Abstract
Determination of the exact criteria for resectability in patients with cholangiocarcinoma and how they are most efficiently evaluated has many limitations. Among many factors taken into account in this decision-making process are: the condition of the patient, the biology of the disease, and the technical expertise of the surgeon and hospital. An attempt is made here to organize recommendations for the work-up of patients and the main criteria for resectability as best possible, keeping in mind that there will always be some limited room for exceptions, especially if the biology is favorable. Work-up and determination of resectability for patients with distal cholangiocarcinoma are more straightforward than at the other two sites of the disease (perihilar and peripheral). In general, these follow the same principles as those for other periampullary carcinomas (pancreas, ampullary, and duodenal). The work-up and determination of resectability for patients with peripheral cholangiocacrcinoma can be relatively straightforward if the lesion is away from the hilus of the liver and does not involve a significant proportion of parenchyma, but can be problematic if it is more central or very large. Patients with perihilar cholangiocarcinomas are perhaps the most challenging, as factors such as patient condition, biology of the disease, local involvement of the major vessels and bile ducts at the hilum, and the future liver remnant all have a bearing in the decision-making process.
Keywords: Cholangiocarcinoma, resectability, staging
Introduction
A unified algorithm for use when staging patients with cholangiocarcinoma and when determining the exact criteria of unresectability has many challenges. First, the three major sites of cholangiocarcinoma (peripheral, hilar, and distal) have different characteristics and unique issues in terms of staging and resectability. Because of the relative rarity of the disease, there is a paucity of randomized controlled clinical trials dealing with issues of staging and resectability. Most publications dealing with staging and resectability of cholangiocarcinoma are either radiological papers on technical aspects of the various modalities, or they are retrospective reports of results of single institutional series (some of which collect data in a prospective database). Therefore, the preponderance of evidence in this report is from observational studies; using “GRADE quality assessment criteria” 1 would be considered low quality evidence. Because of the consistency of multiple reports agreeing, however, some of the data may be considered of moderate quality, but there is great potential bias in these reports. Consensus statements are given at the end of the article.
Peripheral cholangiocarcinoma
Peripheral cholangiocarcinoma, the least frequent type, has been much more aggressively resected over the past 10–15 years at centers specializing in hepatopancreatobiliary diseases. Lang et al. 2 reported on a series of 50 patients who were explored between 1998 and 2005. Twenty-seven patients underwent extended right or left hepatectomy, including resection of the hilar bifurcation in 12, diaphragm in 6, portal vein in 5, vena cava in 4, left hepatic vein and reconstruction in 1, and hepatic artery and reconstruction in 1. This was accomplished with an overall operative mortality of 4% and a margin negative resection rate of 59%. They also nicely summarize the results of multiple other centers that have taken the same approach with similar results.
Two methodologies that have evolved relatively recently are the use of fluorodeoxyglucose positron emission tomography (FDG-PET) and staging laparoscopy. Petrowsky et al. 3 reported on their experience with PET scans in patients with biliary cancers. In patients with cholangiocarcinoma, they demonstrate high sensitivity (93%) for detecting the primary lesion in peripheral cholangiocarcinoma, but moderate sensitivity (55%) for detecting the primary lesion in hilar cholangiocarcinoma. This modality was very sensitive at detecting distant metastatic disease (100%) but poor at detecting locoregional lymphadenopathy (12%). Goere et al. 4 reported on the use of staging laparoscopy in 39 patients with biliary cancers. Fourteen of 39 patients were found to be unresectable, thus saving 38% of the patients from potentially unnecessary laparotomy. However, when the 25 remaining patients were taken to laparotomy, a further 9 were found to be unresectable because of vascular invasion (5), advanced nodal spread (3), liver metastases (2), peritoneal metastases (1), and adjacent organ infiltration (1). They concluded that laparoscopy was sensitive at detecting peritoneal and liver metastases, but not so good at detecting distant lymph node involvement or vascular invasion.
Hilar cholangiocarcinoma
In the past 10 years, there have been increasing reports of aggressive resections for hilar cholangiocarcinoma. Nimura et al. 5 reported on their series of 142 patients who underwent exploration for hilar cholangiocarcinoma. Of these, 108 underwent curative resection and included 100 who underwent hepatectomy, 43 portal vein resections, and 16 pancreatoduodenectomy with hepatectomy. The 5-year survival rate for those undergoing hepatectomy was 26% and 16% for those who did not. Miyazaki et al. 6 reported a similar experience in that patients who underwent portal vein resection to achieve a negative margin did better than those who had a margin-positive resection without vein resection. They stated that portal vein resection had acceptable operative morbidity and could improve prognosis. However, in summarizing the results of the nine patients who underwent hepatic artery resection, they reported that hepatic artery resection could not be justified. Neuhaus et al. 7 reported on the benefit of portal vein resection with extended right hepatectomy. Patients who underwent margin negative portal vein resection with extended right hepatectomy had a 5-year survival rate of 72%, whereas those who did not have portal vein resection but had a margin negative resection with extended right hepatectomy had a 5-year survival rate of 52%.
Conner et al. 8 reported on the role of staging laparoscopy in patients with hilar cholangiocarcinoma. Eighty-four patients underwent laparoscopy for presumed hilar cholangiocarcinoma. Thirty-five where found to be unresectable at laparoscopy and 19 at laparotomy; 20 were resectable. The overall yield of laparoscopy was thus 42%, which is significantly higher than in other reported studies. The authors comment that this could have been because of the extensive use of laparoscopic ultrasound during laparoscopy.
Distal cholangiocarcinoma
Patients with distal cholangiocarcinoma are in general more straightforward in terms of work-up and criteria of resectability. De Oliveira et al. 9 reported on a series of 239 patients explored with distal cholangiocarcinoma. The resection rate was 96%, while the margin-negative resection rate was 82%.
Conclusions
Cholangiocarcinoma comprises a broad spectrum of disease with diverse options for resection depending on location and involvement. Resection provides the only hope of long-term survival. Margin-negative resection is a strong prognostic factor. Resectability at laparotomy remains challenging, but imaging techniques are easing the selection process. Radical resections, including extended hepatectomies for hilar and peripheral tumors, are more common and more often yield negative margins. Efficient but complete staging of patients is paramount to correctly selecting those most likely to benefit from these radical operations and to avoid unnecessary laparotomy in those who will not.
Consensus statements
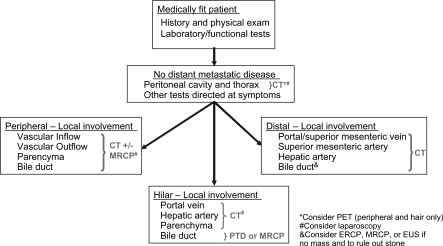
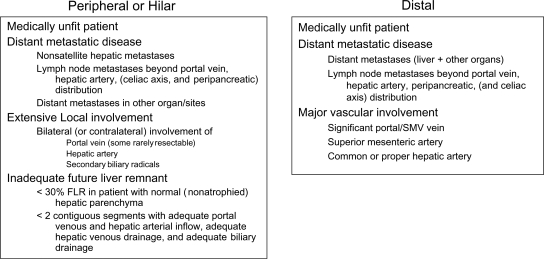
For consideration of resectability for peripheral cholangiocarcinoma (Figure 1), patients must be medically fit for resection. They should be subject to appropriate preoperative studies including those focusing on hepatic function and more direct studies in accordance with their medical history. They should be studied for presence of metastatic disease in the peritoneal cavity and thorax and elsewhere if symptomatic. Typically, a high definition CT scan of the chest, abdomen and pelvis is sufficient. Lastly, local involvement of the main tumor mass should be studied with particular attention to vascular inflow, vascular outflow, hepatic parenchyma, and the biliary tree. This can usually be accomplished with a high definition CT scan with reconstruction. If the tumor encroaches upon the hilus of the liver, magnetic resonance cholangiopancreatography (MRCP) may be of benefit in evaluating the hilus, especially the biliary tree. Consideration should be given to using PET scan to detect distant metastatic disease and laparoscopy to detect metastatic disease in the abdomen, as well as to examine local involvement with laparoscopic ultrasound. The exact criteria for unresectability are listed in Figure 2. The patients must be fit for resection. They should not have metastatic disease. They should not have bilateral or contralateral involvement of the portal vein, hepatic artery, or secondary biliary radicals. In some instances, however, minimal bilateral portal vein involvement can be cleared with resection, if far enough away from the umbilical fissure. Additionally, there should be a sufficient future liver remnant with at least 30% of the liver volume of relatively normal non-atrophied parenchyma that has good vascular inflow, outflow, and biliary drainage.
For consideration of resectability for hilar cholangiocarcinoma (Figure 1), patients must be medically fit for resection. They should be subject to appropriate preoperative studies including those focusing on hepatic function and more directed studies in accordance with their medical history. They should be studied for the presence of metastatic disease in the peritoneal cavity and thorax and elsewhere, if symptomatic. Typically, a high definition CT scan of the chest, abdomen, and pelvis is sufficient. Local involvement of the main tumor mass should be studied with particular attention to the portal vein, hepatic artery, and hepatic parenchyma. This can be accomplished with a high definition CT scan with reconstructions. Biliary involvement should be evaluated with either percutaneous transhepatic cholangiogram (PTC) or MRCP. Consideration should be given to using PET scan to detect distant metastatic disease and laparoscopy to detect metastatic disease in the abdomen, as well as to examine local involvement with laparoscopic ultrasound. The exact criteria for unresectability are listed in Figure 2. The patients must be fit for resection. They should not have metastatic disease nor bilateral or contralateral involvement of the portal vein, hepatic artery, or secondary biliary radicals. In some instances, however, minimal bilateral portal vein involvement can be cleared with resection if far enough away from the umbilical fissure. Additionally, there should be a sufficient future liver remnant with at least 30% of the liver volume of relatively normal non-atrophied parenchyma that has good vascular inflow, outflow, and biliary drainage.
For consideration of resectability for distal cholangiocarcinoma (Figure 1), as before (Figure 1), patients are reviewed to make sure that they are medically fit, and that they do not have metastatic disease. Local involvement generally focuses on the portal/superior mesenteric vein complex, superior mesenteric artery, and hepatic artery. All this can usually be completed with a high definition CT scan with reconstructions. Sometimes endoscopic retrograde pancreatography (ERCP), MRCP, or endoscopic ultrasound can help delineate the disease process if no mass is seen on CT scan, or if a biliary stone is of concern. Patients are unresectable if they are medically unfit, if they have metastatic disease, or if they have significant vascular involvement. If the hepatic artery, or superior mesenteric, is significantly involved (>180 degree involvement), or if the portal or superior mesenteric vein is significantly involved (>2 cm requiring resection), then the patient is considered unresectable.
Figure 1. .
Cholangiocarcinoma – staging.
Figure 2. .
Cholangiocarcinoma – criteria of unresectability.
References
- 1.Atkins D, et al. Systems for grading the quality of evidence and the strength of recommendations II: Pilot study of a new system. BMC Health Services Research. 2005;5:25. doi: 10.1186/1472-6963-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang H, Sotiropoulos GC, Frühauf NR, Dömland M, Paul A, Kind EM, et al. Extended hepatectomy for intrahepatic cholangiocellular carcinoma (ICC): when is it worthwhile? Single center experience with 27 resections in 50 patients over a 5-year period. Ann Surg. 2005;241:134–43. doi: 10.1097/01.sla.0000149426.08580.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto G, Romaneehsen B, Hoppe-Lotichius M, Bittinger F. Hilar cholangiocarcinoma: resectability and radicality after routine diagnostic imaging. J Hepatobil Pancreat Surg. 2004;11:310–18. doi: 10.1007/s00534-004-0912-9. [DOI] [PubMed] [Google Scholar]
- 4.Goere D, Wagholikar GD, Pessaux P, Carrère N, Sibert A, Vilgrain S, et al. Utility of staging laparoscopy in subsets of biliary cancers. Surg Endosc. 2006;20:721–5. doi: 10.1007/s00464-005-0583-x. [DOI] [PubMed] [Google Scholar]
- 5.Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobil Pancreat Surg. 2000;7:155–62. doi: 10.1007/s005340050170. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki M, Kato A, Ito H, Kimura F, Shimizu H, Ohtsuka M, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: Does it work or not? Surgery. 2007;141:581–8. doi: 10.1016/j.surg.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Neuhaus P, Jonas S, Settmacher U, Thelen A, Benckert C, Lopez-Hänninen E, et al. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg. 2003;388:194–200. doi: 10.1007/s00423-003-0383-5. [DOI] [PubMed] [Google Scholar]
- 8.Connor S, Barron E, Wigmore SJ, Madhavan KK, Parks RW, Garden OJ. The utility of laparoscopic assessment in the preoperative staging of suspected hilar cholangiocarcinoma. J Gastrointest Surg. 2005;9:476–80. doi: 10.1016/j.gassur.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: 31-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]