Abstract
Sedum alfredii (Crasulaceae) is the only known Cd-hyperaccumulating species that are not in the Brassica family; the mechanism of Cd hyperaccumulation in this plant is, however, little understood. Here, a combination of radioactive techniques, metabolic inhibitors, and fluorescence imaging was used to contrast Cd uptake and translocation between a hyperaccumulating ecotype (HE) and a non-hyperaccumulating ecotype (NHE) of S. alfredii. The Km of 109Cd influx into roots was similar in both ecotypes, while the Vmax was 2-fold higher in the HE. Significant inhibition of Cd uptake by low temperature or metabolic inhibitors was observed in the HE, whereas the effect was less pronounced in the NHE. 109Cd influx into roots was also significantly decreased by high Ca in both ecotypes. The rate of root-to-shoot translocation of 109Cd in the HE was >10 times higher when compared with the NHE, and shoots of the HE accumulated dramatically higher 109Cd concentrations those of the NHE. The addition of the metabolic inhibitor carbonyl cyanide m-chlorophenylhydrazone (CCCP) resulted in a significant reduction in Cd contents in the shoots of the HE, and in the roots of the NHE. Cd was distributed preferentially to the root cylinder of the HE but not the NHE, and there was a 3–5 times higher Cd concentration in xylem sap of the HE in contrast to the NHE. These results illustrate that a greatly enhanced rate of root-to-shoot translocation, possibly as a result of enhanced xylem loading, rather than differences in the rate of root uptake, was the pivotal process expressed in the Cd hyperaccumulator HE S. alfredii.
Keywords: Cadmium, hyperaccumulation, Sedum alfredii, translocation, uptake
Introduction
Hyperaccumulation of Cd by higher plants, defined as being capable of accumulating and tolerating up to 100 mg Cd kg−1 in shoots (Baker et al., 2000), is a very rare phenomenon. To the best of our knowledge, only three plant species, Thlaspi caerulescens, Arabidopsis halleri, and Sedum alfredii, have been identified as Cd hyperaccumulators (Robinson et al., 1998; Bert et al., 2002; Yang et al., 2004). Sedum alfredii is the only non-brassica to have demonstrated Cd hyperaccumulation. To optimize the potential use of Cd hyperaccumulators for phytoremediation (Salt et al., 1998; Clemens et al., 2002; McGrath and Zhao, 2003), basic information on the physiological, biochemical, and molecular mechanisms of Cd hyperaccumulation is necessary.
Information on the rate of uptake and transport of Cd within the plants is essential to understand better the physiology of Cd accumulation in hyperaccumulators. In the past decade, characteristics and mechanisms of Cd uptake and translocation have been investigated in both T. caerulencens and A. halleri. In the Cd hyperaccumulator T. caerulescens Ganges ecotype, Cd uptake was found to be metabolically dependent, and not inhibited by other divalent ions (Zhao et al., 2002), and may be mediated by a high-affinity Cd transporter in the root cell plasma membranes of T. caerulescens (Lombi et al., 2001). In another Cd hyperaccumulator, A. halleri, Cd uptake appeared to occur partly through the Zn pathway (Zhao et al., 2006). To date, no high-affinity Cd transporter gene has been identified in plants (Cosio et al., 2004).
Hyperaccumulator plants are generally characterized by a highly efficient root-to-shoot translocation system (Baker et al., 1994; Lasat et al., 1996; Shen et al., 1997; Zhao et al., 2006), whereas in non-hyperaccumulator plants (Chan and Hale, 2004) only a fraction of cellular root apoplast Cd is apparently translocated to the shoot. Reduced sequestration of Zn in root vacuoles has been suggested to account for the elevated translocation of Zn to the shoots in T. caerulescens (Lasat et al., 1998) and S. alfredii (Yang et al., 2005); whether it is also responsible for Cd hyperaccmulation needs to be further investigated. Root-to-shoot translocation of Cd probably occurs via the xylem and is driven by transpiration from the leaves (Salt et al., 1995; Hart et al., 1998); efficiency of xylem loading, therefore, may play an important role in the Cd hyperaccumulation of the hyperaccumulating plants. Recently, it was proposed that TcHMA4, a P-type ATPase from T. caerulescens, may play an important role in xylem loading of metals and thus could be important for the hyperaccumulation phenotype expressed in T. caerulescens (Papoyan and Kochian, 2004). HMA4 was also highly expressed in another Cd hyperaccumulator A. halleli, and probably serves as an efficient mechanism for improving Cd tolerance in plants by maintaining low cellular Cd concentrations in the root cytoplasm (Courbot et al., 2007).
Sedum alfredii is a naturally selected Zn/Cd hyperaccumulator belonging to the Crassulaceae family (Yang et al., 2002, 2004), which has significant potential for use in phytoremediation (Yang et al., 2005, 2006). Previous research has revealed that the hyperaccumulating ecotype (HE) of S. alfredii collected from an old Zn/Pb mining area accumulated Cd concentrations of up to 9000 μg g−1 and 6500 μg g−1 in leaves and stems, respectively (Yang et al., 2004), while its contrasting population from an uncontaminated site, non-hyperaccumulating ecotype (NHE), showed severe phytotoxicity at 100 μM Cd exposure (Xiong et al., 2004). Currently our understanding of the mechanisms of Cd hyperaccmulation by plants has focused exclusively on the well-known Cd hyperaccmulators, T. caerulescens and A. halleri, which are both Brassicaceae. A better understanding of the characteristics and physiological mechanisms by which S. alfredii (Crassulaceae) accumulates Cd, may provide additional basic information to aid in the development of these species for phytoremediation purposes.
Materials and methods
Plant culture
Seedlings of two contrasting ecotypes of S. alfredii were cultivated according to Yang et al. (2005). The HE of S. alfredii was obtained from an old Pb/Zn mine area in Zhejiang Province in China, and the NHE of S. alfredii was obtained from a tea garden in Hangzhou, Zhejiang Province, China. Plants were chosen to grow in non-contaminated soil for several generations to minimize the internal metal contents, then uniform and healthy shoots were selected and cultivated in the basal nutrient solution containing: 2 mM Ca2+, 4 mM NO3+, 1.6 mM K+, 0.1 mM H2PO4–, 0.5 mM Mg2+, 1.2 mM SO42–, 0.1 mM Cl–, 10 μM H3BO3, 0.5 μM MnSO4, 1 μM ZnSO4, 0.2 μM CuSO4, 0.01 μM (NH4)6 Mo7O24, and 100 μM Fe–EDTA. Nutrient solution pH was adjusted daily to 5.8 with 0.1 N NaOH or 0.1 N HCl. Plants were grown under glasshouse conditions with natural light, day/night temperature of 26/20 °C and day/night humidity of 70/85%. The nutrient solution was aerated continuously and renewed every 3 d.
Radiotracer 109Cd experiments
Roots of intact 4-week old seedlings of the HE or NHE were rinsed in deionized water, and then treated with a pre-treatment solution containing 2 mM MES-TRIS (pH 5.8) and 0.5 mM CaCl2 (Lasat et al., 1996). After 12 h pre-treatment, the seedlings were used for the different experiments as described subsequently. All the experiments were carried out using vessels filled with an uptake solution identical to the pre-treatment solution, with the addition of Cd as 109Cd-labelled CdCl2, which was added to the uptake solution 24 h before each experiment, and stirred to ensure complete mixing. Before each uptake experiment, 1 ml of uptake solution was collected and 109Cd activity was measured.
Concentration-dependent kinetics of 109Cd influx
Plants were transferred to custom-built hydroponic vessels (three seedlings in each 400 ml vessel) containing the 109Cd-labelled uptake solution. Ten different concentrations of Cd (0.25–50 μM) were used to study the influx kinetics of Cd, and each treatment was replicated three times. An aliquot of a concentrated solution of 109Cd-labelled (1.0 mCi l−1) CdCl2 was added to the uptake solution to achieve the desired final Cd concentration. After 60 min uptake, the seedlings were quickly rinsed with the unlabelled pre-treatment solution, and then transferred to identical vessels containing ice-cold desorption solutions (2 mM MES-TRIS, pH 5.8, 5 mM CaCl2, and 100 μM CdCl2). Lasat et al. (1996) showed that this desorption step was effective in removing most of the Zn adsorbed on cell walls of T. caerulescens and T. arvense roots. After desorption for 15 min, the seedlings were separated into roots and shoots, blotted dry, and weighed. Roots and shoots were transferred into radioactivity counting vials, 109Cd was assayed by gamma spectroscopy (Canberra Packard Auto Gamma 5780).
Effects of Zn, Cu, Fe, Mn, Mg, and high Ca on 109Cd influx
An uptake experiment was conducted to investigate the effect of divalent ions including Zn, Cu, Fe, Mn, Mg, and Ca on Cd uptake by the two ecotypes of S. alfredii; the experimental procedure was the same as described above. The uptake solution contained 0.5 mM CaCl2, 2 mM MES-TRIS (pH 5.8), and 10 μM CdCl2 labelled with 109Cd (2.0 μCi l−1). Nine treatments were included: control, +Zn (10 μM ZnCl2), +Cu (10 μM CuCl2), + Fe (10 μM FeCl2), +Mg (10 μM MgCl2), +Mn (10 μM MnCl2), +La (50 μM LaCl3), +Ca (4.5 mM CaCl2), and +Cl (9 mM NaCl), each with four or five replicates. NaCl was included in these studies to serve as a control to assess the possible confounding effects of Cl on Cd uptake. After 2 h uptake, the plant roots were desorbed as described above. Roots and shoots were then separated, oven-dried at 65 °C for 72 h, and weighed. 109Cd was then measured in plant samples by gamma spectroscopy (Canberra Packard Auto Gamma 5780).
Time-course dynamics of 109Cd uptake and accumulation
Roots of seedlings were immersed in 3 l of aerated uptake solution containing 2 mM MES-TRIS (pH 5.8), 0.5 mM CaCl2, and 10 μM 109CdCl2 (2.0 μCi l−1), At each time interval (0–90 min for short-term, and 2–72 h for long-term time course experiments, respectively), three plants of each ecotype were harvested and desorbed as described above. After desorption, the seedlings were separated into roots and shoots, oven-dried at 65 °C for 72 h, and weighed. 109Cd radioactivity was quantified in both roots and shoots as previously described. For the long-term experiments, fresh 109Cd-labelled solution was added periodically to maintain constant Cd concentrations in the uptake solution.
Effects of low temperature or metabolic inhibitors on Cd uptake
After 4 weeks of pre-cultivation, 10 seedlings of HE or NHE S. alfredii were placed in 1.0 l of aerated uptake solution containing 2 mM MES-TRIS (pH 5.8), 0.5 mM CaCl2, and 10 μM CdCl2 with different treatments including: control, carbonyl cyanide m-chlorophenylhydrazone (CCCP) (100 μM), 2,4-dinitrophenol (DNP) (100 μM), and 2 °C. For the 2 °C treatment, plants were transferred to ice-cold pre-treatment solution 30 min prior to the uptake, and uptake containers were placed in an ice bath and shaded from light. Each ecotype of S. alfredii was replicated four times. At each time interval (0.5, 1, 2, 4, 8, 16, and 24 h), water loss caused by transpiration was measured by weighing and compensated by addition of deionized water. A 2.0 ml aliquot of the uptake solution was taken from each pot for the determination of Cd concentrations by inductively coupled plasma mass spectroscopy (ICP-MS) (Agilent 7500a, USA); 2.0 ml of deionized water was then added to each pot. Total amounts of Cd removed by sampling of the uptake solution were <2% of the initial amounts of Cd in each pot. After 24 h, plants were rinsed, separated into roots and shoots, blotted dry with tissue paper, then oven-dried and weighed. Cumulative uptake of Cd by the two ecotypes of S. alfredii for each treatment were calculated from total cumulative depletion of Cd in the uptake solution.
In a separate experiment, the effect of CCCP on the Cd accumulation in both roots and shoots of S. alfredii was investigated. Two treatments including control (10 μM Cd) and CCCP (10 μM Cd + 100 μM CCCP) were replicated five times. Roots of the plants were immersed in the uptake solution for 2 d. After treatments, roots of the plants were washed and immersed in 5 mM CaCl2 for 10 min then rinsed in deionized water. Roots and shoots were separated, blotted dry with tissue paper, weighed, oven dried at 65 °C for 72 h, and, after determination of dry biomass, ground using a stainless steel mill and passed through a 0.25 mm sieve for elemental analysis. Dry root samples (0.1 g) of each treatment were digested with HNO3–HClO4, and the digest was transferred to a 50 ml volumetric flask, made up to volume, and filtered. Cadmium concentrations were determined by ICP-MS (Agilent 7500a, USA).
Microscopic imaging of Cd in roots
Visualization of Cd distribution in intact roots of S. alfredii was performed in 1-week-old seedlings of both ecotypes. Seedlings were treated with 100 μM Cd2+ for 0, 3, 6, or 24 h. Roots were then washed in 1.0 mM EDTA for 5 min, rinsed and gently blotted dry, and immediately loaded with 5-nitrobenzothiazolecoumarin, in the acetoxymethyl ester form (BTC-5N, AM; Molecular Probes, Leiden, The Netherlands). The solution containing BTC-5N, AM was prepared according to Lindberg et al. (2004), with the following modification. A stock solution of BTC-5N was prepared by dissolving 50 μg of the dye in 39.5 μl [<0.1% o(v/v) water] of dimethylsulphoxide (DMSO). The solution was then mixed with 10 μl of Pluronic F-127 (Molecular Probes) solution (20% w/v) in DMSO. Roots were treated with the BTC-5N solution for 45 min in the dark. A Nikon Eclipse 3000 epifluorescent microscope (Melville, NY, USA) equipped with a green fluorescent filter (excitation 415 nm, emission 500–530 nm) was then used to obtain epifluorescence images. Exposure times were uniform for all samples. Fluorescence and concurrent differential interference contrast images were taken with a SPOT camera (Nikon). No autofluorescence was observed in roots of two S. alfredii ecotypes.
A separate experiment using the Cd probe Leadmium™ Green AM dye (Molecular Probes, Invitrogen, Calsbad, CA, USA) was performed to investigate the distribution of Cd in roots of two S. alfredii ecotypes in plants pre-treated with 100 μM Cd for 24 h or 7 d. A stock solution of Leadmium™ Green AM was made by adding 50 μl of DMSO to one vial of the dye. This stock solution was then diluted with 1:10 of 0.85% NaCl. Roots were immersed in this solution for 90 min in the dark. A Leica DMR series fluorescent microscope equipped with a Chroma 86013 filter set (Chroma Technology, Rockingham, VT, USA) and CoolSNAP-HQ (Roper Scientific, Tucson, AZ, USA was used to visualize the roots. Cd fluorescence was visualized by using filters S484/15 for excitation and S517/30 for emission. All images were taken at ×10 magnification. Images were pseudocoloured with METAMORPH software (Universal Imaging, Downingtown, PA, USA).
Cd analysis in xylem sap and root
Plants of HE and NHE S. alfredii grown hydroponically for 10 weeks were used for xylem sap collection. The effect of Cd exposure on Cd concentrations in xylem sap and root was investigated by replacing the growth solution with a fresh solution containing the respective Cd concentration 4 h before the onset of xylem sap collection. Treatments include: 0, 10, 50, 100, 200, and 400 μM Cd, and were performed in triplicate. Twelve plants from each treatment were de-topped using sharp blades at ∼3.0 cm above the junction point of root and shoot. Immediately after de-topping, each stem was rinsed with deionized water and blotted with absorbent paper to remove contaminants from cut cells. After discarding ∼0.3 ml of sap, each cut surface was blotted again and silicon tubing was fitted over the stem. Sap flowing from the tubing was collected in sterile vials for 1 h. At the end of the collection period, xylem sap samples collected from four plants in each culture vessel were pooled and immediately frozen at 20 °C. Subsequently, roots were harvested, washed in 1.0 mM EDTA for 5 min, rinsed in deionized water, oven-dried at 65 °C for 72 h, weighed, and ground using a stainless steel mill to pass a 0.25 mm sieve. Dry root samples (0.1 g) of each treatment were digested with HNO3–HClO4, and the digest was transferred to a 50 ml volumetric flask, made up to volume and filtered. For xylem sap samples, a subsample of 0.5 ml was mixed with 25 ml of 2% (w/v) nitric acid. Cd concentrations of the samples were determined by ICP-MS (Agilent 7500a, USA).
Statistical analysis
All data were statistically analysed using the SPSS package (Version 11.0); analysis of variance (ANOVA) was performed on the data sets, and the mean and SE of each treatment of corresponding data were calculated.
Results
Cd influx into roots
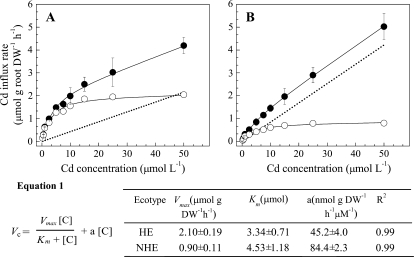
Concentration-dependent Cd influx kinetics in both ecotypes were characterized by smooth, non-saturating curves, although the HE clearly showed a marked saturable component in the low concentration range, which was much less evident in the NHE (Fig. 1). By mathematically resolving these curves using Origin Pro 7.5, linear and saturable components could be derived from experimental data. The linear Cd uptake was believed to reflect the cell wall binding fraction that was not removed by the desorption procedure, whereas the saturable component probably represented carrier-mediated transport across the root cell plasma membranes (Lasat et al., 1996; Cohen et al., 1998; Hart et al., 1998; Lombi et al., 2001). In both ecotypes, the model fitted the experimental data closely, as demonstrated by R2 values of 0.99 (Fig. 1).
Fig. 1.
Concentration-dependent Cd influx kinetics in roots of HE (A) and NHE (B) Sedum alfredii. Plants were placed in the 109Cd-labelled uptake solutions containing different Cd concentrations as shown in the figure for 1 h, followed by a desorption step for 15 min. Linear (dotted line) and saturable (open circles) components were derived from experimental data (filled circles) by mathematically resolving these curves using Origin Pro 7.5. The best fit for equation 1 was calculated for each curve. Parameters of the linear component and Michaelis–Menten model are summarized in the table. Vmax and Km values of saturable components were calculated by fitting a hyperbolic curve function to the saturable points. Data points and error bars represent means (n=4) and SE, respectively. Error bars do not extend outside some symbols. DW, dry weight. Vc, Cd influx rate; [C], Cd concentration; Vmax and Km, Michaelis–Menten parameters; a, slope of the linear component.
Analysis of the kinetic constants for Cd uptake in the HE and the NHE indicated that influx characteristics were different in the two S. alfredii ecotypes (Fig. 1). Saturable Cd influx for HE and NHE plants exhibited similar Km values, 3.34±0.71 μM and 4.53±1.18 μM, respectively. However, the maximal influx (Vmax) for Cd was significantly different between the two ecotypes. The value of Vmax for the HE was >2-fold higher than that for the NHE (Fig. 1). In contrast, angular coefficients of the linear components (a) were ∼2-fold higher for the NHE than those for the HE.
Effects of other divalent ions on Cd influx
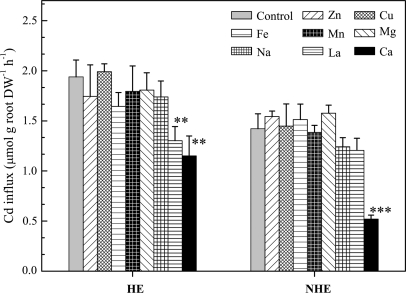
The effects of Zn, Cu, Fe, Mn, Mg, and Ca on Cd influx into roots of the two S. alfredii ecotypes were investigated by adding these divalent ions to the 109Cd uptake solutions (Fig. 2). The results showed that the addition of equal molar (10 μM) Zn, Cu, Fe, Mn, and Mg had no significant effect on Cd influx in either ecotype. However, Cd influx into roots decreased 41% (P < 0.01) and 63% (P < 0.001) in HE and NHE plants, respectively, when high concentrations of CaCl2 treatments (5.0 mM) were provided, in comparison with control plants (0.5 mM). Similarly, treatments of 50 μM LaCl3 decreased Cd uptake by 31% in the HE (P < 0.01) and the 15% in NHE (not significant). Increasing Cl– (as NaCl) from 1.0 mM to 10.0 mM had no significant effect on Cd influx, suggesting that the effects of elevated CaCl2 or LaCl3 levels in the uptake solutions was a consequence of the Ca or La ions.
Fig. 2.
Effects of ZnCl2, CuCl2, FeCl2, MnCl2, MgCl2, NaCl, LaCl3, and CaCl2 on 109Cd uptake by the two ecotypes of S. alfredii. The concentrations of Cd and other divalent cations were 10 μM, except for LaCl3 (50 μM), CaCl2 (4.5 mM), and NaCl (9.0 mM). Uptake of 109Cd was for 2 h, followed by a desorption step with unlabelled Cd for 15 min. Error bars represent SEs (n=4–5). Means marked with two or three asterisks indicate significant difference at P < 0.01 or P < 0.001, respectively. DW, dry weight.
Time-dependent kinetics of Cd influx
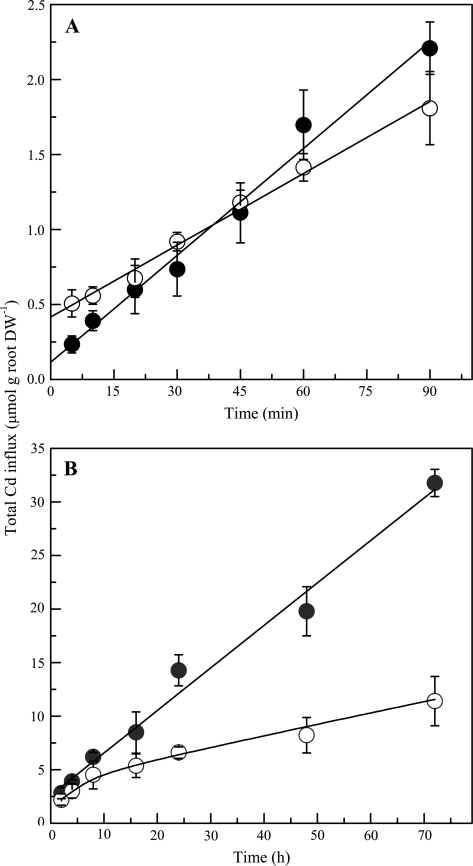
Uptake solutions containing radiolabelled 10 μM Cd were selected to study the short-term and long-term Cd influx in the two ecotypes, as concentration-dependent experiments indicated that symplastic uptake of Cd accounted for at least half of the whole Cd influx in both ecotypes at 10 μM. The uptake period in time-course experiments was 5–90 min and 2–72 h, respectively (Fig. 3). The 90 min uptake period showed no significant difference in terms of unidirectional influx rate of Cd in roots of the two ecotypes. Cd influx into roots of both ecotypes was more or less linear within 90 min during the time-course of the experiment. The observation that linear, time-dependent Cd accumulation intersected the y-axis above the origin in both ecotypes indicated that quite an amount of Cd was not completely removed from roots with the desorption regime used in these experiments. As a consequence of the greater symplastic influx rates in the HE, roots of the HE seedlings accumulated greater concentrations of Cd than those of the NHE after 60 min uptake times. The slope of the Cd uptake into HE seedlings was steeper when compared with the NHE (Fig. 3).
Fig. 3.
Cumulative uptake of Cd by both HE (filled circles) and NHE (open circles) Sedum alfredii, as determined of 109Cd in the roots plus shoots of plants. The uptake period in the time-course experiments was 90 min (A) and 72 h (B), respectively. Roots of intact seedlings were immersed in the 10 μM 109Cd uptake solutions, and plants were harvested and analysed after exposure periods as shown in the figure. Data points and error bars represent means (n=4) and SE, respectively. Error bars do not extend outside some symbols. DW, dry weight.
After 24 h of uptake, the two ecotypes began to show a significant difference in radiolabelled Cd uptake and accumulation, and this difference became more pronounced with time (48–72 h; Fig. 3B). At the end of the uptake experiments, 3-fold higher Cd was accumulated in whole plants of the HE than those of the NHE. It is significant that Cd continued to accumulate more or less linearly for at least 72 h in plants of the HE (Fig. 3A), while the Cd influx rate into the NHE began to decrease after ∼8 h (Fig. 3B).
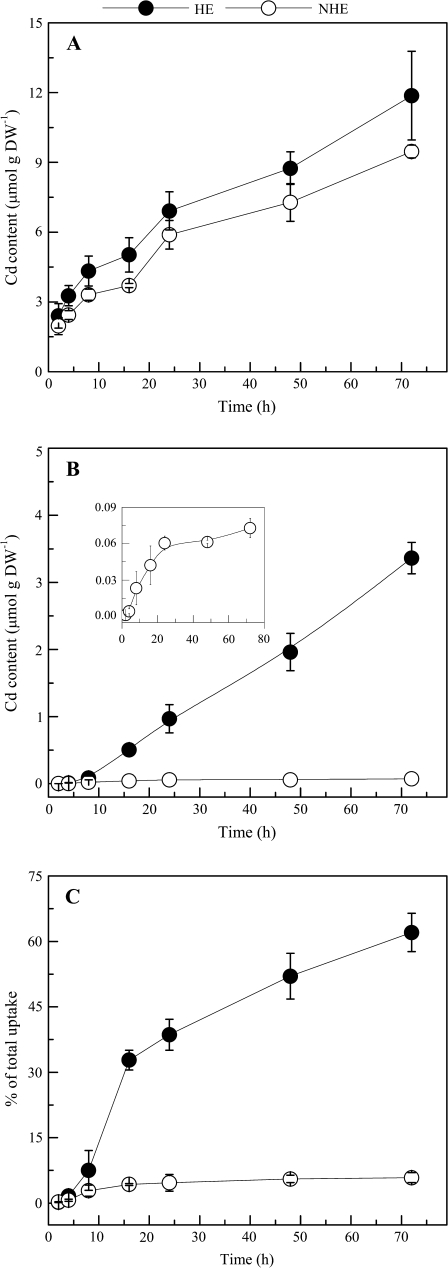
Cd root-to-shoot translocation
Cd in shoots of both ecotypes was not detected when plants were exposed to 109Cd solution for <4 h. Afterwards, differential distribution of Cd in roots and shoots of two ecotypes was seen (Fig. 4) though no significant difference of Cd accumulation in roots was observed (Fig. 4A). Cadmium in shoots of the HE increased significantly with time, while root-to-shoot translocation of Cd in the NHE was quite low (Fig. 4B). When roots were immersed continuously in radiolabelled Cd solution, shoot Cd levels of the HE increased linearly for at least 72 h, while Cd content in the NHE increased over 24 h and then plateaued at longer exposure times (Fig. 4B). At the end of the uptake experiment, Cd content in shoots of the HE was almost 46-fold higher than that of the NHE. Root-to-shoot translocation rates in the HE increased dramatically during the first 48 h of exposure to high Cd (Fig. 4C), and >50% of the total Cd absorbed by the HE roots was translocated to the shoots after 48 h, while prolonged exposure to Cd has little effect on the translocation rate in the NHE, and only 5% Cd was allocated to the shoots at the end of the uptake experiments.
Fig. 4.
Time-course of Cd accumulation in roots (A), shoots (B) and the root-to-shoot translocation rate (C) of HE (filled circles) and NHE (open circles) S. alfredii. Roots of intact seedlings were immersed in the 10 μM 109Cd uptake solutions, and roots and shoots of the plants were harvested and analysed after the exposure periods shown in the figure. The curve in the inset in (B) represents Cd accumulation in shoots of NHE. Data points and error bars represent means (n=4) and SE, respectively. Error bars do not extend outside some symbols. DW, dry weight.
Effects of low temperature or metabolic inhibitors on Cd uptake and translocation
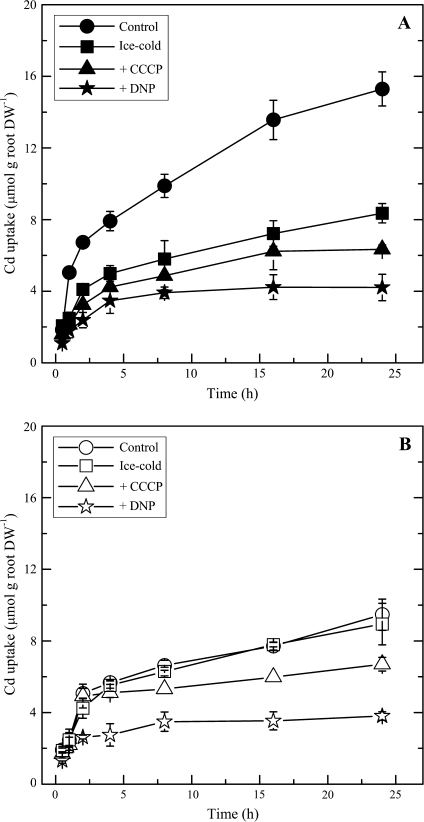
Uptake by active mechanisms into the symplastic pathway is predicted to be minimal when roots are bathed in ice-cold solutions or exposed to metabolic inhibitors. The results demonstrate that the addition of CCCP or DNP significantly inhibited the apparent uptake of Cd in both S. alfredii ecotypes. Apparent uptake of Cd was determined from the depletion of Cd in the uptake solution at each period as shown in Fig. 5. Regardless of the treatments, Cd uptake by the HE was much greater than that by the NHE, with 2.0-fold higher total cumulative uptake of Cd at the end of the experiment (Fig. 4). This difference between two ecotypes was highly consistent with the results obtained by radiotracer techniques (Figs 1, 3). The two ecotypes showed marked difference in their responses to low temperature treatments. Cumulative accumulation of Cd in the HE was decreased 45% by the ice-cold treatment after 24 h (Fig. 5A), whereas Cd uptake by the NHE was essentially unaffected (Fig. 5B). Additionally, inhibition of Cd uptake by metabolic inhibitors was also more pronounced in the HE, as 59% and 73% Cd uptake in the HE was inhibited by CCCP and DNP, respectively (Fig. 5A), while there was only 28% and 60% inhibition in the case of the NHE (Fig. 5B).
Fig. 5.
Cumulative uptake of Cd by HE (A) and NHE (B) S. alfredii with treatments of control (filled circles), ice-cold (open circles), +100 μM CCCP (filled triangles) and +100 μM DNP (open triangles), as determined from the depletion of Cd in the uptake solution [2 mM MES-TRIS (pH 5.8), 0.5 mM CaCl2, and 10 μM CdCl2]. Data points and error bars represent means (n=5) and SE, respectively. Error bars do not extend outside some symbols. DW, dry weight.
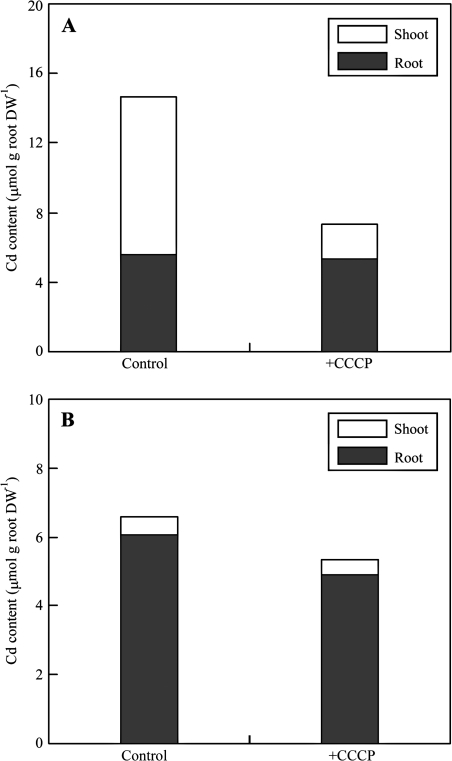
To investigate further the role of active uptake in Cd accumulation in roots and shoots of two S. alfredii ecotypes, CCCP treatment was employed for its relatively lower toxicity to the plants and efficiency in metabolic inhibition. Inhibition of Cd accumulation by CCCP was seen as a decrease in whole plant Cd uptake by 48% in the HE, which was 2-fold greater inhibition than that observed in the NHE (Fig. 6). In comparison, there was an ∼80% reduction in Cd in shoots of the HE in the presence of CCCP (P < 0.01), while no significant change of Cd level was observed in roots (Fig. 6A). In the NHE, CCCP-induced inhibition of Cd accumulation was negligible in shoots, but was significant in roots (25%, P < 0.05) (Fig. 6B).
Fig. 6.
Cadmium contents in roots (filled column) and shoots (open column) of HE (A) and NHE (B) S. alfredii grown in the uptake solution containing 2 mM MES-TRIS (pH 5.8), 0.5 mM CaCl2, and 10 μM CdCl2, with or without addition of 100 μM of the metabolic inhibitor CCCP. Cadmium content in shoots was calculated based on the weight of corresponding roots. Data points represent means (n=5); error bars are not shown in the figure. DW, dry weight.
Localization of Cd in roots
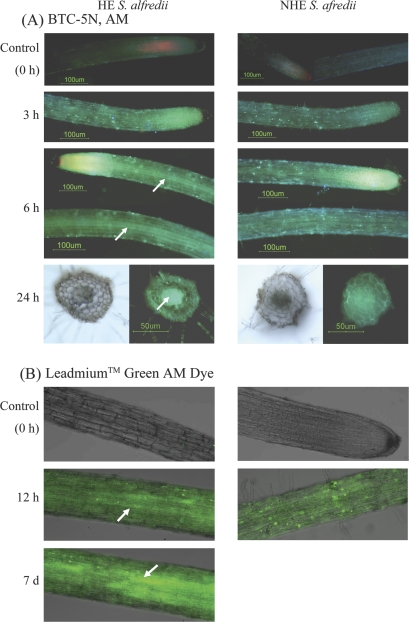
The acetoxymethyl ester of the dye, BTC-5N, AM, has been successfully used to detect Cd in protoplasts (Lindberg et al., 2004), and was employed here to investigate the Cd localization in roots of two S. alfredii ecotypes. The fluorescent dye was loaded into the intact roots of both ecotypes within 45 min and showed a clear and bright green florescence in roots of Cd-treated HE and NHE plants, while a weak fluorescence was observed in roots of control plants (Fig. 7A). In roots from HE plants pre-treated with 100 μM Cd for 6 h, preferential localization of Cd in the stele cylinder was observed, and this effect was more pronounced when Cd exposure was increased to 24 h. However, there was no noticeable localization of Cd in the stele cylinder of HE roots pre-treated with Cd for 3 h, and no noticeable localization in roots of the NHE, regardless of Cd exposure time.
Fig. 7.
Micrographs of roots from seedlings of two S. alfredii ecotypes exposed to 100 μM Cd for different periods, by using 5-nitrobenzothiazolecoumarin, in the acetoxymethyl ester form (BTC-5N, AM) (A) or Leadamium™ Green AM dye (B). (A) Roots from plants pre-treated with 100 μM Cd for 0 h (control), 3, 6, and 24 h, respectively, were loaded with BTC-5N for 45 min. Images were taken in fluorescent light using a fluorescein filter; the bright green fluorescence indicates the binding of the probe with Cd. Scale bars: 100 μm or 50 μm as indicated in the figure. (B) In a separate experiment, roots from plants pre-treated with 100 μM Cd for 0 h (control), 24 h, and 7 d, respectively, were loaded with Leadamium™ Green AM dye for 90 min. All images were taken at ×10 magnification, and green fluorescence represents the binding of the dye to Cd.
A second Cd probe, Leadmium™ Green AM dye, was used to confirm the results observed using BTC-5N, AM. This dye has lower affinity for Cd but is insensitive to other divalent ions (except for lead) as compared with BTC-5N, AM. A very low level of green fluorescence was observed in the roots of both ecotypes grown in the absence of added Cd (Fig. 7B), indicating that this dye does not react with divalent ions such as Ca2+ present in control roots. In contrast, a bright and green fluorescence was observed in Cd-pre-treated roots for both ecotypes (Fig. 7B). In contrast to NHE roots, Cd was consistently observed to be preferentially localized in vascular tissues of HE roots, after exposure to 100 μM Cd for 24 h. As Cd exposure was prolonged to 7 d, a greater intensity of fluorescence was observed in HE roots, and was highly concentrated in vascular tissues. Seven days of Cd treatment resulted in root death of NHE plants.
Cd concentrations in xylem sap
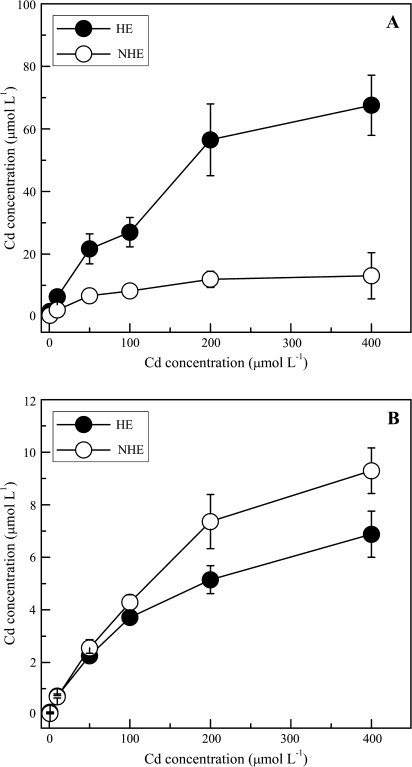
Cd concentrations in the xylem sap of the HE followed a biphasic curve with increasing Cd levels in solution (Fig. 8A), first increasing rapidly up to 50 μM then levelling off up to 100 μM. At Cd supply levels >100 μM, the Cd concentration in the xylem sap again increased sharply and then plateaued at higher external Cd levels (from 200 μM to 400 μM). In contrast, the Cd concentration in xylem sap of the NHE was less variable, with a linear increase up to 50 μM with saturation above 50 μM at least until 400 μM. Additionally, regardless of treatments, Cd concentration in xylem sap of the HE was consistently much higher than that of the NHE (P <0.01). At low external Cd supply (≤100 μM), the xylem sap Cd concentration of the HE was ∼3-fold higher than that of the NHE, while the Cd concentration in xylem sap of the HE was 5-fold higher than in the NHE at high Cd level (≥200 μM). In contrast to the Cd concentration in xylem sap, there was no significant difference in Cd concentration in roots of the two ecotypes (Fig. 8B). At higher Cd exposure level, root Cd concentrations in the NHE were slightly higher than that of the HE, which may be caused by the higher deposition of Cd in the apoplast of NHE root, or perhaps due to the depletion of Cd in roots of the HE caused by the rapid root-to-shoot translocation.
Fig. 8.
Cadmium concentrations in xylem sap (A) and roots (B) of HE (filled circles) and NHE (open circles) S. alfredii at different Cd exposures. Data points and error bars represent means (n=9) and SE, respectively. Error bars do not extend outside some symbols. DW, dry weight.
Discussion
Root uptake of divalent cations typically exhibits two phases: apoplastic binding and symplastic uptake (Hart et al., 1998; Zhao et al., 2002). To analyse Cd influx into the symplast, apoplastic binding to reactive apoplastic sites of root cells must be taken into account and minimized by the desorption steps. According to Zhao et al. (2002), however, complete removal of apoplastically bound Cd by desorption, without risking efflux of symplastic Cd, is probably unachievable. In this study, the desorption step used also did not fully remove apoplastic Cd. It is interesting to note that there is significant difference in apoplastically bound Cd between two ecotypes of S. alfredii; the factors responsible for the greater level of apoplastic Cd binding in the NHE are not known. The higher amount of apoplastic binding may help to explain the greater Cd accumulation in roots of the NHE at the first uptake time (Fig. 3) or when the plants were exposed to higher Cd levels (Figs 1, 8).
Symplastic uptake of Cd in wheat (Hart et al., 1998) has been shown to occur via a concentration-dependent process exhibiting saturable kinetics in plants, suggesting that Cd is taken up via a carrier-mediated system. In this study, the effect of metabolic inhibitors or cold treatment (Fig. 5) illustrates that Cd influx into the roots of both S. alfredii ecotypes at least partly depends on metabolism. The saturable component of uptake curves also indicates that Cd influx into the symplast of roots in both ecotypes is probably controlled by transport proteins in the plasma membrane. The Km values for the two ecotypes appeared to be similar, while a 2-fold increase in Vmax was observed for the HE. The larger Vmax for the HE was confirmed by the greater inhibition of Cd influx in the HE by low temperature or metabolic inhibitors (Fig. 5). These results imply that the two contrasting ecotypes probably use similar Cd transport mechanisms at the root cell membranes, and that there is a higher density or higher activity of these transporters in the plasma membrane of root cells in the HE. The Km values of Cd influx kinetics for both ecotypes of S. alfredii are much higher than previously reported, either for normal non-accumulating species (Cohen et al., 1998; Hart et al., 1998; Lombi et al., 2002), or for Cd hyperaccumulators, T. caerulescens (Lombi et al., 2001; Zhao et al., 2002) and A. halleri (Zhao et al., 2006). In the Cd-hyperaccumulator, Ganges ecotype T. caerulescens, low Km values were consistently exhibited (<0.5 μM), regardless of the concentration used (0–3 μM; Zhao et al., 2002) (0–100 μM; Lombi et al., 2001).
As a non-essential element, Cd is often assumed to be taken up by transporters for essential elements such as Zn, Fe, and Ca as a consequence of a lack of specificity of these transport proteins (Welch and Norvell, 1999). In the Cd-hyperaccumulator A. halleri, Cd uptake partly occurred through the Zn pathway (Zhao et al., 2006). Cd uptake in T. caerulescens, however, was probably mediated by specific Cd transporters (Lombi et al., 2001; Zhao et al., 2002), as well as by ZNT1, a high-affinity Zn transporter, with low affinity for Cd (Lasat et al., 2000; Pence et al., 2000). The results observed here for S. alfredii suggest that this species does not use the same mechanisms as the other two Cd hyperaccmulators. Cd influx into roots of HE S. alfredii was significantly suppressed by addition of high Ca or Ca channel inhibitor (La), suggesting that Cd uptake by HE S. alfredii, together with its contrasting ecotype NHE, is probably regulated by Ca transporters or channels in root cell plasma membrances, with low affinity (high Km values). The transport pathway of Ca in non-accumulator plants has been suggested to be involved in the uptake of Cd, albeit with a low affinity (Clemens et al., 1998); however, inhibition of Cd uptake by Ca in hyperaccmulators has not been previously demonstrated and the mechanisms responsible need further investigation.
Despite the higher apoplastic Cd binding rate in NHE roots (Fig. 1), there was a 3-fold higher amount of Cd accumulated in HE whole plants after 72 h (Fig. 3). Thus, the 2-fold higher symplastic uptake in the HE is insufficient to explain the difference in Cd accumulation observed between the two ecotypes. Our study indicated that large variations in root-to-shoot distribution occurred between the two S. alfredii ecotypes (Fig. 4). The rapid root-to-shoot translocation through the xylem, rather than increased Cd uptake rates in roots, is largely responsible for Cd hyperaccumulation in the HE. This is highly consistent with observations made for other metals in hyperaccumulators, which were generally characterized by a highly efficient translocation of heavy metals from roots to shoots (Baker et al., 1994; Lasat et al., 1996; Shen et al., 1997; Zhao et al., 2006). Meanwhile, a significant decline in Cd accumulation rate occurred in NHE shoots after exposure to Cd for 24 h, whereas the root-to-shoot translocation rate of Cd in the HE remained high for at least 72 h (Fig. 4B). Together, these results suggest the possible existence of differential root-to-shoot transport mechanisms and regulatory pathways during xylem loading for Cd in two S. alfredii ecotypes. This is further indicated by the significant increase in Cd concentration in xylem sap of the HE when plants were exposed to very high Cd levels (Fig. 8A).
The efficiency of root-to-shoot translocation is theoretically dependent on four processes (Lasat et al., 1996, 1998): (i) symplastic uptake by roots; (ii) root sequestration; (iii) xylem loading; and (iv) xylem unloading and uptake of metals by foliar cells. In the Cd-hyperaccumulator, Ganges ecotype T. caerulescens, Lombi et al. (2000) demonstrated that the large difference between this ecotype and the Prayon ecotype in Cd accumulation is related mainly to the differences in metal uptake, rather than to a difference in root sequestration or efficiency in xylem loading. In T. caerulescens and A. halleri, both hyperaccumulators of Cd, the constitutive transport capacities at the leaf protoplast are probably not responsible for hyperaccumulation (Cosio et al., 2004). These results contrast with those reported here in which the HE exhibited both a reduced sequestration of Cd in root cells and an apparently enhanced xylem transport rate. The addition of the metabolic inhibitor CCCP resulted in the greatest reduction in Cd concents in the shoots, but not the roots of the HE, whereas CCCP mainly decreased Cd concents in the roots of the NHE (Fig. 6). These results suggest that the principle difference between the HE and NHE ecotypes is the rate at which root-acquired Cd is translocated to shoots in the HE. This conclusion is consistent with the observation of enhanced fluorescence in the vascular cylinder of HE roots after exposure to Cd for >6 h (Fig. 7), which indicates that Cd ions were rapidly transported into vascular tissues by the symplastic pathway, and then became available for subsequent translocation to HE shoots. The results from the xylem sap analyses are also in agreement with the above conclusions, as a 3–5 times higher Cd concentration in xylem sap of the HE was measured (Fig. 8A). Though it was not explicity measured in these studies, xylem unloading, and the rapid uptake or sequestration of Cd by leaf and stem cells, may also be required to maintain the high rates of root-to-shoot Cd translocation observed, as a 3–5 times higher Cd concentration in xylem transport is inadequte to explain the extremely high translocation rate in the HE.
In conclusion, this work provides clear evidence for carrier-mediated Cd influx into the root symplasm in both HE and NHE S. alfredii, with a 2-fold higher Cd symplastic uptake rate in the HE, and a significant suppression of Cd by Ca in roots of both ecotypes. Furthermore, Cd portioning between roots and shoots varied significantly between the two ecotypes, and the rapid root-to-shoot translocation, possibly involving reduced root cell sequestration and enhanced xylem loading, may be a crucial process in hyperaccumulation of Cd by the HE. Enhanced translocation alone is not, however, a hyperaccumulation mechanism per se since it would not explain the ability of shoot tissues to tolerate Cd at levels that would be expected to be toxic to cellular function. Kochian et al. (2002) hypothesized that enhanced storage of toxic metals in the leaf vacuole might be a critical characterisitc in the hyperaccumulation of heavy metals. Using radioactive techniques, Yang et al. (2005) also suggested that increased Zn uptake in the leaf cells is one of the major mechanisms involved in Zn hyperaccumulation in HE S. alfredii. Here it was not determined how the root and shoot cells limit the toxicity of Cd that would be expected from this degree of Cd accumulation. Additional studies to increase our understanding of the mechanism of Cd hyperaccumulation in this plant specie, HE S. alfredii, are underway.
Acknowledgments
The authors sincerely thank Hongyun Peng, Jiguang Li, Huagang Huang, and Ming Zhang, Zhejiang University, for excellent technical assistance, and DK Gupta, Zhejiang University, for his comments on manuscript correction. This work was supported by the Key Project from the National Natural Science Foundation of China (30630046), the Program for Changjiang Scholars and Innovative Research Team in University (IRT0536), and by the ‘973’ Project from the Science and Technology Ministry of China (2002CB410804).
Glossary
Abbreviations
- BTC-5N
AM, 5-nitrobenzothiazolecoumarin, in the acetoxymethyl ester form
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- ICP-MS
inductively coupled plasma mass spectroscopy
- DMSO
dimethylsulphoxide
- DNP
2,4-dinitrophenol
- HE
hyperaccumulating ecotype
- NHE
non-hyperaccumulating ecotype
References
- Baker AJM, McGrath SP, Reeves RD, Smith JAC. Metal hyperaccumulator plants: a review of the ecology and physiology of a biochemical resource for phytoremediation of metal-polluted soils. In: Terry N, Bañuelos G, editors. Phytoremediation of contaminated soil and water. Boca Raton, FL: Lewis Publishers; 2000. pp. 85–107. [Google Scholar]
- Baker AJM, Reeves RD, Hajar ASM. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl (Brassicaceae) New Phytologist. 1994;127:61–68. doi: 10.1111/j.1469-8137.1994.tb04259.x. [DOI] [PubMed] [Google Scholar]
- Bert V, Bonnin I, Saumitou-Laprade P, de Laguerie P, Petit D. Do Arabidopsis halleri from nonmetallicolous populations accumulate zinc and cadmium more effectively than those from metallicolous populations? New Phytologist. 2002;155:47–57. doi: 10.1046/j.1469-8137.2002.00432.x. [DOI] [PubMed] [Google Scholar]
- Chan DY, Hale BA. Differential accumulation of Cd in durum wheat cultivars: uptake and retranslocation as sources of variation. Journal of Experimental Botany. 2004;55:2571–2579. doi: 10.1093/jxb/erh255. [DOI] [PubMed] [Google Scholar]
- Clemens S, Antosiewicz DM, Ward JM, Schachtman DP, Schroeder JI. The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proceedings of the National Academy of Sciences, USA. 1998;95:12043–12048. doi: 10.1073/pnas.95.20.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Palmgren MG, Krämer U. A long way ahead: understanding and engineering plant metal accumulation. Trends in Plant Science. 2002;7:309–315. doi: 10.1016/s1360-1385(02)02295-1. [DOI] [PubMed] [Google Scholar]
- Cohen CK, Fox TC, Garvin DF, Kochian LV. The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiology. 2004;116:1063–1072. doi: 10.1104/pp.116.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio C, Martinoia E, Keller C. Hyperaccumulation of cadmium and zinc in Thlaspi caerulescens and Arabidopsis halleri at the leaf cellular level. Plant Physiology. 2004;134:716–725. doi: 10.1104/pp.103.031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courbot M, Willems G, Motte P, Arvidsson S, Roosens N, Saumitou-Laprade P, Verbruggen N. A major quantitative trait locus for cadmium tolerance in Arabidopsis halleri colocalizes with HMA4, a gene encoding a heavy metal ATPase. Plant Physiology. 2007;144:1052–1065. doi: 10.1104/pp.106.095133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JJ, Welch RM, Norvell WA, Sullivan LA, Kochian LV. Characterization of cadmium binding, uptake, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiology. 1998;116:1413–1420. doi: 10.1104/pp.116.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Pence NS, Letham DLD, Pineros M, Magalhaes JV, Hoekenga O, Garvin D. Mechanisms of metal resistance in plants: aluminum and heavy metals. Plant and Soil. 2002;247:109–119. [Google Scholar]
- Lasat MM, Baker AJM, Kochian LV. Physiological characterization of root Zn2+ absorption and translocation to shoots in Zn hyperaccumulator and nonaccumulator species of Thlaspi. Plant Physiology. 1996;112:1715–1722. doi: 10.1104/pp.112.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasat MM, Baker AJM, Kochian LV. Altered Zn compartmentation in the root symplasm and stimulated Zn absorption into the leaf as mechanisms involved in Zn hyperaccumulation in Thlaspi caerulescens. Plant Physiology. 1998;118:875–883. doi: 10.1104/pp.118.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasat MM, Pence NS, Garvin DF, Ebbs SD, Kochian LV. Molecular physiology of zinc transport in the Zn hyperaccumulator Thlaspi caerulescens. Journal of Experimental Botany. 2000;51:71–79. [PubMed] [Google Scholar]
- Lindberg S, Landberg T, Greger M. A new method to detect cadmium uptake in protoplasts. Planta. 2004;219:526–532. doi: 10.1007/s00425-004-1256-z. [DOI] [PubMed] [Google Scholar]
- Lombi E, Tearall KL, Howarth JR, Zhao FJ, Hawkesford MJ, McGrath SP. Influence of iron status on cadmium and zinc uptake by different ecotypes of the hyperaccumulator Thlaspi caerulescens. Plant Physiology. 2002;128:1359–1367. doi: 10.1104/pp.010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombi E, Zhao FJ, McGrath SP, Young SD, Sacchi GA. Physiological evidence for a high-affinity cadmium transporter highly expressed in a Thlaspi caerulescens ecotype. New Phytologist. 2001;149:53–60. doi: 10.1046/j.1469-8137.2001.00003.x. [DOI] [PubMed] [Google Scholar]
- McGrath SP, Zhao FJ. Phytoextraction of metals and metalloids from contaminated soils. Current Opinion in Biotechnology. 2003;14:277–282. doi: 10.1016/s0958-1669(03)00060-0. [DOI] [PubMed] [Google Scholar]
- Papoyan A, Kochian LV. Identification of Thlaspi caerulescens genes that may be involved in heavy metal hyperaccumulation and tolerance: characterization of a novel heavy metal transporting ATPase. Plant Physiology. 2004;136:3814–3823. doi: 10.1104/pp.104.044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence NS, Larsen PB, Ebbs SD, Letham DLD, Lasat MM, Garvin DF, Eide D, Kochian LV. The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proceedings of the National Academy of Sciences, USA. 2000;97:4956–4960. doi: 10.1073/pnas.97.9.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BH, Leblanc M, Petit D, Brooks RR, Kirkman JH, Gregg PEH. The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant and Soil. 1998;203:47–56. [Google Scholar]
- Salt DE, Prince RC, Pickering IJ, Raskin I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiology. 1995;109:1427–1433. doi: 10.1104/pp.109.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Smith RD, Raskin I. Phytoremediation. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Shen ZG, Zhao FJ, McGrath SP. Uptake and transport of zinc in the hyperaccumulator Thlaspi caerulescens and the non-hyperaccumulator Thlaspi ochroleucum. Plant, Cell and Environment. 1997;20:898–906. [Google Scholar]
- Welch RM, Norvell WA. Mechanisms of cadmium uptake, translocation and deposition in plants. In: McLaughlin MJ, Singh BR, editors. Cadmium in soils and plants. Dordrecht: Kluwer Academic Publishers; 1999. pp. 125–150. [Google Scholar]
- Xiong YH, Yang XE, Ye ZQ, He ZL. Characteristics of cadmium uptake and accumulation by two contrasting ecotypes of Sedum alfredii Hance. Journal of Environmental Science and Health. 2004;39:2925–2940. [PubMed] [Google Scholar]
- Yang XE, Li TQ, Long XX, Xiong XH, He ZH, Stoffella PJ. Dynamics of zinc uptake and accumulation in the hyperaccumulating and non-hyperaccumulating ecotypes of Sedum alfredii Hance. Plant and Soil. 2006;284:109–119. [Google Scholar]
- Yang XE, Li TQ, Yang JC, He ZH, Lu LL, Meng FH. Zinc compartmentation in root, transport into xylem, and absorption into leaf cells in the hyperaccumulating species of Sedum alfredii Hance. Planta. 2005;224:185–195. doi: 10.1007/s00425-005-0194-8. [DOI] [PubMed] [Google Scholar]
- Yang XE, Long XX, Ni WZ, Fu CX. Sedum alfredii H: a new Zn hyperaccumulating plant first found in China. Chinese Science Bulletin. 2002;47:1634–1637. [Google Scholar]
- Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ. Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance) Plant and Soil. 2004;259:181–189. [Google Scholar]
- Zhao FJ, Hamon RE, Lombi E, McLaughlin MJ, McGrath SP. Characteristics of cadmium uptake in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. Journal of Experimental Botany. 2002;53:535–543. doi: 10.1093/jexbot/53.368.535. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Jiang RF, Dunham SJ, McGrath SP. Cadmium uptake, translocation and tolerance in the hyperaccumulator Arabidopsis halleri. New Phytologist. 2006;172:646–654. doi: 10.1111/j.1469-8137.2006.01867.x. [DOI] [PubMed] [Google Scholar]