the greenhouse throughout the day; light irradiance did not exceed 40 μmol photons m−2 s−1. Only on 31 August, did the rain stop and the upper part of the liana was exposed to sunshine for 1–2 h and thus small T and r.h. gradients developed. The following days of 2 and 3 September were partly cloudy and sunny and the peaking of T and r.h. occurred at c. 13.00 h. The upper, but not the lower part of the liana was exposed to sunshine for several hours. This resulted in the formation of T and r.h. gradients. The size of the gradients was similar to that measured on 29 August.
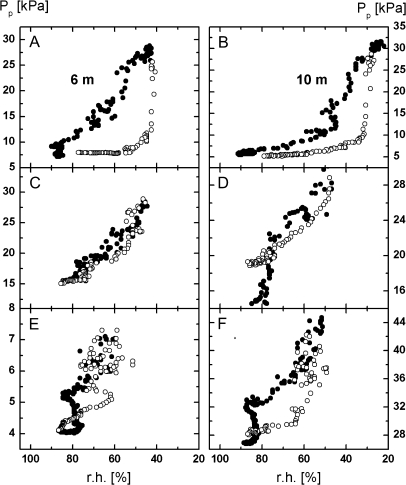
Inspection of Fig. 3A indicates that peaking of the Pp values coincided with peaking of r.h. and T in Fig. 3B and C. Figure 3A shows further that the peak amplitude of the Pp values recorded at the three measuring sites decreased continuously from 28 August whereas the low Pp values at the nights remained unaltered. The peak amplitude measured on 29 August was only slightly lower than that measured on the day before, the peak amplitude was rather low on 30 August. It remained at this low level until 2 September, except that on 31 August the peak amplitude at the three measuring sites increased slightly, presumably because of the short-time exposure of the upper leaves to sun (see above). Comparison of the diurnal changes in the Pp values with the corresponding T and r.h. changes in Fig. 3B and C indicates that part of the changes in the peak amplitude of the Pp values were induced by changes in the environmental parameters. However, part of the changes must obviously be attributed to irrigation. This conclusion can be drawn if, for example, the peaking of Pp on 2 and 3 September is compared with the peaking of Pp on 29 August. Despite the comparable size of the T and r.h. gradients, the peak Pp values were much smaller, particularly at 0.2 m height, on 2 and 3 September compared with 29 August. Plots of the Pp values measured at 6 m and 10 m height on 24 May, 28 August, and 2 September against r.h. (or against T since both parameters were usually closely related) also support the view that the local microclimate is not the only factor which determined the magnitude of the Pp values at the three measuring sites. Figure 4 shows that the Pp values recorded at 6 m and 10 m height increased with decreasing r.h. from c. 09.00 h until r.h. reached the minimum value (and T the maximum value, respectively), when with the progression of the day r.h. increased and T decreased, Pp decreased again. This decrease was much faster than expected in the light of the preceding r.h.-induced increase of Pp if the plant was well-watered (24 May; Fig. 4A, B). Refilling of the leaf cells was apparently enhanced by the high hydration state of the plant. By contrast, when the liana had not been irrigated well (28 August, Fig. 4C, D) both kinetics of the changes of Pp with r.h. were comparable. The low hydration state of the plant apparently delayed cell refilling. After 4 d irrigation (2 September), the low value of Pp recovered at relatively low r.h. as in May at 10 m height (Fig. 4F), but not at 6 m height (Fig. 4E). Recovery of the low Pp values at this height occurred, however, at significantly lower r.h. than on 28 August, indicating that rehydration of the leaves at this height had not been completed. Evaluation of other data sets yielded similar results (not shown).
Fig. 4.
Typical dependencies of Pp on r.h. measured at 6 m and 10 m height on 24 May (A, B), on 28 August (C, D) and on 2 September (E, F). Data were calculated partly from the data sets shown in Figs 2 and 3 as well as from other data sets not shown. Filled circles represent the dependency of Pp on r.h. until Pp peaking, open circles the relationship between Pp and r.h. after Pp peaking. Note that under conditions of efficient watering the low Pp values were established at much lower r.h. after peaking than expected from the preceding increase of Pp with decreasing r.h. (B, F). For more details, see text. Note also, that similar results were obtained when Pp was plotted against T (not shown) because of the close relationship between T and r.h..
Patch clamp pressure versus cell turgor pressure measurements
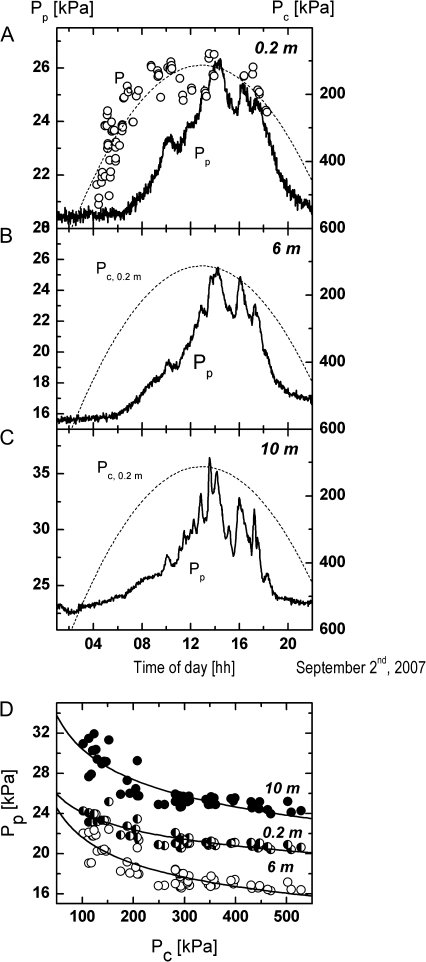
The leaf patch clamp pressure probe measures the pressure transfer function of a defined leaflet area. The above theoretical considerations have shown that the transfer function is mainly determined by turgor pressure provided that the structural elements do not contribute or constantly contribute to the pressure signal transfer. In order to prove the theory parallel measurements of leaf patch clamp pressure (Pp) and cell turgor pressure (Pc) were performed on a leaflet at 0.2 m height (Fig. 5A). Simultaneously, the Pp values were recorded at 6 m and 10 m height (Fig. 5B, C). Measurements were performed after several days of irrigation. In these experiments, the microcapillary of the turgor pressure probe was inserted into parenchymal cells located close to the main vein on the abaxial side of a leaflet, i.e. c. 10 cm away from the leaf patch clamp pressure probe near the leaflet periphery.
Fig. 5.
Diurnal recordings of cell turgor pressure, Pc, at 0.2 m height (A) and of leaf patch clamp pressure, Pp, at 0.2 m (A), 6 m (B), and 10 m height (C). The Pc recordings (circles; 77 data points) were performed on the same leaflet as the Pp measurement at 0.2 m height. Measurements were performed at the beginning of September after several days of irrigation. The microcapillary of the turgor pressure probe was inserted into parenchymal cells located close to the main vein on the abaxial side of a leaflet whereas the leaf patch clamp pressure probe was clamped about 2 cm away from the leaflet periphery as in the experiments shown in Figs 2 and 3. The delay between the Pp responses at the different heights and the changes in Pc at 0.2 m height increased from 10 m height (1 h) to 6 m height (1.5 h) and 0.2 m height (2.5 h). (D) shows that the relationships between Pp and Pc (56 data points measured during the morning hours) could be fitted by equation 8 if the delay between the response of Pc and Pp values upon environmental changes were not taken into account (fitting parameters: 0.2 m: Pin=50 kPa, a=9.38, b=0.99 kPa; 6 m: Pin=65 kPa, a=5.41, b=1.40 kPa; 10 m: Pin=70 kPa, a=6.58, b=2.73 kPa).
Turgor pressure measurements on leaf cells of T. voinierianum were difficult to perform because of the presence of mucilage. Mucilage resulted in clogging of the tip of the microcapillary of the cell turgor pressure probe. For this reason, measurements with the Scholander pressure chamber were not very reliable. It was extremely difficult to determine exactly the balancing pressure at which water appears at the cut end of the petiole of the leaf. In Fig. 5A the average turgor pressure values are plotted which were calculated from 3–8 min long measurements.
Inspection of Fig. 5A–C shows that Pc exhibited similar diurnal changes as the Pp values at 0.2 m, 6 m, and 10 m height. Changes in Pc after sunrise preceded changes in Pp, most likely due to the different measuring sites on the leaflets. After the onset of transpiration the loss of turgor pressure of cells located at the periphery of the leaflets (where Pp is recorded) is apparently immediately compensated by water shifting from the xylem and the cells located close to the main vein (where Pc is measured). Consistent with this explanation, towards the afternoon when all cells throughout the leaflets exhibit low turgor pressure, the increase in Pc and the decrease in Pp occurred nearly concomitantly.
Interestingly, the delay between the response of Pc and Pp upon changes in T and r.h. in the early morning hours decreased from about 2.5 h at 0.2 m height to 1.5 h at 6 m height, and to 1 h at 10 m height. In Fig. 5D the Pp values measured at the three heights are plotted against the corresponding Pc values (i.e. neglecting the delayed response of Pp). The curves could be fitted quite well by equation 8. Fitting of the curves required the assumption of different values for the constants a and b in equation 4 (see legend to Fig. 5) indicating that the elastic properties of the leaves were different, presumably due to changes of the ambient temperature with height (see above).
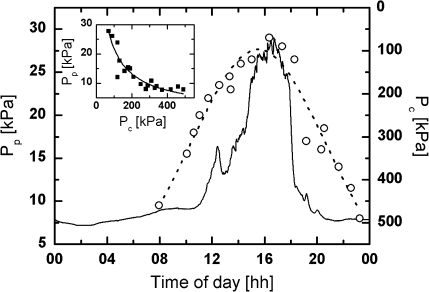
Turgor pressure measurements at 5 m height failed because of abundant mucilage in the leaflet tissue at this time of the year. Mucilage in the cells and xylem vessels was less abundant when turgor pressure measurements were performed during the summer of 1999. Plot of the Pc data measured by Thürmer et al. (1999) at 5 m height against online recordings of Pp in May 2007 revealed very good agreement between both parameters, even though the microcapillary was inserted into cells located on the upper leaflet surface between the main vein and the leaflet edge (Fig. 6). On both measuring days in 1999 and 2007, similar environmental conditions existed. Inspection of Fig. 6 shows that Pp measured at 6 m height was delayed by c. 1.5 h. A plot of the Pp values against the Pc values according to Fig. 5D could also be fitted very well by equation 8 (see inset of Fig. 6) indicating the validity of equation 8 to describe properly the relationship between Pp and Pc.
Fig. 6.
Comparison of diurnal turgor pressure measurements of Thürmer et al. (1999) performed at 5 m height with the diurnal Pp curve measured at 5 m height on 24 May, 2007. The microclimate conditions on both measuring days were similar, but leaflets contained much less mucilage in 1999 than in 2007. Inset: Plot of Pp versus Pc; data were fitted by equation 8 using Pin=75 kPa, a=1.1, and b=37.7 kPa. Note that in the experiments of Thürmer et al. (1999) the microcapillary of the turgor pressure probe was inserted into cells located on the adaxial and not on the abaxial leaflet surface. Despite this, similar results were obtained as in Fig. 5 suggesting hydraulic connection between the tissue cells.
Discussion
The results presented here demonstrate that the novel patch clamp pressure probe is a very sensitive, versatile, and easy-to-handle plant-based tool for online monitoring of the effects of evapotranspiration and/or of irrigation regimes on the water relations of leaves (Figs 2, 3). The probe measures the output signal upon application of a constantly clamped pressure, i.e. the pressure transfer function of leaf patches. Since the application of the probe is not restricted to special leaves, patch clamp pressure measurements will readily show which crop varieties can be grown with the least water for the most yield. It has also been demonstated here that the plant-based data can be sent by small telemetric units connected to the patch clamp pressure probes to a receiver base station which logs and stores the data pending transfer to a GPRS modem linked to an Internet server. The telemetric systems operated faultlessly. This is a very important result because there is agreement between scientists and growers (Jones, 2004) that control of irrigation must be tied to remote sensing devices in order to reduce manpower and to conserve water. The patch clamp pressure probe technique introduced here meets these demands.
A further advantage is that the probe is cheap and easy to handle. Some care is required only when clamping the leaf. Leaves must be clean and any rough surface topography must be avoided. This implies that the dimensions of the pads of the spring clamp must be adjusted to the size of the intercostal area since non-uniform contact between leaf surface and pads lowered the output pressure signal. Experience collected with patch clamp pressure measurements on T. voinierianum and on preliminary results of other plants (e.g. grapevines, bananas, Eucalyptus, and Arabidopsis) showed that a smooth intercostal leaf area for clamping can readily be found. Equally, optimum values for the clamp pressure can also be relatively quickly selected empirically if the guidelines mentioned above are followed. For many purposes calibration of the leaf patch clamp pressure probe against the cell turgor pressure probe is not stringent. It may be sufficient to know that the transfer function assumes small values at full turgescence and is nearly unity at low turgescence.
However, for scientific reasons and for the application of the probe in basic research, the dependency of the transfer function on cell turgor pressure might be of great interest. The theoretical considerations have demonstrated that the relationship between the transfer function and the turgor pressure can obviously be described by equation 8, i.e. by a power function. Concomitant measurements of turgor pressure and patch clamp pressure have confirmed the theoretical considerations (Fig. 5). Analogous recordings of turgor pressure and patch clamp pressure on grapevines, bananas, and Eucalyptus trees also yielded results (D Zimmermann, unpublished results) which were consistent with the predictions of equation 8. Patch clamp pressure measurements allow, therefore, calculation of turgor pressures if the constants a and b defined by equation 4 are known. These parameters can be obtained by numerical fitting of Pp versus Pc curves.
Online patch clamp pressure measurements open up new possibilities to unravel the hydraulic communication among leaves, including tall trees. It is shown here that the patch clamp pressure can simultaneously be monitored in shaded or sun-exposed leaflets at different heights under greenhouse conditions by using several probes. It was even possible to measure the turgor pressure at different sites on the leaf. Therefore, water shifting between leaf cells via the apoplastic and symplastic pathways can be studied. Physically sound theories are available (Molz and Ikenberry, 1974; Molz et al., 1979), but experimental facts are not available or are mainly based on speculations (Steudle and French, 1996). The first evidence for the dynamics of the hydraulic communication between leaflets at different height and within leaflets is given here. Thürmer et al. (1999) have shown by using the xylem and turgor pressure probe that the decrease in local xylem pressure after daybreak from positive, sub-atmospheric or slightly above-atmospheric values to negative values was associated with an almost 1:1 decrease in turgor pressure of the cells located close to the main vein of the leaflets (see also Zimmermann et al., 2004). Similarly, in the afternoon, the increase of xylem pressure in non-cavitated vessels towards more positive values correlated with a 1:1 increase in cell turgor pressure. Obviously, changes in transpiration resulted in a very rapid establishment of a new local water equilibrium state between the xylem and the turgor pressure of the adjacent cells. The turgor pressure measurements of Thürmer et al. (1999) were performed at 1 m and 5 m height. No significant differences in the turgor pressure values were found over the day. The turgor pressure measurements at 0.2 m height reported here confirmed the finding of Thürmer et al. (1999) that the turgor pressure in the cells located near the main vein dropped immediately upon daybreak. However, our patch clamp pressure measurements at the periphery of the leaflets imply that the turgor pressure of these cells apparently had a significantly delayed response upon the onset of transpiration. This finding can be taken as evidence that turgor pressure gradients are temporarily generated within the leaf during the early morning hours.
This delay in the response of patch clamp pressure after daybreak was c. 1 h for leaves at the top, but c. 2.5 h for leaves at the base. The data suggest that with progression of the day the gradients collapsed first at the top of the liana, where transpiration was high, before it occurred in leaves further down. During the afternoon hours the increase in the turgor pressure values measured close to the main vein coincided with the values measured by the patch clamp pressure probe (Fig. 5). The unbalanced osmotic pressure throughout the leaflets obviously favoured simultaneous turgor pressure regeneration in all leaflet cells. The process seemed to be very similar to the refilling of the cells of the resurrection plant Myrothamnus flabellifolia with water after desiccation (Wagner et al., 2000). Uniform radial refilling of cells with water in this species is also driven by the unbalanced osmotic pressure throughout the tissue. As indicated by Fig. 4A and B, restoration of full turgescence in T. voinierianum was faster than the preceding turgor pressure loss if the lianas were well-watered. By contrast, limitations in water supply resulted in the coincidence of the phases of turgor pressure loss and regeneration.
Interestingly, watering of the lianas after non-irrigation seemed to lead preferentially to water shifting to the top of the plant, because, after 3 d of irrigation, turgor pressure regeneration during the afternoon was very fast again at 10 m height (Fig. 4F), but not at 5 m height (Fig. 4E). Rapid water movement to the top is expected if hydrostatic pressure, osmotic pressure, and/or gel-based gradients throughout the vessels exist or are generated rapidly in the afternoon when cavitation has occurred. This was demonstrated by Benkert et al. (1995) and Thürmer et al. (1999) by using the xylem pressure probe. Continuous xylem pressure gradients during the morning hours can also explain why changes in Pp at lower heights were frequently found which could not be correlated with changes in local temperature or relative humidity. Water loss at the top of the plant induces a drop in xylem pressure which is transferred rapidly to the basal leaves where it induces a temporary turgor pressure loss that is soon compensated by water uptake from the roots (arrow in Fig. 2).
Even though more work is required, the present study shows that the leaf patch clamp pressure probe is a promising tool to elucidate short- and long-distance water transport in T. voinierianum and other plants.
Acknowledgments
We are very grateful to St Nieft, B Eberhardt, and A Pustlauck for very skilful technical assistance and A Gessner for engineering assistance and guidance. We are also very grateful to G Kunze, Raumedic, for supply of the pressure sensor chips.
References
- Balling A, Zimmermann U. Comparative measurements of the xylem pressure of Nicotiana plants by means of the pressure bomb and pressure probe. Planta. 1990;182:325–338. doi: 10.1007/BF02411382. [DOI] [PubMed] [Google Scholar]
- Benkert R, Zhu J-J, Zimmermann G, Türk R, Bentrup F-W, Zimmermann U. Long-term xylem pressure measurements in the liana Tetrastigma voinierianum by means of the xylem pressure probe. Planta. 1995;196:804–813. [Google Scholar]
- Burquez A. Leaf thickness and water deficits in plants: a tool for field studies. Journal of Experimental Botany. 1987;38:109–114. [Google Scholar]
- Edelmann HG, Neinhuis C, Bargel H. Influence of hydration and temperature on the rheological properties of plant cuticles and their impact on plant organ integrity. Journal of Plant Growth Regulation. 2005;24:116–126. [Google Scholar]
- Geitmann A. Experimental approaches used to quantify physical parameters at cellular and subcellular levels. American Journal of Botany. 2006;93:1380–1390. doi: 10.3732/ajb.93.10.1380. [DOI] [PubMed] [Google Scholar]
- Grant OM, Tronina L, Jones HG, Chaves MM. Exploring thermal imaging variables for the detection of stress responses in grapevine under different irrigation regimes. Journal of Experimental Botany. 2007;58:815–825. doi: 10.1093/jxb/erl153. [DOI] [PubMed] [Google Scholar]
- Hogan CJ, Niklas KJ. Temperature and water content effects on the viscoelastic behaviour of Tilia americana (Tiliaceae) sapwood. Trees. 2004;18:339–345. [Google Scholar]
- Jones HG. Irrigation scheduling: advantages and pitfalls of plant-based methods. Journal of Experimental Botany. 2004;55:2427–2436. doi: 10.1093/jxb/erh213. [DOI] [PubMed] [Google Scholar]
- Lintilhac PM, Wei C, Tanguay JJ, Outwater JO. Ball tonometry: a rapid, non-destructive method for measuring cell turgor pressure in thin-walled plant cells. Journal of Plant Growth Regulation. 2000;19:90–97. doi: 10.1007/s003440000009. [DOI] [PubMed] [Google Scholar]
- Malone M. Rapid inhibition of leaf growth by root cooling in wheat: kinetics and mechanisms. Journal of Experimental Botany. 1993;44:1663–1669. [Google Scholar]
- McBurney T. The relationship between leaf thickness and plant water potential. Journal of Experimental Botany. 1992;43:327–335. [Google Scholar]
- Meinzer FC, Clearwater MJ, Goldstein G. Water transport in trees: current perspectives, new insights and some controversies. Environmental and Experimental Botany. 2001;45:239–262. doi: 10.1016/s0098-8472(01)00074-0. [DOI] [PubMed] [Google Scholar]
- Molz FL, Ikenberry E. Water transport through plant cells and cell walls: theoretical development. Soil Science Society of America Proceedings. 1974;38:699–704. [Google Scholar]
- Molz FJ, Kerns DV, Peterson CM, Dane JH. A circuit analog model for studying quantitative water relations of plant tissues. Plant Physiology. 1979;64:712–716. doi: 10.1104/pp.64.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ. Plant biomechanics. Chicago: University of Chicago Press; 1992. [Google Scholar]
- Petersen NO, McConnaughey WB, Elson EL. Dependence of locally measured cellular deformability on position on the cell, temperature, and cytochalasin B. Proceedings of the National Academy of Sciences, USA. 1982;79:5327–5331. doi: 10.1073/pnas.79.17.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip JR. The osmotic cell, solute diffusibility, and the plant water economy. Plant Physiology. 1958;33:264–271. doi: 10.1104/pp.33.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA. Sap pressure in vascular plants. Science. 1965;148:339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Sherf AF, MacNab AA. Vegetable diseases and their control. 2nd edn. New York: John Wiley & Sons Inc; 1986. [Google Scholar]
- Steudle E, French J. Water transport in plants: role of the apoplast. Plant and Soil. 1996;187:67–79. [Google Scholar]
- Thürmer F, Zhu J-J, Gierlinger N, Schneider H, Benkert R, Gessner P, Herrmann B, Bentrup F-W, Zimmermann U. Diurnal changes in xylem pressure and mesophyll cell turgor pressure of the liana Tetrastigma voinierianum: the role of cell turgor in long-distance water transport. Protoplasma. 1999;206:152–162. [Google Scholar]
- Tomos AD. Cellular water relations of plants. In: Franks F, editor. Water science reviews 3. Cambridge: Cambridge University Press; 1988. pp. 186–277. [Google Scholar]
- Wagner H-J, Schneider H, Mimietz S, Wistuba N, Rokitta M, Krohne G, Haase A, Zimmermann U. Xylem conduits of a resurrection plant contain a unique lipid lining and refill following a distinct pattern after desiccation. New Phytologist. 2000;148:239–255. doi: 10.1046/j.1469-8137.2000.00755.x. [DOI] [PubMed] [Google Scholar]
- Wendler S, Zimmermann U, Bentrup F-W. Relationship between cell turgor pressure, electrical membrane potential, and chloride efflux in Acetabularia mediterranea. Journal of Membrane Biology. 1983;72:75–84. [Google Scholar]
- Zimmermann D, Westhoff M, Zimmermann G, et al. Foliar water supply of tall trees: evidence for mucilage-facilitated moisture uptake from the atmosphere and the impact on pressure bomb measurements. Protoplasma. 2007;232:11–34. doi: 10.1007/s00709-007-0279-2. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Hüsken D. Turgor pressure and cell volume relaxation in Halicystis parvula. Journal of Membrane Biology. 1980;56:55–64. [Google Scholar]
- Zimmermann U, Räde H, Steudle E. Kontinuierliche Druckmessung in Pflanzenzellen. Naturwissenschaften. 1969;56:634. [Google Scholar]
- Zimmermann U, Schneider H, Wegner LH, Haase A. Water ascent in tall trees: does evolution of land plants rely on a highly metastable state? New Phytologist (Tansley Review) 2004;162:575–615. doi: 10.1111/j.1469-8137.2004.01083.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Steudle E. Physical aspects of water relations of plant cells. Advances in Botanical Research. 1978;6:45–117. [Google Scholar]