Abstract
Buckwheat (Fagopyrum esculentum Moench) is an important annual plant cultivated for grain or as a cover crop in many countries, and it is also used for weed suppression in agro-economic systems through its release of allelochemicals. Little is known, however, concerning the mode of action of allelochemicals or plant defence response against them. Here, microarrays revealed 94, 85, and 28 genes with significantly higher expression after 6 h of exposure to the allelochemicals fagomine, gallic acid, and rutin, respectively, compared with controls. These induced genes fell into different functional categories, mainly: interaction with the environment; subcellular localization; protein with binding function or cofactor requirement; cell rescue; defence and virulence; and metabolism. Consistent with these results, plant response to allelochemicals was similar to that for pathogens (biotic stress) or herbicides (abiotic stress), which increase the concentration of reactive oxygen species (ROS; with consequent oxidative stress) in plant cells. The data indicate that allelochemicals might have relevant functions, at least in part, in the cross-talk between biotic and abiotic stress signalling because they generate ROS, which has been proposed as a key shared process between these two stress mechanisms.
Keywords: Abiotic stress, allelopathy, biotic stress, fagomine, Fagopyrum esculentum Moench, gallic acid, gene expression, microarray, mode of action, rutin
Introduction
Allelopathy, the chemical inhibition of one plant species by another, represents a form of chemical warfare between neighbouring plants competing for limited light, water, and nutrient resources. Allelopathic interactions have been proposed to have profound effects on the evolution of plant communities through the loss of susceptible species via chemical interference and by imposing selective pressure favouring individuals resistant to inhibition from a given allelochemical (Baerson et al., 2005). Most plant species, including crops, are capable of producing and releasing biologically active compounds (allelochemicals) into the environment to suppress the growth of other plants. Allelochemicals have usually been considered to be secondary metabolites or waste products of the main metabolic pathways in plants, and they do not appear to play a role in the primary metabolism essential for plant survival (Swain, 1977). Although most plants produce phytotoxic allelochemicals, relatively few, including buckwheat (Fagopyrum esculentum Moench), have strong allelopathic properties (Campbell, 1997; Tsuzuki, 2001; Bhowmik and Inderjit, 2003; Fujii et al., 2005; Khanh et al., 2005; Xuan et al., 2005; Golisz et al., 2007a).
Buckwheat is used for weed suppression in agro-economic systems through its release of allelochemicals, and it is also an important annual plant cultivated for grain or as a cover crop in many countries. Certain compounds that can inhibit the growth of other plants have been identified in buckwheat. In a previous study, Golisz et al. (2007b) identified eight allelochemicals including rutin, quercetin, (+)-catechin, (–)-epicatechin, chlorogenic acid, caffeic acid, ferulic acid, and gallic acid. The total activity of rutin, as calculated by concentration and growth inhibitory potential, suggests that this compound is the major allelochemical in buckwheat. However, Iqbal et al. (2007) have suggested that gallic acid might play a key role and act synergistically with minor allelochemicals in buckwheat to produce other allelopathic activities. Moreover, they also proposed that fagomine is a significant growth inhibitor of lettuce seedlings.
The present study employed three allelochemicals, fagomine (a piperidine alkaloid), gallic acid (a phenolic acid), and rutin (a flavonoid), which are responsible for the allelopathic potential of buckwheat (Golisz et al., 2007b; Iqbal et al., 2007). Most studies have focused on the relevance of the activities of these compounds to mammalian physiology, but not that of plants. Fagomine has been found in the aerial parts of buckwheat (Iqbal et al., 2002), but also in leaves and roots of Xanthocercis zambesiaca (Leguminosae) (Ramalingam, 2005). Fagomine reduces the blood glucose level and increases plasma insulin levels in diabetic mice. The fagomine-induced potentiation of insulin release may contribute in part to its antihyperglycaemic action (Bnouham et al., 2006). In another study, fagomine showed potent inhibitory activity toward rice α-glucosidase (Asano et al., 2000). Gallic acid has been identified as an allelopathic agent in a number of studies, including those of Rudrappa et al. (2007) and Weidenhamer and Romeo (2004). Moreover, Abdelwahed et al. (2007) assessed its antimutagenic and antioxidant activities on human leukaemia cells and concluded that it acts as an antimutagen by directly influencing the activity of DNA repair enzymes by modulating their gene expression. Also, Soobrattee et al. (2005) reported that gallic acid is the most potent antioxidant amongst simple phenolics. Among these compounds, much more information is available on rutin because it is an important flavonoid—especially for human health—because it has antibacterial, antiviral, antihypertensive, and antioxidant activities. Moreover, flavonoids have functions that are critical to various aspects of plant life related to interactions with the environment: they protect the plant against ultraviolet radiation, they have antimicrobial properties, and they act as a deterrent for herbivores by limiting assimilation of dietary protein and inhibiting digestive enzymes (Pourcel et al., 2006). Furthermore, flavonoids act as scavengers of free radicals such as reactive oxygen species (ROS) and also prevent their formation by chelating metals (Pourcel et al., 2006).
Relatively little is known about the molecular mode of action of allelochemicals and the plant defence response against them (Duke et al., 2005). To address such issues, Arabidopsis thaliana L. has been an excellent model to study plant response to allelochemicals and other environmental toxins (Baerson et al., 2005). Moreover, microarray techniques have become a standard tool for genome-wide monitoring of gene expression. Hence, the present work uses microarrays to analyse gene expression profiles of Arabidopsis thaliana L. exposed to fagomine, gallic acid, and rutin. To the best our knowledge, this is the first study to assess gene responses to these compounds using microarrays. This study may lead to a better understanding of the mode of action of tested allelochemicals.
Materials and methods
Plants and growth condition
Seeds of A. thaliana L. (Col-4, source NASC N933) from LEHLE SEEDS Company (Round Rock, TX, USA) were sterilized for 1 min in 70% ethanol, following by 1% sodium hypochlorite with Tween-20 (Sigma-Aldrich Group, St Louis, MO, USA) for 6 min, and finally rinsed 10 times with distilled water. Sterilized seeds were placed on 1% solidified agar (Nacalai Tesque, Inc., Kyoto, Japan) with Murashige and Skoog Plant Salt Mixture (Wako Pure Chemical Industries, Ltd, Japan) and 1% sucrose (Wako Pure Chemical Industries) in Agripot (Iwaki, Tokyo, Japan). The seeds in Agripot were cold treated (4 °C) for 3 d in darkness and then transferred to a growth chamber. Plants were maintained in the growth chamber on a schedule of 16 h light (22 °C) and 8 h dark (20 °C) (Boyes et al., 2001; Baerson et al., 2005).
Allelochemical treatment
Arabidopsis plants were grown in Agripot as described above. After 20 d in the growth chamber, plants were removed from agar and transferred to solution culture in distilled water containing one of the following allelochemicals: fagomine (10 mg kg−1 of water), gallic acid (20 mg kg−1), or rutin (200 mg kg−1). These concentrations reflect specific activities (EC50—the effective concentration of the compound to induce half-maximum inhibition) as determined in previous studies (Golisz et al., 2007b; Iqbal et al., 2007). Plants transferred to distilled water without any compounds were used as a control. After 6 h of treatment, plants were collected and immediately frozen in liquid nitrogen and then stored at –80 °C prior to analysis. Each treatment was replicated three times in each of three independent experiments.
Total RNA
The total RNA from 20-d-old Arabidopsis plants was isolated using the RNeasy Plant Mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The quantity and quality of isolated total RNA was assessed by spectrophotometry and gel electrophoresis, respectively.
Microarray data analysis
Isolated total RNA was amplified and labelled as described in the GeneChip Expression Analysis Technical Manual, Rev.5 (Affymetrix, Santa Clara, CA, USA). Total RNA (1 μg) was converted into double-stranded cDNA using the One-Cycle cDNA Synthesis kit (Affymetrix). In vitro transcription reactions were performed using a GeneChip IVT labelling kit, which includes T7 RNA polymerase and biotin-labelled ribonucleotides. The cDNA (15 μg) was hybridized to an Affymetrix GeneChip Arabidopsis ATH1 Genome Array. The array was incubated for 16 h, then automatically washed and stained using the GeneChip Hybridization, Wash and Stain kit (Affymetrix). The Probe Array was scanned using a GeneChip Scanner 3000 7G.
GeneSpring Viewer 5.2 software (Agilent Technologies, Santa Clara, CA, USA) was used for expression analysis. Genes were defined as responsive when transcripts were detected in at least two of three independent experiments (biological replicates) and when the signals were significantly different (P ≤0.05) compared with control plants.
Microarray validation: quantitative real-time RT-PCR analysis
The total RNA was isolated using TRIZOL (Invitrogen, Carlsbad, CA, USA). Synthesis of cDNA was carried out using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). All kits were used according to the manufacturer's instructions. Primers for quantitative real-time RT-PCR were designed using PRIMER EXPRESS software (v 2.0, Applied Biosystems, Foster City, CA, USA). A primer pair was used to amplify the constitutively expressed control gene encoding elongation factor 1α (EF1α; At5g60390, Becher et al., 2004). The sequences of all primers are presented in Supplementary Table S1 available at JXB online.
Quantitative real-time RT-PCR was performed on the ABI Prism 7700 Sequence Detection System using SYBR Green. cDNA was diluted 1:50 with nuclease-free water. Reactions were in 20 μl containing 9 μl of qPCR Master Mix (Eurogentec, Liege, Belgium), 0.6 μl of SYBR Green (Eurogentec), and 0.5 μmol of forward and reverse primers (Operon Biotechnologies, Tokyo, Japan). The following standard thermal profile was used: 2 min at 50 °C, 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 60 s at 60°C. Each sample was analysed in three technical replicates, and the resulting data were analysed using ABI Prism 7000 SDS Software. For the calculation of the threshold cycle (CT) values, the auto-CT function was used. For further calculations, the mean value of each triplicate was used. To normalize the target gene expression, the difference between the CT of the target gene and the CT of EF1α (constitutive control) for the respective template was calculated (ΔCT value). To calculate fold changes in gene expression, the ΔCT value was calculated as follows: ΔCT=CT (target gene)–CT (constitutive control gene). Relative transcript levels were calculated as: 1000×2–ΔCT.
Results
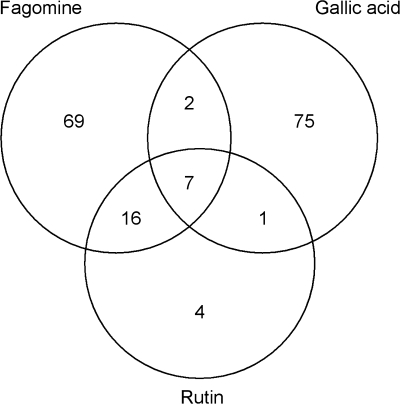
Microarray analysis demonstrates that A. thaliana plants respond to each of the three allelochemicals added to the culture medium by changes in gene expression. Culturing for 6 h caused up-regulation (≥2-fold) of 94 genes for fagomine, 85 for gallic acid, and 28 for rutin, compared with the control. Among these genes, seven were common for all three compounds, 23 for fagomine and rutin, nine for fagomine and gallic acid, and eight for rutin and gallic acid (Fig. 1). Supplementary Table S2 at JXB online presents all the genes that were significantly (P ≤0.05) up-regulated upon exposure to each of the three allelochemicals.
Fig. 1.
Venn diagrams displaying Arabidopsis thaliana genes significantly (P ≤ 0.5) induced by ≥2-fold (normalized) after 6 h of exposure to fagomine, gallic acid, or rutin in the aquaculture medium. Seven common genes were up-regulated by each tested compound (representative public ID/probe set identifier): ankyrin repeat family protein (At4g14400/245265_at), disease resistance protein (At4g16890/245451_at), pathogenesis-related protein (At2g14610/266385_at), glutathione S-transferase (At1g78370/260745_at), and three expressed proteins (At1g17870/255891_at; At3g22231/256766_at; At2g32160/265698_at).
The seven common genes up-regulated by ≥2-fold by fagomine, gallic acid, and rutin were as follows: ankyrin repeat family protein (At4g14400/245265_at), disease resistance protein (At4g16890/245451_at), pathogenesis-related protein (At2g14610/266385_at), glutathione S-transferase (GST) (At1g78370/260745_at), and three expressed proteins of unknown function (At1g17870/255891_at; At3g22231/256766_at; At2g32160/265698_at) (Fig. 1, Table 1).
Table 1.
The 26 common Arabidopsis thaliana genes significantly (P ≤ 0.05) induced by ≥2-fold (normalized) by at least two of the allelochemicals fagomine, gallic acid, and rutin after 6 h of exposure of plants to one allelochemical in the aquaculture medium
| ID_Affymetrix | Fold-change |
Gene title_Affymetrix | ||
| Fagomine | Gallic acid | Rutin | ||
| At4g14400 | 3.44 | 2.27 | 3.56 | Ankyrin repeat family protein |
| At4g16890 | 2.07 | 3.72 | 2.98 | Disease resistance protein (TIR-NBS-LRR class), putative |
| At1g17870 | 3.17 | 2.77 | 2.71 | Expressed protein |
| At3g22231 | 2.12 | 2.12 | 2.59 | Expressed protein |
| At1g78370 | 3.99 | 2.04 | 2.00 | Glutathione S-transferase, putative |
| At2g32160 | 3.09 | 2.00 | 2.53 | Expressed protein |
| At2g14610 | 4.06 | 2.25 | 2.98 | Pathogenesis-related protein 1 (PR-1) |
| At2g15120 | 2.31 | 2.39 | Pseudogene, disease resistance family protein/fatty acid elongase-related | |
| At2g32570 | 2.30 | 2.31 | F-box family protein | |
| At5g04190 | 2.32 | 2.51 | Phytochrome kinase substrate-related | |
| At5g59680 | 2.52 | 2.45 | Leucine-rich repeat protein kinase, putative | |
| At5g56080 | 3.72 | 3.12 | Nicotianamine synthase, putative | |
| At3g62040 | 3.36 | 2.17 | Haloacid dehalogenase-like hydrolase family protein | |
| At3g56585 | 2.00 | 2.31 | Hydroxyproline-rich glycoprotein family protein | |
| At4g08780 | 2.19 | 2.55 | Peroxidase, putative | |
| At1g48750 | 2.45 | 2.00 | Protease inhibitor/seed storage/lipid transfer protein (LTP) family protein | |
| At3g08770 | 7.45 | 3.38 | Lipid transfer protein 6 (LTP6) | |
| At1g21250 | 2.20 | 2.82 | Wall-associated kinase 1 (WAK1) | |
| At1g64360 | 2.67 | 2.18 | Expressed protein | |
| At1g74670 | 2.87 | 2.88 | Gibberellin-responsive protein, putative | |
| At1g24020 | 2.29 | 2.02 | Bet v I allergen family protein | |
| At2g17580 | 2.44 | 2.00 | Polynucleotide adenylyltransferase family protein | |
| At2g21050 | 2.26 | 2.05 | Amino acid permease, putative | |
| At2g28780 | 3.51 | 2.00 | Expressed protein | |
| At3g21720 | 2.79 | 2.83 | Isocitrate lyase, putative | |
| At2g43620 | 3.19 | 2.64 | Chitinase, putative | |
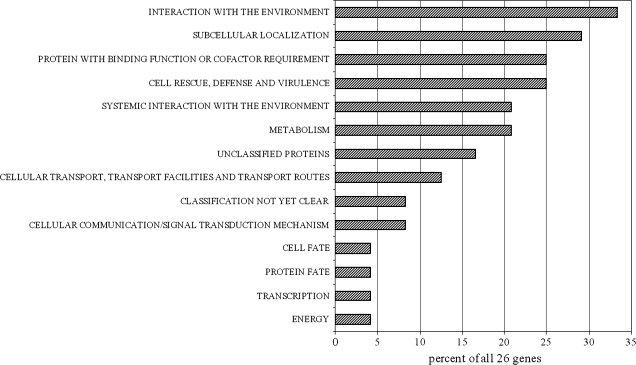
The 26 genes up-regulated by ≥2-fold by two or three individual compounds (Table 1) were distributed into 14 different functional categories (Fig. 2) based on FunCat assignments available through the MIPS A. thaliana database (http://mips.gsf.de/proj/funcatDB/search_main_frame.html). The most populated categories were ‘interaction with the environment’ and ‘subcellular localization’, representing 33% and 29%, respectively, of all functions assigned. Also noteworthy are the categories of ‘protein with binding function or cofactor requirement’ and ‘cell rescue, defence and virulence’, representing 25%, or genes associated with ‘systemic interaction with the environment’ and ‘metabolism’, representing 21% of all functions assigned.
Fig. 2.
Functional categorization of 26 Arabidopsis thaliana genes induced by ≥2-fold (normalized) by at least two of the three allelochemicals fagomine, gallic acid, and rutin (these genes are listed in Table 1). Genes were categorized based on the FunCat scheme devised by the Munich Information Center for Protein Sequences (http://mips.gsf.de/proj/funcatDB/search_main_frame.html).
Gallic acid, which up-regulated 20 genes by ≥3-fold, was a relatively more potent allelochemical in this regard than fagomine (13 genes) and rutin (six genes) (Table 2). The highest measured fold change (15.57) was with gallic acid for the gene encoding the class II heat shock protein (At5g12030). Among the 20 genes highly up-regulated by gallic acid, eight encode heat shock proteins (Table 2). Other gallic acid-induced genes of interest were leucine-rich repeat protein, chitinase, and ubiquinol–cytochrome c reductase complex (Table 2), transcription factors WRKY and MYB15, and Cu–Zn superoxide dismutase, peroxidase, and cytochrome P450 (Supplementary Fig. S2 at JXB online). Some of these gene products are involved in generating ROS during oxidative stress. In the case of fagomine treatment, lipid transfer protein (LTP; At3g08770) had the highest fold-induction of 7.45, whereas in response to rutin this gene was induced only by 3.38-fold (Table 2). Moreover, fagomine and rutin induced nicotianamine synthase (At5g56080) expression by 3.72- and 3.12-fold, respectively (Table 2).
Table 2.
Arabidopsis thaliana genes significantly (P≤0.05) induced ≥3-fold (normalized) after 6 h of exposure of plants to fagomine, gallic acid, or rutin in the aquaculture medium
| ID_Affymetrix | Gene title_Affymetrix | Fold-change | P-value |
| Fagomine | |||
| At3g08770 | Lipid transfer protein 6 (LTP6) | 7.45 | 0.02 |
| At2g14610 | Pathogenesis-related protein 1 (PR-1) | 4.06 | 0.05 |
| At1g78370 | Glutathione S-transferase, putative | 3.99 | 0.03 |
| At4g02290 | Glycosyl hydrolase family 9 protein | 3.95 | 0.02 |
| At5g56080 | Nicotianamine synthase, putative | 3.72 | 0.05 |
| At2g28780 | Expressed protein | 3.51 | 0.01 |
| At4g14400 | Ankyrin repeat family protein | 3.44 | 0.02 |
| At1g78970 | Lupeol synthase (LUP1)/2,3-oxidosqualene-triterpenoid cyclase | 3.41 | 0.01 |
| At3g62040 | Haloacid dehalogenase-like hydrolase family protein | 3.36 | 0.05 |
| At1g17870 | Expressed protein | 3.17 | 0.05 |
| At1g52400 | Glycosyl hydrolase family 1 protein/beta-glucosidase, putative (BG1) | 3.15 | 0.01 |
| At2g32160 | Expressed protein | 3.09 | 0.01 |
| At1g32450 | Proton-dependent oligopeptide transport (POT) family protein | 3.00 | 0.00 |
| Gallic acid | |||
| At5g12030 | 17.7 kDa class II heat shock protein 17.6A (HSP17.7-CII) | 15.57 | 0.02 |
| At5g12020 | 17.6 kDa class II heat shock protein (HSP17.6-CII) | 10.33 | 0.02 |
| At3g46230 | 17.4 kDa class I heat shock protein (HSP17.4-CI) | 6.57 | 0.02 |
| At2g02010 | Glutamate decarboxylase, putative | 5.91 | 0.05 |
| At5g48570 | Peptidyl-prolyl cis-trans isomerase, putative/FK506-binding protein, putative | 5.58 | 0.03 |
| At5g52640 | Heat shock protein 81-1 (HSP81-1)/heat shock protein 83 (HSP83) | 5.36 | 0.02 |
| At3g12580 | Heat shock protein 70, putative/HSP70, putative | 5.08 | 0.03 |
| At2g29500 | 17.6 kDa class I small heat shock protein (HSP17.6B-CI) | 4.63 | 0.05 |
| At1g74310 | Heat shock protein 101 (HSP101) | 4.53 | 0.01 |
| At1g54050 | 17.4 kDa class III heat shock protein (HSP17.4-CIII) | 4.22 | 0.01 |
| At4g22610 | Protease inhibitor/seed storage/lipid transfer protein (LTP) family protein | 3.86 | 0.01 |
| At5g09530 | Hydroxyproline-rich glycoprotein family protein | 3.73 | 0.03 |
| At4g16890 | Disease resistance protein (TIR-NBS-LRR class), putative | 3.72 | 0.01 |
| At2g39200 | Seven transmembrane MLO family protein (MLO12) | 3.56 | 0.05 |
| At1g51800 | Leucine-rich repeat protein kinase, putative | 3.47 | 0.03 |
| At1g02430 | ADP-ribosylation factor, putative | 3.29 | 0.05 |
| At5g25450 | Ubiquinol–cytochrome c reductase complex 14 kDa protein, putative | 3.11 | 0.03 |
| At3g02240 | Expressed protein | 3.10 | 0.03 |
| At5g55050 | GDSL-motif lipase/hydrolase family protein | 3.05 | 0.03 |
| At3g54420 | Class IV chitinase (CHIV) | 3.00 | 0.04 |
| Rutin | |||
| At4g14400 | Ankyrin repeat family protein | 3.56 | 0.01 |
| At3g08770 | Lipid transfer protein 6 (LTP6) | 3.38 | 0.05 |
| At3g49870 | ADP-ribosylation factor, putative | 3.35 | 0.05 |
| At5g56080 | Nicotianamine synthase, putative | 3.12 | 0.05 |
| At2g14610 | Pathogenesis-related protein 1 (PR-1) | 3.00 | 0.05 |
| At4g16890 | Disease resistance protein (TIR-NBS-LRR class), putative | 3.00 | 0.04 |
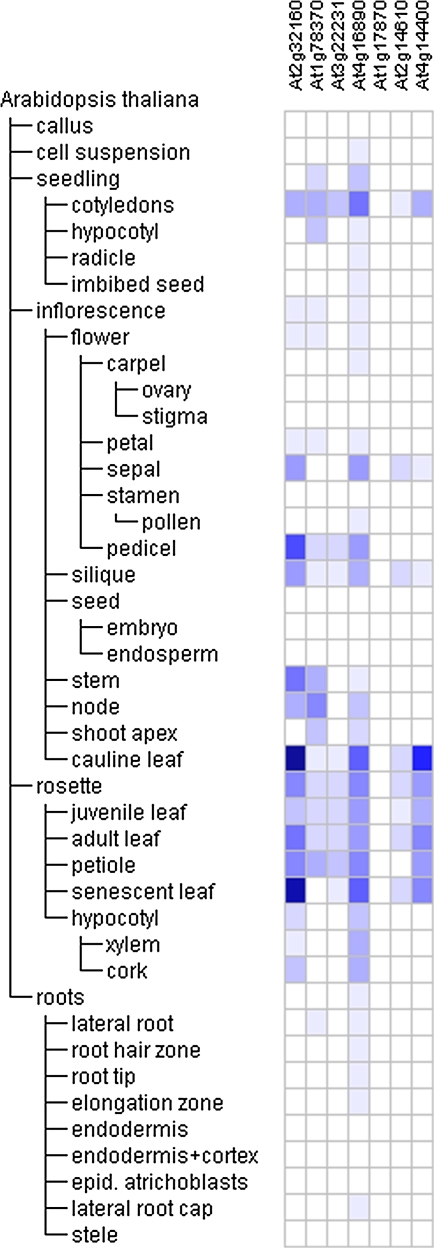
The Genevestigator online search tool Meta-profile analysis (https://www.genevestigator.ethz.ch/) reports expression as a heat map where a colour spectrum defines the relative expression of each gene. Querying the database using a list of seven common genes that were up-regulated by each of the three tested allelochemicals tentatively identified the developmental tissues (Fig. 3) in which these compound-responsive genes are expressed during the normal life cycle of Arabidopsis. These seven common genes are preferentially expressed in seedling cotyledons, the inflorescence pedicel and cauline leaf, and the rosette senescent leaf; expression is particularly high for disease resistance protein (At4g16890), ankyrin repeat protein (At4g14400), and an expressed protein (At2g32160) (Fig. 3).
Fig. 3.
Heat map showing the levels of Arabidopsis gene expression throughout one plant life cycle. The Meta-Profile Analysis tool of the Genevestigator software (https://www.genevestigator.ethz.ch/) was utilized with a probe set for seven genes up-regulated by all three allelochemicals. The blue–white scheme was chosen by the software developers to visualize gene expression levels and discern slight changes in signal intensity. The colour/intensity for each gene expression profile was normalized such that the highest signal intensity was defined as 100% (dark blue), and the absence of signal was defined as 0 (white).
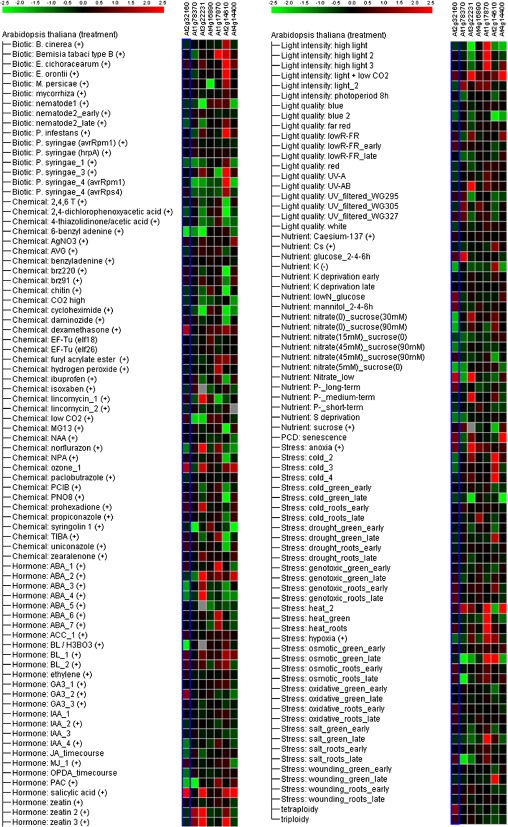
Figure 4 presents Genevestigator compilation data for stimulus-responsive expression of gene clusters for which the data are consistent across many microarray experiments. These compilations were derived from independent experiments that may have used different stimulus durations and/or application methods. Thus, these heat maps represent averages of behaviour and are colour coded by the average expression level change between stimulated and unstimulated conditions. Among seven common genes up-regulated by all three allelochemicals, pathogenesis-related protein (At2g14610) is one of most highly regulated genes; the most potent stimuli affecting the expression of this gene are organisms that elicit biotic stress, such as Erysiphe orontii or Phytophthora infestans, and abiotic stresses such as wounding, cold, and osmotic shock (Fig. 4). The gene encoding expressed protein At1g17870 is also up-regulated by abiotic stress such as heat, osmotic shock, salt stress, or exposure to abscisic acid. Four genes were up-regulated upon stimulation with the plant hormone, salicylic acid: pathogenesis-related protein (At2g14610), ankyrin (At4g14400), and two expressed proteins (At3g22231, At2g32160). Among the seven common genes identified, the disease resistance protein (At4g16890) was up-regulated to a lesser degree based on this analysis (Fig. 4).
Fig. 4.
Heat map showing the levels of gene expression in response to external stimuli. The Meta-Profile Analysis tool of the Genevestigator software (https://www.genevestigator.ethz.ch/) was utilized with a probe set for seven genes up-regulated by all three allelochemicals as follows, from the left side: At2g32160, At1g78370, At3g22231, At4g16890, At1g17870, At2g14610, and At4g14400. The heat map displays the results for a given list of genes, with colour coding representing relative expression values. Columns represent probe sets, and rows represent stimuli. All gene expression profiles were normalized for colour from red through black to green. Red and green indicate relatively higher and lower expression levels, respectively, upon stimulation. Black indicates no change between conditions.
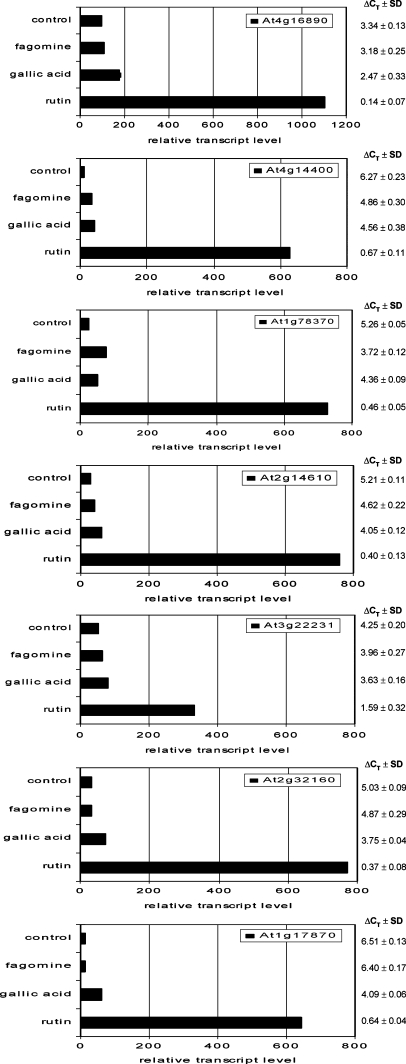
To verify the microarray results, quantitative real-time RT-PCR assays were carried out for the seven common genes that were up-regulated by fagomine, gallic acid, and rutin (Fig. 5). In general, the two methods gave similar results in terms of fold change, and all genes were shown to be up-regulated. In the case of rutin, real-time RT-PCR yielded considerably higher transcript levels compared with the microarray results (Fig. 5), suggesting that rutin is the most effective of the three tested compounds, in agreement with a previous study using another method (Golisz et al., 2007b). Relative transcript levels in plants exposed to fagomine or gallic acid were rather similar in both methods, although the levels for both compounds were lower compared with rutin. Among the tested genes, expressed protein (At1g17870) had the highest expression level for rutin and gallic acid with respect to the control plants, and for fagomine, it was GST (At1g78370) (Fig. 5). Conversely, the lowest fold increases was for expressed protein (At3g22231) for rutin and gallic acid, and another expressed protein (At1g17870) for fagomine compared with the control expression level (Fig. 5).
Fig. 5.
Real-time RT-PCR analysis of expression of seven common genes: disease resistance protein (At4g16890), ankyrin repeat family protein (At4g14400), glutathione S-transferase (At1g78370), pathogenesis-related protein (At2g14610), and three expressed proteins (At3g22231, At2g32160, At1g17870) in Arabidopsis thaliana. Transcript levels were assessed by real-time RT-PCR in plants following 6 h of exposure of 20-d-old plants to fagomine, gallic acid, or rutin in the aquaculture medium. Values are mean ΔCT ±SD, and the relative transcript level was calculated from three technical replicates. The ΔCT values were calculated as follows: ΔCT (target gene) = CT (target gene)–CT (constitutive control gene, EF1α), whereby the CT value is the cycle number at which the PCR product exceeded a set threshold. Relative transcript levels were calculated as: 1000× 2–ΔCT.
Discussion
The role of allelopathy in weed management has gained a great deal of attention over the last two decades (Wu et al., 1999; Bertholdsson, 2005). Weed suppression by crop allelopathy during the early establishment period could reduce the need for early-season application of commercial herbicides, with late-season weed control provided by the relative competitiveness of crops over weeds. The genetic enhancement of crop allelopathy for weed management has been demonstrated (Duke et al., 2001). Recent reports have highlighted the response of the Arabidopsis transcriptome upon exposure of plants to allelochemicals such as (–)-catechin (Bais et al., 2003) and benzoxazolin-2(3H)-one (BOA) (Baerson et al., 2005).
Plant response to allelochemicals, according to these microarray results, may be caused by reactions that are similar to those occurring during biotic and/or abiotic stress. Furthermore, a few papers have suggested cross-talk between abiotic and biotic stress response pathways (Narusaka et al., 2004; Fujita et al., 2006) as well as cross-talk among various signalling pathways under each type of stress (Knight and Knight, 2001; Chinnusamy et al., 2004; Taylor et al., 2004; Kaur and Gupta, 2005). Biotic and abiotic stresses regulate the expression of different but overlapping sets of genes. The generation of ROS has been proposed as a key process shared between biotic and abiotic stress responses (Apel and Hirt, 2004; Narusaka et al., 2004; Fujita et al., 2006). Notably, several ROS-related gene products, such as ubiquinol–cytochrome c reductase complex, Cu–Zn superoxide dismutase, peroxidase, and cytochrome P450, were up-regulated in plants exposed to gallic acid in this study (Supplementary Table S2 at JXB online). These results thus suggest that gallic acid which is a potent antioxidant (Soobrattee et al., 2005) generated elevated levels of ROS. This is somewhat counterintuitive, but it is worth noting and is in agreement with other reports that antioxidant compounds such as gallic acid (Rudrappa et al., 2007) and (±)-catechin (Prithiviraj et al., 2007) increased production of ROS in Arabidopsis plants.
Data from these studies show that brief exposures to low doses of fagomine, gallic acid, and rutin result in a significant genetic response in target plants. This implies that these compounds may exert a significant effect on the growth of target plants even though they are present at low concentration. However, it is important to note that the phytotoxic activity of allelochemicals in natural ecosystems and agroecosystems is affected by many factors through their effects on the behaviour in soil and both the donor and receiver plants (Kobayashi, 2004).
The common genes that were up-regulated by each of fagomine, gallic acid, and rutin (see Table 1, top) have been described as stress-related genes—some of which have been well characterized in this regard. For example, ankyrin repeats form a domain in accelerated cell death 6 (ACD6), overexpression of which confers enhanced disease resistance by priming stronger and quicker defence responses during pathogen infection or plant development, or upon treatment with an antagonist of the key defence regulator salicylic acid (Lu et al., 2005). The best characterized ankyrin protein from plants is NPR1/NIM1, which is involved in salicylic acid-dependent disease resistance and in a salicylic acid-independent resistance response elicited by certain root-associated bacteria (Lu et al., 2003). GST, another common up-regulated protein in this study, appears to be ubiquitous in plants and has defined roles in herbicide detoxification (Crawford et al., 2000; Dixon et al., 2002; Wagner et al., 2002; Blokhina et al., 2003). The primary biochemical function of many GSTs is conjugation, either of xenobiotics or of intermediates and secondary metabolites. In addition, certain GSTs play roles as peroxidases or in regenerating ascorbate from dehydroascorbate (Foyer and Noctor, 2005). Pathogenesis-related protein (At2g14610) is induced in response to salicylic acid and a variety of pathogens. Among three proteins up-regulated by all three allelochemicals, one (At3g22231) is distinct because its expression is regulated by the circadian clock and it is up-regulated in response to both virulent and avirulent strains of Pseudomonas syringae pv. tomato (Sauerbrunn and Schlaich, 2004). A second protein (At1g17870) contains transmembrane helices near its C-terminus and has a conserved zinc-binding motif, and it is involved in response to abiotic stresses such as heat and high-intensity light (Chen et al., 2005). The function of a third protein (At2g32160) is unknown. Further annotation of this protein, however, may provide important clues toward understanding the mode of action of allelochemicals.
Among genes up-regulated by ≥3-fold by each of the allelochemicals of interest was heat shock protein induced by gallic acid (Table 2). These proteins are expressed in plants not only when they experience high temperature stress but also in response to a wide range of other environmental insults, such as water stress, salinity, osmotic stress, and cold and oxidative stress (Grover et al., 2001; Wang et al., 2004). Heat shock proteins/chaperones play a crucial role in protecting plants against stress and in the re-establishment of cellular homeostasis by binding to and sequestering misfolded or unfolded proteins. Moreover, they also act synergistically with other cellular stress response pathways to limit cellular damage (Wang et al., 2004). In the case of fagomine and rutin, of interest was LTP6 and nicotianamine synthase. LTPs have been suggested to participate in cutin assembly and in plant defence against pathogens (Arondel et al., 2000). Nicotianamine synthase is a highly important enzyme for the precise regulation of the iron metabolism that is required for the control of basic cellular processes such as electron transport in photosynthesis and respiration (Herbik et al., 1999), and it is also a cytosolic chelator of Zn2+ (Trampczynska et al., 2006).
The present data suggest that allelochemicals such as fagomine, gallic acid, and rutin cause reactions in plants that are similar to those involved in response to pathogens or herbicides—environmental factors that increase ROS levels inside plant cells, which results in oxidative stress. Oxidative stress may arise from any abiotic or biotic stress that generates ROS (Bray et al., 2000; Blokhina et al., 2003). ROS include not only free radicals (superoxide radical and hydroxyl radical), but also molecules such as hydrogen peroxide, singlet oxygen, and ozone (Blokhina et al., 2003; Mittler et al., 2004). Depending on the nature of the ROS species, some are highly toxic and rapidly detoxified by various cellular enzymatic and non-enzymatic mechanisms (Apel and Hirt, 2004). ROS control and regulate many different processes in plants, such as growth, cell cycle, programmed cell death, pathogen defence, hormone signalling, stomatal behaviour, development, and biotic and abiotic stress responses (Apel and Hirt, 2004; Mittler et al., 2004; Laloi et al., 2007). Under steady-state conditions, ROS are scavenged by various antioxidative defence mechanisms (Laloi et al., 2007). However, under stress conditions, ROS have been proposed to affect stress responses in two different ways. First, they may react with a large variety of biomolecules, and thus cause irreversible damage that can lead to tissue necrosis and ultimately death of plants. On the other hand, ROS influence the expression of a number of genes and signal transduction pathways. The cells have evolved strategies to utilize ROS as environmental indicators and biological signals that activate and control various genetic stress response programmes (Apel and Hirt, 2004). It is also known that when plants are exposed to various abiotic stress conditions, a large part of the stress-induced transient increase in ROS concentration takes place within chloroplasts, when the balance between light absorption and the use of light energy is disturbed and excess light energy will lead to the inhibition of photosynthesis (Laloi et al., 2006, 2007).
The present results with Arabidopsis, especially when plants were treated by gallic acid, suggest that environmental factors should be added to the category of allelochemicals/allelopathy. The authors assume that a comparative analysis might reveal valuable information that otherwise may not be obtainable by identifying responses that are shared (or different) across species. However, these results revealed that most of these genes are up-regulated during oxidative stress; these include Cu–Zn superoxide dismutase, calmodulin kinase, GST, heat-shock proteins, and transcription factors (WRKY and myb). Detoxification enzymes such as GST, catalases, superoxide dismutase, and ascorbate peroxidases are involved in protection from reactive singlet oxygen species; moreover, proteins involved in regulatory functions and in signal transduction, including various protein kinases and transcription factors, have a broader role in governing stress responses (Grover et al., 2001). On the other hand, the allelochemicals used in this study also affected the expression of genes that are regulated in response to pathogen attack or wounding—for example, pathogenesis-related protein, disease resistance protein, and leucine-rich repeat protein. Notably, plant hormones such as nitric oxide and salicylic acid are key regulators of responses to pathogen attack or wounding, and also of the activation of programmed cell death (Mittler, 2002; Zottini et al., 2007). Defence-related stress may also induce cell death.
In conclusion, the results of this study show that allelopathy may involve genes that are up-regulated during biotic and/or abiotic stresses. This suggests that the plant pathways involved in response to allelochemicals may be shared between abiotic and biotic stresses. It is clear, however, that future studies are needed to understand better the mode of action of allelochemicals in plants with regard to their potential contribution to the biological control of weeds.
Supplementary data
The following supplementary data can be found on JXB online.
Table S1. Primers used for quantitative real-time RT-PCR assays.
Table S2. Arabidopsis thaliana genes significantly (P ≤0.05) up-regulated (≥2-fold) after 6 h of exposure to fagomine, gallic acid, or rutin in the aquaculture medium.
Supplementary Material
Acknowledgments
This work was partially supported by the Japanese Society for the Promotion of Science (JSPS fellowship award to AG, ID No. P 05643) and by the Research and Development Program for Resolving Critical Issues, Risk Assessment of Alien Plants and their Control in the Field granted to YF. The authors thank Professor Naoki Asano from the Faculty of Pharmaceutical Sciences, Hokuriku University, Japan, for providing a standard for fagomine.
References
- Abdelwahed A, Bouhlel I, Skandrani I, et al. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus confirmation by microarray expression profiling. Chemico-Biological Interactions. 2007;165:1–13. doi: 10.1016/j.cbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Arondel V, Vergnolle C, Cantrel C, Kader JC. Lipid transfer proteins are encoded by a small multigene family in Arabidopsis thaliana. Plant Science. 2000;157:1–12. doi: 10.1016/s0168-9452(00)00232-6. [DOI] [PubMed] [Google Scholar]
- Asano N, Nishida M, Miyauchi M, Ikeda K, Yamamoto M, Kizu H, Kameda Y, Watson AA, Nash RJ, Fleet GWJ. Polyhydroxylated pyrrolidine and piperidine alkaloids from Adenophora triphylla var. japonica (Campanulacease) Phytochemistry. 2000;53:379–382. doi: 10.1016/s0031-9422(99)00555-5. [DOI] [PubMed] [Google Scholar]
- Baerson SR, Sanchez-Moreiras A, Pedrol-Bonjoch N, Schulz M, Kagan IA, Agarwal AK, Reigosa MJ, Duke SO. Detoxification and transcriptome response in Arabidopsis seedlings exposed to the allelochemical benzoxazolin-2(3H)-one. Journal of Biological Chemistry. 2005;280:21867–21881. doi: 10.1074/jbc.M500694200. [DOI] [PubMed] [Google Scholar]
- Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM. Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science. 2003;301:1377–1380. doi: 10.1126/science.1083245. [DOI] [PubMed] [Google Scholar]
- Becher M, Talke IN, Krall L, Kramer U. Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. The Plant Journal. 2004;37:251–268. doi: 10.1046/j.1365-313x.2003.01959.x. [DOI] [PubMed] [Google Scholar]
- Bertholdsson NO. Early vigour and allelopathy—two useful traits for enhanced barley and wheat competitiveness against weeds. Weed Research. 2005;45:94–102. [Google Scholar]
- Bhowmik PC, Inderjit Challenges and opportunities in implementing allelopathy for natural weed management. Crop Protection. 2003;22:661–671. [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bnouham M, Ziyyat A, Mekhfi H, Tahri A, Legssyer A. Medicinal plants with potential antidiabetic activity— review of ten years of herbal medicine research (1990–2000) International Journal of Diabetes and Metabolism. 2006;14:1–25. [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Gorlach J. Growth stage-based phenotypic analysis of arabidopsis: a model for high throughput functional genomics in plants. The Plant Cell. 2001;13:1499–1510. doi: 10.1105/TPC.010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray EA, Bailey-Serres J, Weretilnyk E. Responses to abiotic stresses. In: Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Biologists; 2000. pp. 1158–1203. [Google Scholar]
- Campbell CG. Promoting the conservation and use of underutilized and neglected crops 19. Gatersleben: Institute of Plant Genetics and Crop Plant Research/Rome: International Plant Genetic Resources Institute; 1997. Buckwheat Fagopyrum esculentum Moench. 26. [Google Scholar]
- Chen G, Bi YR, Li N. EGY1 encodes a membrane-associated and ATP-independent metalloprotease that is required for chloroplast development. The Plant Journal. 2005;41:364–375. doi: 10.1111/j.1365-313X.2004.02308.x. [DOI] [PubMed] [Google Scholar]
- Chinnusamy V, Schumaker K, Zhu JK. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. Journal of Experimental Botany. 2004;55:225–236. doi: 10.1093/jxb/erh005. [DOI] [PubMed] [Google Scholar]
- Crawford NM, Kahn ML, Leustek T, Long SR. Nitrogen and sulphur. In: Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Biologists; 2000. pp. 841–845. [Google Scholar]
- Dixon DP, Lapthorn A, Edwards R. Plant glutathione transferases. Genome Biology. 2002;3:1–10. doi: 10.1186/gb-2002-3-3-reviews3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke SO, Baerson SR, Pan Z, Kagan IA, Sanchez-Moreiras A, Reigosa MJ, Pedrol-Bonjoch N, Schulz M. Proceedings of the 4th World Congress on Allelopathy, August 2005. 2005. Genomic approaches to understanding allelochemical modes of action and defenses against allelochemicals. Wagga Wagga, Australia, 107–113. [Google Scholar]
- Duke SO, Scheffler BE, Dayan FE, Ota E. Strategies for using transgenes to produce allelopathic crops. Weed Technology. 2001;15:826–834. [Google Scholar]
- Foyer CH, Noctor G. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell and Environment. 2005;28:1056–1071. [Google Scholar]
- Fujii Y, Golisz A, Furubayashi A, Iqbal Z, Nasir H. Proceedings the 20th Asian-Pacific Weed Science Society Conference. 2005. Allelochemicals from buckwheat and tartary buckwheat and practical weed control in the field. Ho Chi Minh City, Vietnam, 227–233. [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Current Opinion in Plant Biology. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Golisz A, Gawronska H, Gawronski SW. Influence of buckwheat allelochemicals on crops and weeds. Allelopathy Journal. 2007a;19:337–350. [Google Scholar]
- Golisz A, Lata B, Gawronski SW, Fujii Y. Specific and total activities of allelochemicals identified in buckwheat. Weed Biology and Management. 2007b;7:164–171. [Google Scholar]
- Grover A, Kapoor A, Lakshmi OS, Agarwal S, Sahi C, Katiyar-Agarwal S, Agarwal M, Dubey H. Understanding molecular alphabets of the plant abiotic stress responses. Current Science. 2001;80:206–216. [Google Scholar]
- Herbik A, Koch G, Mock HP, Dushkov D, Czihal A, Thielmann J, Stephan UW, Baumlein H. Isolation, characterization and cDNA cloning of nicotianamine synthase from barley. A key enzyme for iron homeostasis in plants. 1999. European Journal of Biochemistry. 1999;265:231–239. doi: 10.1046/j.1432-1327.1999.00717.x. [DOI] [PubMed] [Google Scholar]
- Iqbal Z, Hiradate S, Noda A, Isojima S, Fujii Y. Allelopathy of buckwheat: assessment of allelopathic potential of extract of aerial parts of buckwheat and identification of fagomine and other related alkaloids as allelochemicals. Weed Biology and Management. 2002;2:110–115. [Google Scholar]
- Iqbal Z, Nasir H, Fujii Y. Allelopathic activity of buckwheat: a ground cover crop for weed control. In: Fujii Y, Hiradate S, editors. Allelopathy new concepts and methodology. Enfield, NH: Science Publishers, Inc.; 2007. pp. 173–183. [Google Scholar]
- Kaur N, Gupta AK. Signal transduction pathways under abiotic stresses in plants. Current Science. 2005;88:1771–1780. [Google Scholar]
- Khanh TD, Chung MI, Xuan TD, Tawata S. The exploitation of crop allelopathy in sustainable agricultural production. Journal of Agronomy and Crop Science. 2005;191:172–184. [Google Scholar]
- Knight H, Knight MR. Abiotic stress signalling pathways: specificity and cross-talk. Trends in Plant Science. 2001;6:262–267. doi: 10.1016/s1360-1385(01)01946-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biology and Management. 2004;4:1–7. [Google Scholar]
- Laloi C, Przybyla D, Apel K. A genetic approach towards elucidating the biological activity of different reactive oxygen species in Arabidopsis thaliana. Journal of Experimental Botany. 2006;57:1719–1724. doi: 10.1093/jxb/erj183. [DOI] [PubMed] [Google Scholar]
- Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K. Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 2007;104:672–677. doi: 10.1073/pnas.0609063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Liu Y, Greenberg JT. Structure–function analysis of the plasma membrane-localized Arabidopsis defense component ACD6. The Plant Journal. 2005;44:798–809. doi: 10.1111/j.1365-313X.2005.02567.x. [DOI] [PubMed] [Google Scholar]
- Lu H, Rate DN, Song JT, Greenberg JT. ACD6, a novel ankyrin protein, is a regulator and an effector of salicylic acid signalling in the arabidopsis defense response. The Plant Cell. 2003;15:2408–2420. doi: 10.1105/tpc.015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Narusaka Y, Narusaka M, Seki M, Umezawa T, Ishida J, Nakajima M, Enju A, Shinozaki K. Crosstalk in the responses to abiotic and biotic stresses in Arabidopsis: Analysis of gene expression in cytochrome P450 gene superfamily by cDNA microarray. Plant Molecular Biology. 2004;55:327–342. doi: 10.1007/s11103-004-0685-1. [DOI] [PubMed] [Google Scholar]
- Pourcel L, Routaboul JM, Cheynier V, Lepiniec L, Debeaujon I. Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends in Plant Science. 2006;12:29–36. doi: 10.1016/j.tplants.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Prithiviraj B, Perry LG, Badri DV, Vivanco JM. Chemical facilitation and induced pathogen resistance mediated by a root-secreted phytotoxin. New Phytologist. 2007;173:852–860. doi: 10.1111/j.1469-8137.2006.01964.x. [DOI] [PubMed] [Google Scholar]
- Ramalingam S. Synthetic studies towards bioactive molecules using asymmetric dihydroxylation and organic transformation using yttris-zirconia and other heterogeneous catalysts. 2005. [Google Scholar]
- Rudrappa T, Bonsall J, Gallagher JL, Seliskar DM, Bais HP. Root-secreted allelochemical in the noxious weed Phragmites Australis deploys a reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. Journal of Chemical Ecology. 2007;33:1898–1918. doi: 10.1007/s10886-007-9353-7. [DOI] [PubMed] [Google Scholar]
- Sauerbrunn N, Schlaich NL. PCC1: a merging point for pathogen defence and circadian signaling in Arabidopsis. Planta. 2004;218:552–561. doi: 10.1007/s00425-003-1143-z. [DOI] [PubMed] [Google Scholar]
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutation Research. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Swain T. Secondary compounds as protective agents. Annual Review of Plant Physiology. 1977;28:479–501. [Google Scholar]
- Taylor JE, Hatcher PE, Paul ND. Crosstalk between plant responses to pathogens and herbivores: a view from the outside in. Journal of Experimental Botany. 2004;55:159–168. doi: 10.1093/jxb/erh053. [DOI] [PubMed] [Google Scholar]
- Trampczynska A, Bottcher C, Clemens S. The transition metal chelator nicotianamine is synthesized by filamentous fungi. FEBS Letters. 2006;580:3173–3178. doi: 10.1016/j.febslet.2006.04.073. [DOI] [PubMed] [Google Scholar]
- Tsuzuki E. Application of buckwheat as a weed control. Agriculture Horticulture. 2001;76:55–62. [Google Scholar]
- Wagner U, Edwards R, Dixon DP, Mauch F. Probing the diversity of the arabidopsis glutathione S-transferase gene family. Plant Molecular Biology. 2002;49:515–532. doi: 10.1023/a:1015557300450. [DOI] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends in Plant Science. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Weidenhamer JD, Romeo JT. Allelochemicals of Polygonella myriophylla: chemistry and soil degradation. Journal of Chemical Ecology. 2004;30:1067–1082. doi: 10.1023/b:joec.0000028468.97851.7a. [DOI] [PubMed] [Google Scholar]
- Wu H, Pratley J, Lemerle D, Haig T. Crop cultivars with allelopathic capability. Weed Research. 1999;39:171–180. [Google Scholar]
- Xuan TD, Shinkichi T, Khanh TD, Chung IM. Biological control of weeds and plant pathogens in paddy rice by exploiting plant allelopathy: an overview. Crop Protection. 2005;24:197–206. [Google Scholar]
- Zottini M, Costa A, De Michele R, Ruzzene M, Carimi F, Lo Schiavo F. Salicylic acid activates nitric oxide synthesis in Arabidopsis. Journal of Experimental Botany. 2007;58:1397–1405. doi: 10.1093/jxb/erm001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.