Abstract
Thinopyrum intermedium is resistant to many different pathogens. To understand the roles of ethylene response factors (ERFs) in defence responses, the first member of the ERF family in T. intermedium, TiERF1, was characterized and functionally analysed in this study. The TiERF1 gene encodes a putative protein of 292 amino acids, belonging to the B3 subgroup of the ERF transcription factor family. Biochemical assays demonstrated that the TiERF1 protein is capable of binding to the GCC box, a cis-element present in the promoters of pathogenesis-related (PR) genes, and possessing transactivation activity, as well as localizing to the nucleus. The transcript of TiERF1 in T. intermedium is rapidly induced by infection with Rhizoctonia cerealis, Fusarium graminearum, or Blumeria graminis, and ethylene, jasmonic acid, and salicylic acid treatments. More importantly, the ectopic expression of TiERF1 in tobacco activated the transcript of the PR genes of tobacco with a GCC box cis-element, and ACO and ACS genes key to ethylene synthesis, and in turn improved the resistance level to Alternaria alternata and tobacco mosaic virus, as well as causing some phenotypic changes associated with ethylene response in the transgenic tobacco plants. Taken together, TiERF1 protein as an ERF transcription activator positively regulates defence responses via the activation of some defence-related genes.
Keywords: Ethylene response factor, phenotypic change, resistance, Thinopyrum intermedium, transcription activator, transgenic tobacco plants
Introduction
An ever increasing number of publications indicate that several transcription factor families with various functions are involved in plant development and a variety of stresses. The ethylene response factors (ERFs), formerly known as ethylene-responsive element-binding proteins (EREBPs), belong to one large transcription factor family that contain a single AP2/ERF DNA-binding domain conferring GCC box-binding ability. EREBP1–EREBP4 of tobacco were first identified as GCC box-binding proteins (Ohme-Takagi and Shinshi, 1995). After this finding, more and more proteins in the ERF family were identified, for instance 122 and 139 ERF family genes were identified from Arabidopsis (Arabidopsis thaliana) and rice (Oryza sativa subsp. japonica), respectively, by a comprehensive computational analysis (Nakano et al., 2006). Many ERF members have been shown to take part in hormonal signal transduction (Ohme-Takagi and Shinshi, 1995; Solano et al., 1998), responses to biotic (Zhou et al., 1997; Park et al., 2001; Gu et al., 2002; Berrocal-Lobo et al., 2002; McGrath et al., 2005) and abiotic stresses (Stockinger et al., 1997; Liu et al., 1998), and regulation of metabolism and development processes in various plant species (Nakano et al., 2006). According to the difference in DNA box-binding ability of the single AP2/ERF domain (Sakuma et al., 2002), the ERF family is further divided into two major subfamilies, the ERF subfamily and the CBF/DREB subfamily. CBF/DREB transcription factors function predominantly in response to abiotic stresses. ERF proteins are mainly involved in modulating disease resistance responses in positive or negative mode (Gutterson and Reuber, 2004; McGrath et al., 2005).
Thinopyrum intermedium [Barkworth and Dewey = Agropyron intermedium (Host) Beauvoir; 2n=42; genome E1E1E2E2StSt], a wild wheat relative, possesses traits useful for wheat improvement, including multiple resistances against wheat leaf rust, yellow rust, stem rust (Cauderon et al., 1973), barley yellow dwarf virus, wheat streak mosaic virus (Sharma et al., 1984), powdery mildew caused by Blumeria graminis (Franke et al., 1992), head scab blight caused by Fusarium graminearum (Fedak and Han, 2005), sharp eyespot caused by Rhizoctonia cerealis (ZJ Chang, personal communication), and eyespot (Li et al., 2005). The resistances to wheat rusts, powdery mildew, barley yellow dwarf virus, wheat streak mosaic virus, head scab blight, and eyespot have been introgressed into the wheat background and characterized by different groups. However, whether ERFs are involved in T. intermedium defence responses has not been studied yet. Therefore, the study of the species-specific ERF genes may provide insights into T. intermedium disease resistance mechanisms.
The objectives of this study were: (i) to isolate the first ERF gene of T. intermedium, TiERF1, and characterize the molecular and biochemical properties of the TiERF1 protein; (ii) to examine the expression pattern of TiERF1 in T. intermedium after treatments with pathogens and defence-related hormones; and (iii) to investigate if ectopic expression of TiERF1 in tobacco affects the development and response to biotic stresses.
Materials and methods
Plant materials, growth conditions, and treatments
Seeds of T. intermedium cv. ‘Z1146’ were germinated and the seedlings were grown hydroponically at 22–25 °C with a photoperiod of 12 h. Four-leaf stage seedlings of T. intermedium were treated with the pathogens, R. cerealis, F. graminearum, and B. graminis, and defence-related hormones including 0.1 mM ethylene, 0.1 mM jasmonic acid (JA), and 1 mM salicylic acid (SA), following the protocol of Zhang et al. (2007).
Identification and sequence analysis of the TiERF1 gene
The full-length cDNA sequence (1433 bp) of a putative ERF1 gene in T. intermedium, termed TiERF1, was isolated using RT-PCR and RACE (rapid amplification of cDNA ends) methods from cDNA of T. intermedium leaves at 1 d after inoculation with R. cerealis as described by Zhang et al. (2007). To investigate whether the sequence is correct and determine if there is any intron in the gene, the reconstituted cDNA and DNA sequences of the TiERF1 gene containing the open reading frame (ORF) of 879 bp were amplified from cDNA and genomic DNA of T. intermedium using a pair of gene-specific primers (TiE-CF, 5′-ACGGCGAGGATGCTGCTGAAC-3′ and TiE-CR, 5′-GGTTGCTTTGCCGGATTGCTC-3′) based on the full-length cDNA sequence of TiERF1. The PCR products were cloned. The cDNA and genomic DNA sequences of TiERF1 were confirmed with the sequence information derived from five positive independent clones each using the ABI3730 sequencer (Applied Biosystems, Foster City, CA, USA).
Sequence analysis was performed using DNAMAN software, DNAStar software, and the BLAST online (http://www.ncbi.nlm.gov/blast) public database.
Subcellular localization
The ORF without the termination codon of TiERF1 was fused in-frame to the 5′-terminus of the green fluorescent protein (GFP) gene under the control of the cauliflower mosaic virus (CaMV) 35S promoter in the pGJ280 vector (Kotchoni et al., 2006). The resulting 35S::TiERF1-GFP fusion construct and the control vector 35S::GFP were transformed into white onion epidermal cells for transient expression following the protocol of Zhang et al. (2007). The expression of genes introduced into the onion cells was observed using a confocal laser scanning microscope (Leica TCS SP2, Germany).
GCC-binding assay via gel mobility shift
To test if the AP2/ERF domain of the predicted TiERF1 protein binds to the GCC box, the partial sequence of TiERF1 containing the AP2/ERF domain was amplified by the primers TiAP2F, 5′-ATGGATCCTCGTCGTGCTTCGGTTTC-3′ (with the BamHI site underlined); and TiAP2R, 5′-ATGTCGACTTCCTTCTTTTCGGGGAC-3′ (with the SalI site underlined), and then fused in-frame to the BamHI–SalI sites of the 3′-terminus of the ORF of glutathione S-transferase (GST) in the pGEX-4T-1 vector (Amersham). The GST–TiERF1 fusion protein was expressed in Escherichia coli BL21 cells, and secreted into the culture supernatant after being induced with 0.4 mM isopropyl-β-D-thiogalactopyranoside (IPTG), and purified using GST Purification Modules following the manufacturer's instructions (Amersham). Two copies of the wild-type GCC box 5′-AATTCATAAGAGCCGCCACT-3′ or mutated GCC box 5′-AATTCATAAGATCCTCCACT-3′ sequence were end-labelled with [α-32P]dATP as probe. The binding reaction, gel preparation, and electrophoretic mobility-shift assay (EMSA) were performed following the protocol of Zhang et al. (2007).
Transactivation activity assays in tobacco and yeast
To investigate the transactivation activity of TiERF1 in tobacco, the effector vector pBI35S::TiERF1, the TiERF1 binary expression vector, was constructed, in which the TiERF1 gene was expressed under the control of the CaMV 35S promoter in the sense orientation. The reporter vectors pGCC-GUS and pmGCC-GUS (Zhang et al., 2004), in which four copies of the GCC box or mutated GCC box were inserted upstream of the TATA-box of the CaMV 35S promoter (minimal promoter, mini) and the β-glucuronidase (GUS) gene, were kindly provided by Dr Rongfeng Huang (Institute of Biotechnology, CAAS, China). The tobacco transformation using different combinations of the effector vector with a reporter vector and the histochemical GUS activity assay were performed according to Zhang et al. (2004).
To investigate the transactivation activity of TiERF1 in yeast cells, the effector vector Yepgap::TiERF1 and the reporter constructs pGCC-LacZ and pmGCC-LacZ were constructed as described by Zhang et al. (2007), all of which do not contain any yeast activating domain and binding domain except the domains existing in TiERF1, and can thus be expressed in the yeast strain YM4271 cells. The yeast one-hybrid and the β-galactosidase (encoded by LacZ) filter-lift assay were performed according to the manufacturer's protocol (Clontech).
Expression analysis in T. intermedium by fluorescence quantitative RT-PCR
Fluorescence quantitative RT-PCR (Q-RT-PCR) was used to investigate the expression patterns of TiERF1 in T. intermedium after challenge with R. cerealis, F. graminearum, and B. graminis, and ethylene, JA, and SA treatments in the ABI Prism 7000 Real-Time PCR System (Applied Biosystems). Relative expression levels of TiERF1 were calculated using the standard curve method (User Bulletin 2, ABI PRISM 7000 Sequence Detection System); the serial dilutions of a standard cDNA mixture pooled from each cDNA sample for the times indicated in the experiments (equal aliquots were pooled from each of the samples) were used as standards. Actin was chosen as a reference gene. A standard curve was generated for the TiERF1 gene or actin gene on the same PCR plate that held the time series of experimental samples (linear standard curves with a slope between –3.5 and –3.2 and an R2 value >0.99). The primers specific for TiERF1 (TiRU, 5′-AGCCCGCCTTTCACTGTTG-3′; and TiRL, 5′-GGACCACGACGACCCGATA-3′) can produce the amplicon located in the 3′-untranslated region (UTR) of the TiERF1 gene. The primers specific for actin, the Q-RT-PCR, and the program followed Zhang et al. (2007). The molar amounts of TiERF1 and actin transcripts were calculated based on the standard curves analysis, generated from each standard cDNA. Transcript analysis of the TiERF1 gene was performed at least three times to confirm the accuracy of the results. Data were analysed using Microsoft Excel 2000.
Generation of TiERF1 transgenic tobacco plants
The TiERF1 binary expression vector pBI35S::TiERF1 was first introduced into Agrobacterium tumefaciens strain LBA 4404, then into tobacco (Nicotiuna tabacum cv. W38, nn) using the Agrobacterium-mediated leaf disc transformation method.
The positive transgenic T0 plants from regenerated kanamycin- (Km) resistant plants were screened via detection of the presence of TiERF1 by PCR with specific primers (TiFY, 5′-CCGTCGTGCTTCGGTTTCC-3′; and TiRY, 5′-CCATTGCCATCACCACCTTG-3′), then grown in a greenhouse and allowed to self-fertilize. The T1 seeds germinated in a 1/2 MS medium containing 200 mg l−1 Km, and the survivors were confirmed by PCR detection and then transferred into soil, grown in a growth chamber under a 16 h day/8 h night at 25 °C for 3–4 weeks. The positive T1 plants were grown and produced T2 seeds in a greenhouse. T2 seeds were grown on 1/2 MS medium and the phenotype of T2 plants was scored.
Disease resistance assays of transgenic tobacco plants
The detached leaves of the tobacco plants were inoculated with a plug of the medium containing the fungal mycelia of Alternaria alternata pv. tobacco as described by Jiang and Guo (2000), in which the mycelium side of the medium plug was in contact with the leaf surface at sites wounded using a toothpick. Disease severity on the leaves was evaluated and photographed on the third day after inoculation. The disease rating was scored as an average of the inoculated leaves of the transgenic and wild-type (WT) lines, where ‘IT:1’ indicated a disease lesion <0.2 cm in size, ‘IT:2’ indicated a lesion between 0.3 cm and 0.8 cm, and ‘IT:3’ indicated a lesion >0.8 cm. If there was no disease symptom, the score was ‘IT:0’.
The inoculation with tobacco mosaic virus (TMV) was performed using a standard mechanical rubbing method according to Cao et al. (2006). The inoculated plants were maintained at 25–27°C under cool-white fluorescent lamps. The lesions on the inoculated leaves were photographed at 5 d post-inoculation. The TMV phenotype of leaves in the inoculated plants were scored during the adult period.
The analysis of the resistance to Pseudomonas syringae pv. tobacco was performed according to Cao et al. (2006).
Expression of TiERF1 and defence-related genes in transgenic tobacco plants
Northern blotting was used to analyse the expression of the TiERF1 gene in the transgenic and WT tobacco plants. A 20 μg aliquot of total RNA for each sample was separated through electrophoresis in a 1.2% denaturing agarose gel. The probe from the 3′-UTR of TiERF1 was labelled with [α-32P]dATP according to the prime-α-gene labeling kit (Promega, Madison, WI, USA). Northern hybridization and filter washing conditions were carried out following the protocol of Zhang et al. (2004).
Semi-quantitative RT-PCR analyses were used to analyse the expression of TiERF1, and of tobacco PR genes containing the GCC box in their promoters, including PR1b (GenBank accession no. X66942), PR3 (CHN50, GenBank accession no. XS1599), PR2 (GLA13B, GenBank accession no. M60402), and osmotin (GenBank accession no. X95308), and two genes (ACO, GenBank accession no. AF19945; and ACS, GenBank accession no. EU123522) that are key to ethylene synthesis, in the transgenic and WT tobacco plants with appropriate primers. The tubulin gene as the internal standard was amplified using the gene-specific primers. The specific primers for the above genes are listed in Supplementary Table 1 available at JXB online. Q-RT-PCR was carried out using these gene-specific primers and according to the protocol of Zuo et al. (2007). The relative expression of the target gene is normalized to that of the tubulin gene.
Results
The sequence of TiERF1
The full-length cDNA sequence of TiERF1 (GenBank accession no. EF570121) was obtained from cDNA of T. intermedium leaves challenged by R. cerealis using RT-PCR and RACE methods. The analyses of sequences derived from cDNA and DNA of T. intermedium demonstrated that there is no intron in the genomic transcription unit of the TiERF1 gene.
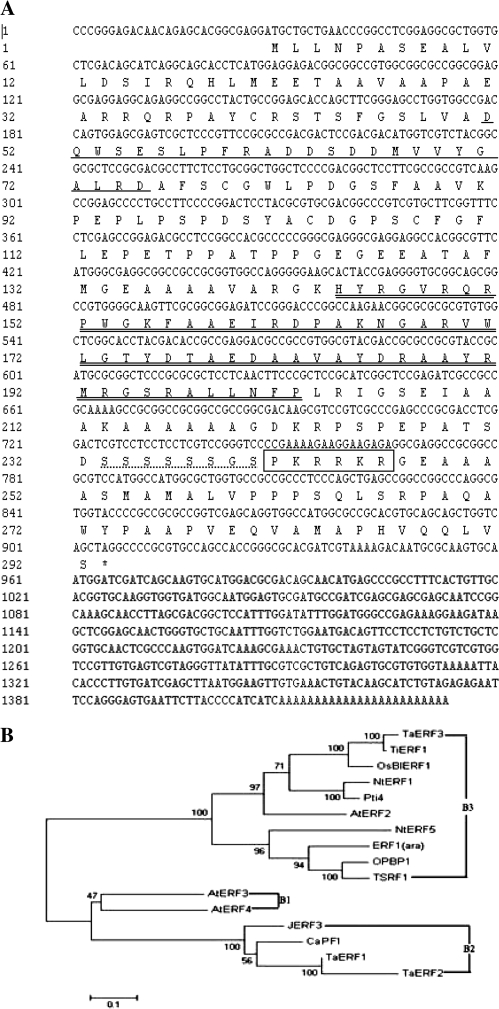
The TiERF1 gene encodes a putative protein TiERF1 consisting of 292 amino acids with a predicted molecular weight of 30.9 kDa and an isoelectric point of 5.84. Database analysis revealed that the TiERF1 protein contains a single conserved AP2/ERF DNA-binding domain extending from amino acid 143 to 202, in which there are two conserved amino acids, namely the 14th alanine (A) and the 19th aspartate (D), characteristic of ERF proteins (Sakuma et al., 2002). In addition, the predicted TiERF1 protein contains a region rich in acidic amino acids (a putative transcription activation domain) from amino acid 51 to 75, and a putative nuclear localization signal (NLS) (P241KRRKR), as well a serine-rich region (Fig. 1A).
Fig. 1.
(A) cDNA sequence of TiERF1 and its deduced amino acid sequence. The AP2/ERF domain is double underlined, the putative acidic domain is underlined, the basic region corresponding to an NLS is shown by a black box, and the serine-rich region is marked by dashed and doted lines. (B) The reconstructed phylogenetic tree. The phylogenetic tree was drawn by the Neighbor–Joining program of MEGA 3.1 software after the ERF protein sequences were aligned using DNAMAN. GenBank accession numbers of the ERF proteins are listed as follows: Arabidopsis ERF1 (ara) (AAM63284), AtERF2 (AAM64544), AtERF3 (O80339), and AtERF4 (O80340); tomato Pti4 (AAC50047), TSRF1 (AN32899), and JERF3 (AAQ91334); tobacco NtERF1(BAA07321), OPBP1(U81157), and NtERF5 (AAU81956); pepper CaPF1 (AAP72289); wheat TaERF1 (AY781352), TaERF2 (APP32468), and TaERF3 (EF570122); and rice OsBiERF3 (aav98702).
Results of protein-blast showed that the TiERF1 protein aligns with different degrees of similarity to other ERF proteins that have been shown to play regulatory roles in defence responses, including rice OsBiERF3, Arabidopsis AtERF2, and tomato Pti4 and TSRF1. Sequence comparison showed that TiERF1 is closely related to OsBiERF3, with an amino acid identity of 66.37%. The reconstructed phylogenetic analysis indicated that TiERF1 belongs to the previously described B3 subgroup of the ERF family (Gutterson and Reuber, 2004) (Fig. 1B), including dicot Pti4, TSRF1, OPBP1, NtERF5, Arabidopsis ERF1 and AtERF2, and monocot OsBiERF3, TaERF3, and TiERF1, coinciding with the view that the major functional diversification within the ERF family pre-dated the monocot–dicot divergence (Nakano et al., 2006).
TiERF1 protein localizes to the nucleus and binds to the GCC box
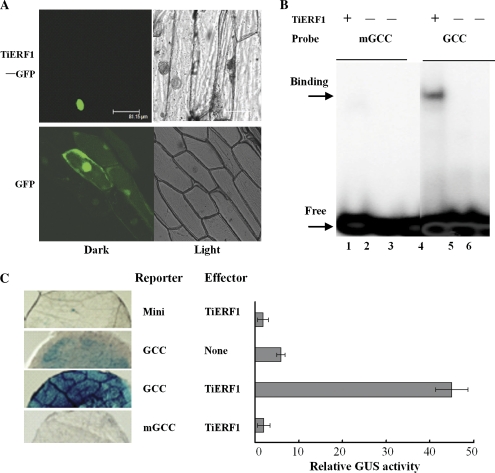
Since the predicted sequence of TiERF1 contains an NLS (P241KRRKR, Fig. 1B), a transient expression experiment was performed to investigate if the TiERF1 protein is targeted to the nucleus. The results showed that the TiERF1–GFP fusion protein was exclusively localized in the nucleus, while the control GFP was distributed throughout the cell (Fig. 2A), demonstrating that the TiERF1 protein localizes to the nucleus.
Fig. 2.
(A) Subcellular localization of TiERF1–GFP fusion protein in onion cells. The GFP fluorescence images in onion epidermal cells are shown for 35S::TiERF1–GFP localization in the nucleus (top) compared with the control 35S::GFP distributed in the whole cell (bottom). (B) Electrophoretic mobility shift assay. Lanes 1 and 4, GST–TiERF1 plus labelled mGCC or GCC, respectively; lanes 2 and 5, GST protein plus labelled mGCC or GCC, respectively; lanes 3 and 6, only labelled mGCC or GCC, respectively. Arrows indicate the TiERF1–GCC box binding band (B) and free probe (F). (C) Transcriptional activation of TiERF1 in tobacco. Reletive histochemical GUS activity as a result of activation of the GUS reporter gene by TiERF1. The bar indicates the standard error of the data (±SE).
An EMSA was performed to verify the possible interaction between TiERF1 and the GCC box in vitro. The results showed that only the recombinant TiERF1 protein with the GCC box probe resulted in a single, discrete DNA–protein binding band that migrated more slowly than the free probe, whereas other combinations did not demonstrate a bandshift, except the free probe (Fig. 2B), indicating that TiERF1 did bind to the GCC box, but not to the mutated GCC box.
TiERF1 is a transcriptional activator
As the predicted sequence of TiERF1 contains the region rich in acidic amino acids that may function as a putative transcription activation domain, the transcription activation activity of TiERF1 in tobacco and in yeast was assessed. The transient expression assays in tobacco showed that the GUS activity of co-expression of pGCC-GUS with the effector TiERF1 was ∼6-fold that of pGCC-GUS in the absence of TiERF1, and ∼40-fold that of the combination of pMini-GUS with TiERF1 (Fig. 2C), indicating that TiERF1 is able to activate the expression of genes following binding to the GCC box cis-element in the gene promoters in planta.
The yeast one-hybrid system is a stable system to study transcription regulation characteristics of transcription factors. The results of yeast one-hybrid and β-galactosidase activity assays indicated that only the hybrid cells containing the combination of pYepgap::TiERF1 and pGCC-LacZ showed much stronger β-galactosidase activity (∼7-fold) compared with other combinations, including pYepgap::TiERF1 with pmGCC-LacZ, pYepgap with pGCC-LacZ, and pYepgap::TiERF1 with pLacZ, demonstrating that TiERF1 as a GCC box-binding ERF transcriptional activator can activate the transcripts of genes with the GCC box cis-element in yeast cells (not shown).
Expression patterns of TiERF1 in T. intermedium following various treatments
The Q-RT-PCR method was used to investigate the transcript profile of TiERF1 in T. intermedium leaves in response to treatments with various pathogens and exogenous hormones.
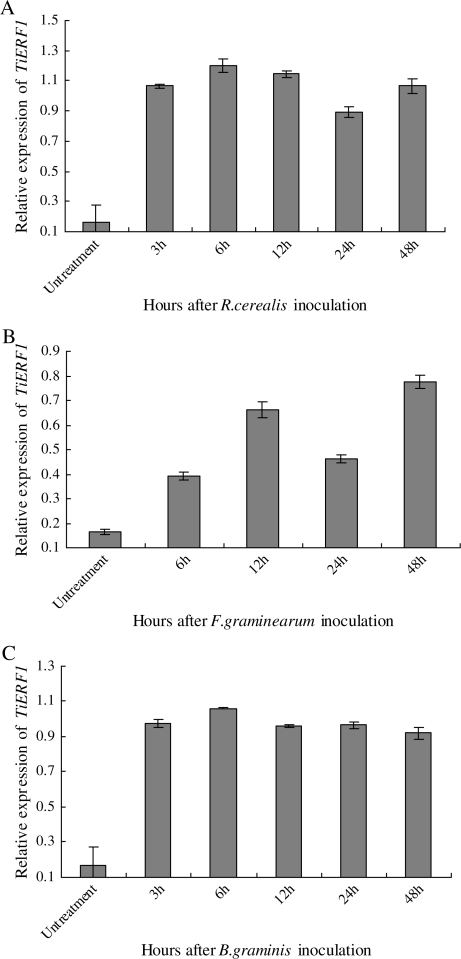
The transcript level of TiERF1 was up-regulated at 3 h after inoculation (hai) with R. cerealis, then peaked at 6 hai, and declined slightly after 24 hai (Fig. 3A). Upon F. graminearum challenge, TiERF1 transcription was induced within 6 hai, and reached a peak at 48 hai (Fig. 3B). With B. graminis challenge, the TiERF1 transcription level increased rapidly within 3 hai, and then remained at a similar level during the period tested (Fig. 3C). The degree of induction of TiERF1 transcription is strongest in response to R. cerealis challenge, and weakest in response to F. graminearum.
Fig. 3.
Q-RT-PCR analyses of the expression of TiERF1 in T. intermedium after challenge with pathogens. (A) R. cerealis, (B) F. graminearum, (C) B. graminis. Gene-specific primers for TiERF1 and actin genes were used. The expression value of the actin gene was used as internal control. The bar indicates the standard error of the data (±SE).
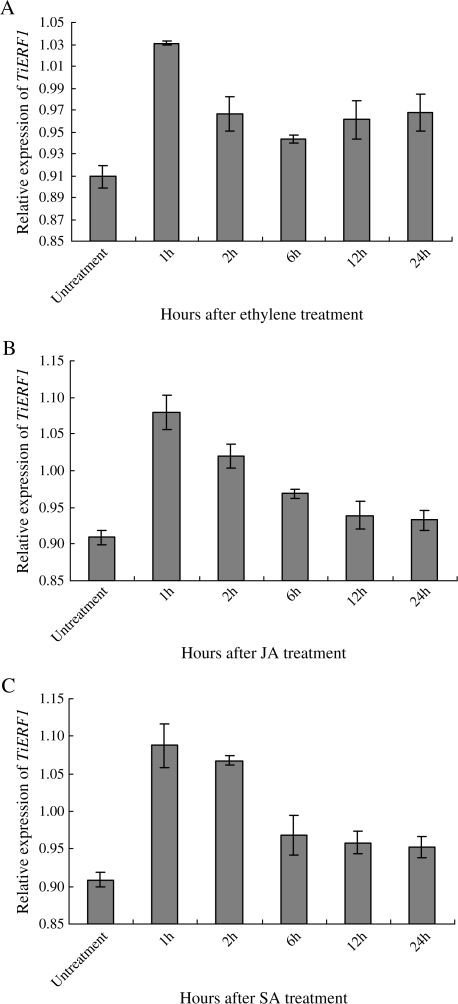
The expression of TiERF1 was rapidly induced after exogenous application of ethylene, JA, and SA, reached a peak at 1 h after the treatments, and then declined slowly (Fig. 4), suggesting that TiERF1 might be a responsive component of the ethylene/JA or SA pathways.
Fig. 4.
Q-RT-PCR analyses on the expression of TiERF1 in T. intermedium after ethylene (ETH), JA, and SA treatments. (A) R. cerealis, (B) F. graminearum, (C) B. graminis. Gene-specific primers for TiERF1 and actin genes were used. The expression value of the actin gene was used as internal control. The bar indicates the standard error of the data (±SE).
Generation and molecular characterization of transgenic tobacco lines overexpressing the TiERF1 gene
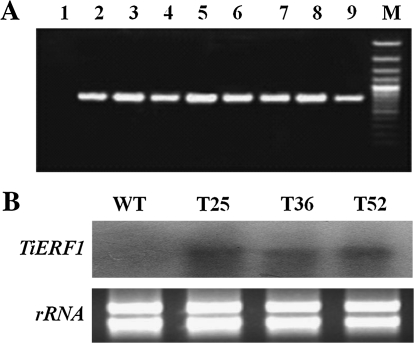
To test if TiERF1 plays important roles in regulating disease resistance, a functional analysis of TiERF1 in transgenic tobacco lines was first performed. Sixteen independent transformants (T0) were identified. The TiERF1-positive transgenic plants in T1 and T2 generations were selected by Km and confirmed by PCR analysis (Fig. 5A), and then subjected to Northern blotting analysis. The Northern blotting results showed that the TiERF1 transcript was overexpressed constitutively in the transgenic plants compared with the WT tobacco plants (Fig. 5B). The positive transgenic tobacco plants showing ovexpression were subjected to phenotype analysis and disease resistance tests.
Fig. 5.
(A) PCR analysis of the TiERF1 gene present in the transgenic lines compared with wild-type (WT) plants. 1, WT plant; 2–9: T2-positive plants derived from the transgenic lines T25, T36, and T52. (B) Northern blotting analysis of expression of TiERF1 in transgenic tobacco T1 plants compared with wild-type (WT) plants.
Overexpression of TiERF1 in tobacco caused phenotypic change associated with the ethylene response
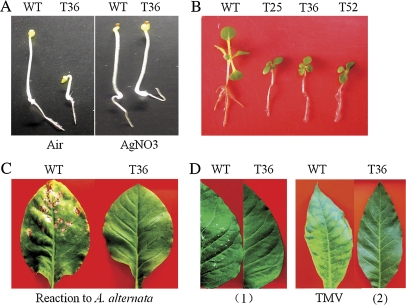
To unravel whether TiERF1 is involved in the ethylene signalling pathway, the ‘triple response’ was examined. This is a well-known effect of ethylene on plant growth, which is characterized by the inhibition of hypocotyl and root cell elongation, radial swelling of the hypocotyl, and exaggerated curvature of the apical hook (Guo and Ecker, 2004). At an early developmental stage (3 d post-germination), the ‘triple response’ was observed in the transgenic tobacco lines (T25, T36, and T52) overexpressing TiERF1 (OE-T), in which the etiolated OE-T seedlings in air displayed inhibition of root and hypocotyl elongation, and more curvature of the apical hook compared with the WT plants, whereas AgNO3, a potent inhibitor of ethylene action, could inhibit the ‘triple response’ of the OE-T seedlings (Fig. 6A). Furthermore, at 2–3 weeks post-germination, the OE-T plants could not form lateral roots, and had smaller leaves and lower fresh weight compared with the WT plants (Fig. 6B). In a later development period, the leaves of OE-T plants were darker green and slightly smaller, and the plants were shorter on average compared with the WT plants.
Fig. 6.
Phenotype changes in the TiERF1-overexpressiong (OE-T) tobacco lines compared with the wild-type (WT) tobacco plants. (A) The ‘triple response’ of the OE-T line on 1/2 MS medium (air) and the eliminated ‘triple response’ on 1/2 MS+AgNO3 (15 μM) medium at 3 d post-germination in the dark. (B) TiERF1 overexpression inhibits the lateral roots and leaves in young seedlings of transgenic tobacco. The seedlings of the OE-T transgenic and WT plants at 2 weeks post-germination on 1/2 MS medium. T25, T36, and T52 indicate the OE-T transgenic lines. (C) Analysis of disease resistance of the OE-T leaf compared with the wild type (WT) after inoculation with A. alternata at the adult stage. (D) Analysis of disease resistance of the OE-T and wild-type (WT) leaves inoculated with TMV for 5 d (1), and heart leaves at the adult stage (2).
Transgenic TiERF1 tobacco lines show enhanced resistance to A. alternata and TMV
The detached leaves of the OE-T tobacco lines (T25, T36, and T52) were inoculated with A. alternata. The results of the resistance analysis showed that the average lesion sizes of OE-T lines T25, T36, and T52 were 0.16, 0.11, and 0.13 cm, respectively; namely, their ITs were 1, whereas the average lesion sizes of WT leaves were 1.03 cm (IT:3). Furthermore, at the adult stage, the OE-T lines did not show the disease phenotype while WT plants had serious disease symptoms caused by A. alternata (Fig. 6C), suggesting that the overexpression of TiERF1 enhanced the resistance to A. alternata.
The resistance to TMV in the transgenic plants (T25, T36, and T52) and the WT W38 was examined. No visible lesions of the leaves in the OE-T transgenic plants (T25, T36, and T52) were observed at 5 d post-inoculation in seedlings, while there were necrotic lesions on leaves of WT plants with a lesion size >2.5 mm in diameter. About 1 month later, there were typical mosaic leaves of TMV in WT plants whereas no typical mosaic leaves of TMV were seen in OE-T plants (Fig. 6D). The results suggested that the overexpression of TiERF1 improved the level of resistance to TMV.
Expression of TiERF1 in tobacco induced expression of defence-related genes
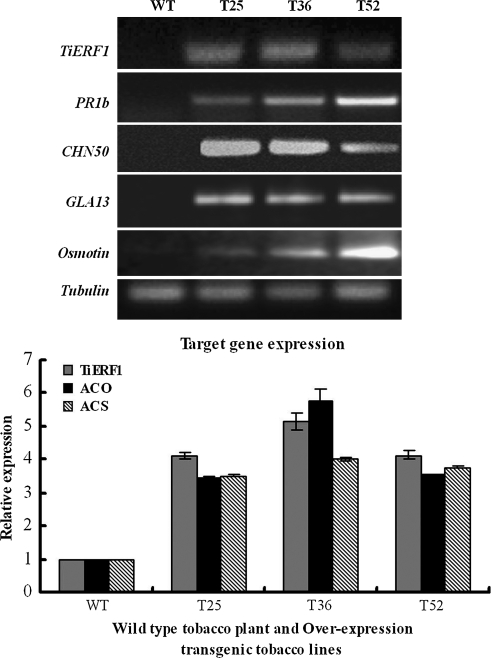
The gene expression analysis showed that overexpression of TiERF1 in tobacco increased the transcript levels of tobacco PR genes, including PR1b, CHN50 (PR2), GLA13B (PR3), and osmotin, and even ACS and ACO key to ethylene synthesis, in the OE-T transgenic lines compared with the WT plants (Fig. 7), suggesting that the overexpression of TiERF1 in tobacco activates the transcripts of the tested tobacco genes directly or indirectly.
Fig. 7.
Expression of TiERF1 and the tobacco PR, ACS, and ACO genes in OE-T and WT tobacco plants by semi-quantitative RT-PCR and quantitative RT-PCR analyses. The relative gene expression is normalized to that of the tubulin gene.
Discussion
Accumulating ERF proteins in several plant species have been shown to play important roles in the defence response through modulating the transcripts of a series of defence-related genes (Zhou et al., 1997; Park et al., 2001; Gu et al., 2000, 2002; Berrocal-Lobo et al., 2002; Chakravarthy et al., 2003; Lorenzo et al., 2003; Fischer and Droge-Laser, 2004; Guo et al., 2004; Gutterson and Reuber, 2004; Zhang et al., 2004; McGrath et al., 2005; Mazarei et al., 2007; Oňate-Sánchez et al., 2007). However, it is not known if and how ERFs in T. intermedium regulate the defence response to the pathogens. Given that across species ERF proteins may have different regulatory roles in defence responses, it is necessary to study the species-specific ERF genes in order to understand the plant defence mechanisms. Since T. intermedium is a wild relative of wheat, the wheat expressed sequence tags (ESTs) homologous to ERF genes help in the isolation of ERF genes from T. intermedium cDNA expressed under stress. Based on this strategy, the first ERF gene of T. intermedium, namely TiERF1, has been successfully isolated using RT-PCR and RACE methods. Sequence comparison and phylogenetic analysis indicated that the deduced TiERF1 protein had 66.37% amino acid identity to OsBiERF3 from rice (Cao et al., 2006) and 91% identity to TaERF3 from wheat (Zhang et al., 2007).
Sequence analysis showed that the predicted TiERF1 protein sequence contains the traits commonly associated with ERFs, including a single AP2/ERF DNA-binding domain typical of ERF proteins, a putative transcription activation domain, an NLS, and a serine-rich region. In this study, the results of EMSA and transient expression assays demonstrated that the TiERF1 protein does have the capability of binding to the GCC box (a cis-element responsive to ethylene) in vitro and in vivo (in tobacco and yeast), can enhance the transcripts of genes with the GCC box cis-element, and is targeted to the nucleus. The biochemical assay results of TiERF1 coincide with its sequence traits. The data demonstrated that TiERF1 indeed functions as an activator-type ERF transcription factor following binding of the GCC box cis-element, implying that TiERF1 should play modulating roles in the defence response through up-regulating transcripts of a subset of genes with the GCC box present in their promoters. In the present study, the expression analysis further demonstrated that the ectopic overexpression of TiERF1 in tobacco activated the transcripts of some PR genes with the GCC box cis-element in the transgenic tobacco plants, such as PR1b, CHN50, GLA13B, and osmotin.
Phylogenetic analysis indicated that the TiERF1 protein belongs to the B3 subgroup of the ERF family. Some activator-type ERF proteins in the B3 subgroup have been shown to confer enhanced disease resistance when overexpressed in plants (Gu et al., 2000, 2002; Berrocal-Lobo et al., 2002; Berrocal-Lobo and Molina, 2004; Guo et al., 2004; Zhang et al., 2004; McGrath et al., 2005; Cao et al., 2006; Oňate-Sánchez et al., 2007). For example, Arabidopsis ERF1 and AtERF2, as activator-type ERF transcription factors, positively regulate resistance to some necrotrophic and soil-borne fungal pathogens (Berrocal-Lobo et al., 2002; Berrocal-Lobo and Molina, 2004; McGrath et al., 2005). However, AtERF4, as a repressor-type ERF in the B1 subgroup of the ERF family, is a negative regulator of resistance to necrotrophic pathogens and negatively regulates root sensitivity to JA in Arabidopsis (McGrath et al., 2005). Interestingly, the expression of the activator-type ERF genes mentioned above is also induced by pathogen- and defence-related hormones, consistent with their function in defence against pathogens (Oňate-Sánchez and Singh, 2002, 2007; Chakravarthy et al., 2003; Berrocal-Lobo et al., 2004; Zhang et al., 2004; McGrath et al., 2005; Oňate-Sánchez et al., 2007). In this report, Q-RT-PCR analyses showed that the transcript of the TiERF1 gene in T. intermedium was induced to a different extent after challenge with R. cerealis, F. graminearum, and B. graminis. The distinct induction of TiERF1 in response to different pathogens may have several explanations; for instance, it may defend the plant infected with three kinds of wheat pathogens to different extents, or TiERF1 may play a different role in the defence response to R. cerealis, F. graminearum, and B. graminis. Since no T. intermedium cultivars susceptible to R. cerealis, F. graminearum, and B. graminis were found, it is impossible to analyse the expression of TiERF1 after infection with virulent pathogens. Therefore, it is necessary to develop TiERF1-overexpressing T. intermedium or other species of plants in order to investigate the defence function and regulatory mechanism of TiERF1 in detail.
To investigate if TiERF1 functions in regulating defence responses, a functional analysis of TiERF1 in the transgenic tobacco lines was first performed because tobacco can be easily transformed, and has a well-characterized disease resistance assay system and defence-related genes regulated by ERFs (Cao et al., 2006). In this study, overexpression of TiERF1 in the transgenic tobacco plants enhanced the resistance to A. alternata and TMV, but did not change the level of resistance to P. syringae pv. tobacco compared with the WT plants. Additionally, the OE-T transgenic tobacco plants did not show any lesion resulting from the hypersensitive response (HR), inferring that it is also possible that TiERF1 functions through a different pathway from HR. Furthermore, overexpression of TiERF1 in tobacco caused phenotypic changes associated with ethylene response, such as the ‘triple response’ and inhibition of lateral root growth in early seedlings. Accordingly, in the OE-T TiERF1 transgenic plants, the transcript levels of ACO and ACS, two key genes related to ethylene biosynthesis, were increased, implying that the ectopic overexpression of TiERF1 in tobacco is potentially involved in ethylene biosynthesis, contributing to the above phenotypic changes. Previous reports have shown that the ectopic overexpression of tomato TERF1 in tobacco (Huang et al., 2004) and Pti4 in Arabidopsis (Gu et al., 2002) was also involved in ethylene biosynthesis and caused phenotypic change associated with the ethylene response. An understanding of the role of ERFs in ethylene biosynthesis might provide a view of ERFs in the ethylene signalling pathway. However, the highly homologous ERF OsBiERF3 overexpression enhanced the transgenic tobacco resistance to TMV and P. syringae pv. tobacco, but did not have phenotypic change associated with ethylene response (Cao et al., 2006). The main reasons for the differences are probably that the different ERF proteins from different plant species regulate distinct defence-related genes or that the tobacco cultivars transformed are different. Previous reports also showed that overexpression of different ERF proteins produced a different spectrum of disease resistance and had different phenotypic changes to some extent (Cao et al., 2006). These studies support the view that there are a large number of ERF genes in various plant species for dealing with specific environmental stress conditions (Fischer and Dröge-Laser, 2004).
Members of the ERF family in various plant species have been shown to be involved in ethylene/JA or SA signalling pathways (Ohme-Takagi and Shinshi, 1995; Solano et al., 1998; Park et al., 2001; Chakravarthy et al., 2003; Lorenzo et al., 2003; Guo et al., 2004; Huang et al., 2004; Zhang et al., 2004; McGrath et al., 2005; Mazarei et al., 2007). For example, Arabidopsis ERF1 is a point of integration of the ethylene/JA signalling pathways. In this report, TiERF1 expression could be induced by exogenous ethylene/JA or SA treatments, suggesting that TiERF1 might be a responsive component of the ethylene/JA or SA signalling pathways. Overexpression of TiERF1 in the transgenic tobacco plants increased the transcript levels of the tobacco PR genes, including PR1b, CHN50 (PR2), GLA13B (PR3), and osmotin. Analysis of the promoters of these PR genes showed that their promoters contain the GCC box that is related to the ethylene responsive cis-element (Ohme-Takagi and Shinshi, 1995). Interestingly, the promoters of CHN50 and GLA13B also contain the JA responsive cis-element (TGACG) (Mazarei et al., 2007). The GCC box and JA-responsive cis-element present in the promoters of the PR genes could be the cause of the observed transcript induction of the PR genes by TiERF1, implying that TiERF1 plays modulating roles in defence responses via the activation of some PR genes through ethylene/JA signalling pathways. The present results provide a starting point for understanding the functions of ERFs in T. intermedium defence mechanisms.
In conclusion, the first ERF gene of T. intermedium, TiERF1, was isolated using RT-PCR and RACE methods. It encodes a GCC-binding ERF transcription activator. The ectopic expression of TiERF1 in tobacco indicated that it can directly or indirectly regulate the expression of PR genes functioning downstream of the ethylene/JA signalling pathways, in turn positively modulating defence against some pathogens. These data will contribute to a further understanding of the characteristics and function of the ERF family of transcription factors in different species.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Material
Acknowledgments
The authors are grateful to Professor Francesco Salamini (Max-Planck Institute for Plant Breeding Research, Germany) and anonymous reviewers for kind suggestions on the manuscript, and our colleagues Dr Ming Chen and Dr Rongfeng Huang for providing some vectors and tobacco cv. W38. This study was supported by the National Natural Science Foundation of China (grant no. 30671292) and the National ‘Hi-Tech’ project (grant no. 2006AA10A104).
References
- Berrocal-Lobo M, Molina A. ETHYLENE RESPONSE FACTOR 1 mediates Arabidopsis resistance to soilborne fungus Fusarium oxysporum. Molecular Plant-Microbe Interactions. 2004;17:763–770. doi: 10.1094/MPMI.2004.17.7.763. [DOI] [PubMed] [Google Scholar]
- Berrocal-Lobo M, Molina A, Solano R. Constitutive expression of ETHYLENE- RESPONSE-FACTOR 1 in Arabidopsis confers resistance to several necrotrophic fungi. The Plant Journal. 2002;29:23–32. doi: 10.1046/j.1365-313x.2002.01191.x. [DOI] [PubMed] [Google Scholar]
- Cao YF, Wu YF, Zhang Z, Song FM. Overexpression of the rice EREBP-like gene OsBIERF3 enhances disease resistance and salt tolerance in transgenic tobacco. Physiological and Molecular Plant Pathology. 2006;67:202–211. [Google Scholar]
- Cauderon Y, Saigne B, Dauge M. The resistance to wheat rusts of Agropyron intermedium and its use in wheat improvement. In: Sears ER, Sears LMS, editors. Proceedings of the International 4th Wheat Genetics Symposium on Wheat Improvement. 1973. University of Missouri, Columbia, MD, pp. 401–407. [Google Scholar]
- Chakravarthy S, Touri RP, D'Ascenzo MD, Fobert PR, Despres C, Martin GB. The tomato transcription factor Pti4 regulates defense-related gene expression via GCC box and non-GCC box cis elements. The Plant Cell. 2003;15:3033–3050. doi: 10.1105/tpc.017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedak G, Han F. Characterization of derivatives from wheat–Thinopyrum wide crosses. Cytogenetic and Genome Research. 2005;109:360–367. doi: 10.1159/000082420. [DOI] [PubMed] [Google Scholar]
- Fischer U, Dröge-Laser W. Overexpression of NtERF5, a new member of the tobacco ethylene response transcription factor family enhances resistance to tobacco mosaic virus. Molecular Plant-Microbe Interactions. 2004;17:1162–1171. doi: 10.1094/MPMI.2004.17.10.1162. [DOI] [PubMed] [Google Scholar]
- Franke R, Nestrowicz R, Senula A, Staat B. Intergeneric hybrids between Triticum aestivum L. and wild Triticeae. Hereditas. 1992;116:225–231. [Google Scholar]
- Gu YQ, Yang C, Thara VK, Zhou J, Martin GB. Pti4 is induced by ethylene and salicylic acid, and its product is phosphorylated by the Pto kinase. The Plant Cell. 2000;12:771–785. doi: 10.1105/tpc.12.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu YQ, Wildermuth MC, Chakravarthy S, Loh YT, Yang C, He X, Han Y, Martin GB. Tomato transcription factor Pti4, Pti5, and Pti6 activate defense responses when expressed in Arabidopsis. The Plant Cell. 2002;14:817–831. doi: 10.1105/tpc.000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HW, Ecker JR. The ethylene signaling pathway: new insights. Current Opinion in Plant Biology. 2004;7:40–49. doi: 10.1016/j.pbi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Guo ZJ, Chen XJ, Wu XL, Ling JQ, Xu P. Overexpression of the AP2/EREBP transcription factor OPBP1 enhances disease resistance and salt tolerance in tobacco. Plant Molecular Biology. 2004;55:607–618. doi: 10.1007/s11103-004-1521-3. [DOI] [PubMed] [Google Scholar]
- Gutterson N, Reuber TL. Regulation of disease resistance pathways by AP2/ERF transcription factors. Current Opinion in Plant Biology. 2004;7:465–471. doi: 10.1016/j.pbi.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Zhang ZJ, Zhang XL, Zhang HB, Huang DF, Huang RF. Tomato TERF1 modulates ethylene response and enhances osmotic stress tolerance by activating expression of downstream genes. FEBS Letters. 2004;573:110–116. doi: 10.1016/j.febslet.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Jiang DH, Guo ZJ. Disease resistance and inheritance analysis of cryptogein transgenic tobacco plants. Acta Phytopathologia Sinica. 2003;33:237–242. (in Chinese) [Google Scholar]
- Kotchoni SO, Kuhns C, Ditzer A, Kirch HH, Bartels D. Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant, Cell and Environment. 2006;29:1033–1048. doi: 10.1111/j.1365-3040.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- Li HJ, Arterburn M, Jones SS, Murray TD. Resistance to eyespot of wheat, caused by Tapesia yallundae, derived from Thinopyrum intermedium homoeologous group 4 chromosome. Theoretical and Applied Genetics. 2005;11:932–940. doi: 10.1007/s00122-005-0025-0. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREBl and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal responsive gene expression, respectively, in Arabidopsis. The Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. ETHYLENE- RESPONSE-FACTOR 1 integrates signals from ethylene and jasmonate pathways in plant defense. The Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarei M, Elling AA, Maier TR, Puthoff DP, Baum TJ. GmEREBP1 is a transcription factor activating defense genes in soybean and Arabidopsis. Molecular Plant-Microbe Interactions. 2007;20:107–119. doi: 10.1094/MPMI-20-2-0107. [DOI] [PubMed] [Google Scholar]
- McGrath KC, Dombrecht B, Manners JM, Schenk PM, Edgar CI, Maclean DJ, Scheible WR, Udvardi MK, Kazan K. Repressor- and activator-type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome-wide screen of Arabidopsis transcription factor gene expression. Plant Physiology. 2005;139:949–959. doi: 10.1104/pp.105.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi N. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiology. 2006;140:411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme-Takagi M, Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. The Plant Cell. 1995;14:2565–2575. doi: 10.1105/tpc.7.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oňate-Sánchez L, Jonathan AP, Young J, Singh KB. AtERF14, a member of the ERF family of transcription factors, plays a nonredundant role in plant defense. Plant Physiology. 2007;143:400–409. doi: 10.1104/pp.106.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oňate-Sánchez L, Singh KB. Identification of Arabidopsis ethylene-responsive element binding factors with distinct induction kinetics after pathogen infection. Plant Physiology. 2002;128:1313–1322. doi: 10.1104/pp.010862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Park CJ, Lee SB, Ham BK, Shin R, Peak KH. Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. The Plant Cell. 2001;9:49–60. doi: 10.1105/tpc.13.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaka K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochemical and Biophysical Research Communications. 2002;290:998–1009. doi: 10.1006/bbrc.2001.6299. [DOI] [PubMed] [Google Scholar]
- Sharma HC, Gill BS, Uyemoto JK. High levels of resistance in Agropyron species to barley yellow dwarf virus and wheat streak mosaic viruses. Phytopathology. 1984;119:143–147. [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE 3 and ETHYLENE-RESPONSE-FACTOR 1. Genes and Development. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proceedings of the National Academy of Sciences, USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HB, Zhang DB, Chan J, Yang YH, Huang ZJ, Huang DF, Wang XC, Huang RF. Tomato stress-responsive factor TSRF1 interacts with ethylene responsive element GCC box and regulates pathogen resistance to Ralatonia solanacearum. Plant Molecular Biology. 2004;55:825–834. doi: 10.1007/s11103-004-2140-8. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Yao WL, Dong N, Liang HX, Liu HX, Huang RF. A novel ERF transcription activator in wheat and its induction kinetics after pathogen and hormone treatments. Journal of Experimental Botany. 2007;58:2993–3003. doi: 10.1093/jxb/erm151. [DOI] [PubMed] [Google Scholar]
- Zhou J, Tang X, Martin GB. The Pto kinase conferring resistance to tomato bacterial speck disease interacts with proteins that bind a cis-element of pathogensis-related genes. EMBO Journal. 1997;16:3207–3218. doi: 10.1093/emboj/16.11.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo KJ, Qin J, Zhao JY, Ling H, Zhang LD, Cao YF, Tang KX. Over-expression of GbERF2 transcription factors in tobacco enhanced brown spots disease resistance by activating expression of downstream genes. Gene. 2007;391:80–90. doi: 10.1016/j.gene.2006.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.