Abstract
Background and aim: Iron overload and inflammation might participate in the pathogenesis of insulin resistance in community. The improvement of insulin resistance in hemodialysis (HD) patients is frequently seen after correction of anemia. The aim of this study was to investigate the influence of recobinant humam erythropoietin (Epo) treatment on insulin resistance in non-diabetic HD patients.
Patients and methods: We investigated the effects of 6 months-duration treatment with Epo on insulin resistance and inflammatory parameters in 16 (6 male/10 female) patients on maintenance HD with renal anemia (hemoglobin concentration ≤105 g/l). The control group consisted of 15 patients on HD with renal anemia, without Epo treatment. Further clinical and laboratory variables were observed: fasting blood glucose (FBG), insulin, albumin, iron, total iron binding capacity (TIBC), transferrin saturation (TSAT), ferritin, TNF-alpha, and IL-6. Independent predictors for changes of calculated insulin resistance index by homeostasis model assessment (HOMA-IR) were identified by multivariate linear regression analysis.
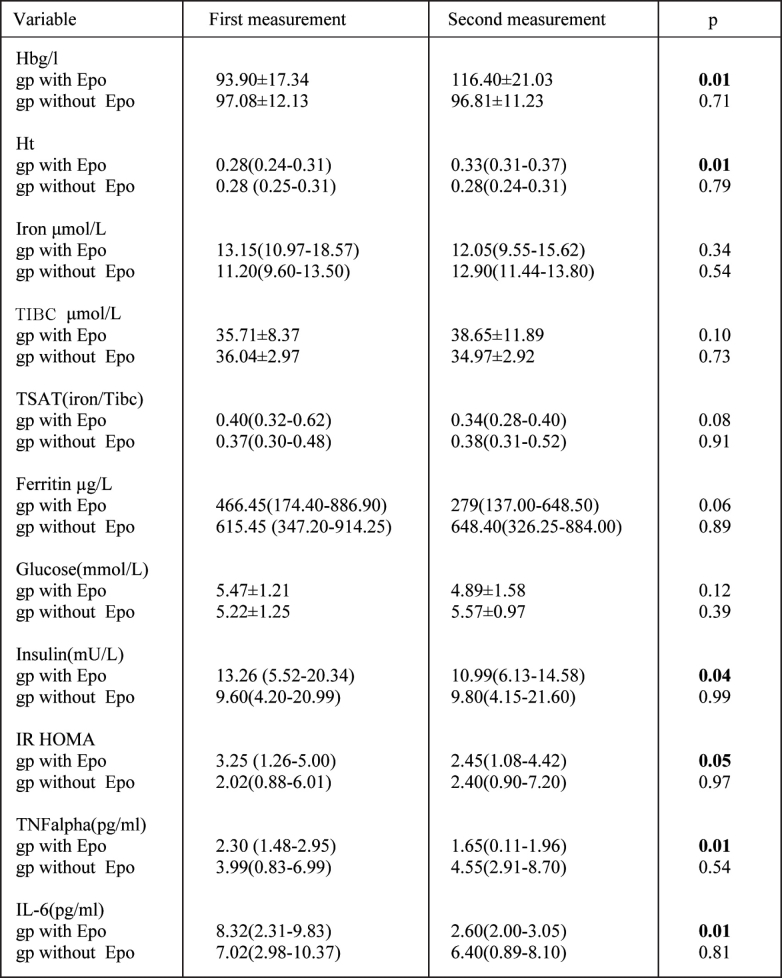
Results: A significant reduction of insulin levels and therefore significant lowering of HOMA-IR was registered in Epo treated group. It was observed improvement of anemia [Hb 93.90±17.34 g/L vs. 116.40±21.03 g/L, p: 0.01; Hct 0.28 (0.24-0.31) vs. 0.33% (0.31-0.37), p: 0.01] as well as a trend toward iron stores decrease [ferritin 466.45 (174.40-886.90) vs. 279 μg/L (137.00-648.50), p: 0.06]. A significant decrease of TNF-alpha [2.30 pg/ml (1.48-2.95) vs. 1.65 pg/ml (0.11-1.96), p: 0.01] and IL6 levels [8.32 pg/ml (2.31-9.83) vs. 2.60 pg/ml (2.00-3.05), p: 0.01] was presented. After adjustment for confounding variables (age, sex, and Kt/v), a model consisting of BMI, ferittin, and TNF alpha accounted for 96% of the variance in HOMA-IR in Epo treated patients.
Conclusions: The present study demonstrated that Epo treatment could participate in reducing insulin resistance through iron stores reduction and improvement of chronic inflammation in patients on maintenance HD.
Keywords: TNF-alpha, IL-6, end stage renal disease, erythropoietin, insulin resistance, iron
Patients with end-stage renal disease (ESRD) are at high-risk of cardiovascular disease-induced death1,2. Renal failure is associated with multiple metabolic and endocrinology abnormalities and these alterations are involved in advanced atherosclerosis and high cardiovascular risk1–6. It has been shown that insulin resistance may contribute to the pathogenesis of atherosclerotic cardiovascular disease6, so we should devote more attention to insulin resistance in uraemic patients. It was demonstrated that HOMA-IR could be an independent predictor of cardiovascular mortality in non-diabetic patients on maintenance HD7. Recently, we have shown that out of BMI, serum iron participates as an independent predictor for calculated IR index in patients on maintenance HD8. Our finding supports the statement that insulin resistance, as non-classic cardiovascular risk factor, could be also the consequence of iron therapy in ESRD patients9. However, it is likely that improvement of anemia with regular Epo treatment and adjusted dose of intravenous iron may decrease a high level of serum iron and furthermore iron stores. Hence, a reduction of insulin resistance can be seen. In the present study, we test the potential efficiency of Epo administration on iron status, insulin resistance and chronic inflammation in observed patients, by conventional once weekly schedule as a subcutaneous injection.
Patients and Methods
This single-centre prospective study was designed to verify the effectiveness of administration of Epo on insulin resistance and inflammatory status. The study involved 31 ESRD patients on maintenance HD, with renal anemia. These patients were divided into two groups. Sixteen patients with Epo treatment and of fifteen pts without Epo treatment consisted the first and second group respectively. The patients fulfilled all inclusion criteria: a) age >18 years; b) HD treatment for >12 months; c) exclusion of serious adverse event, known malignancies, inflammatory or hematological diseases and acute or active phase of chronic infectious diseases; d) the presence of anemia (Hb ≤105 g/l) and without diabetes (fasting glycemia < than 7 mmol/l). The patients gave written consent to participate in the study.
The study was conducted according to the Declaration of Helsinki and was approved by the Zemun Clinical Hospital local Ethical Committee. All patients received s.c. Epo-b three times weekly during a 4-week run-in period, with starting dose mean 95±44 IU/kg BW/week. Following the run-in period, patients were randomly assigned to treatment with once weekly regimen. Dose titrations were permitted 6 weeks after randomization and every 4 weeks thereafter. Epoetin-b dose was increased by 20% if the serum Hb concentration decreased below 100 g/l, or redunced by 20% if it rose above 125 g/l. After the stable Epo dose was attained, the patients from the first group were included in the study and thereafter followed for 6 months. All patients were regularly supplemented with i.v. iron, folic acid, and vitamin B12.
Blood samples for Hb and Hct measurements were collected on fortnight, before a midweek dialysis session. Anthropometric measurements, including body mass index (BMI) and waist circumference, were recorded at the start and the end of the study by using standardized protocol [dry weight or after-dialysis weight (dry weight / height2) for calculation of BMI]. Furthermore, the following parameters were measured: iron, TIBC, TSAT, ferritin, FBG, insulin, TNF-alpha, IL-6 levels. Kt/V was calculated by Daugirdas method.
The levels of FBG, triglyceride, total cholesterol, HDL-cholesterol, Hb, Hct and iron were measured with conventional autoanalyzer, by using blood samples obtained on midweek, after overnight fasting and immediately prior to dialysis. Serum ferritin was measured by using an immunoradiometric assay (IRMA). The plasma insulin level was measured by using a radioimmunoassay method (RIA, INEP Zemun, Belgrade). IL-6 and TNF-alpha levels were measured in duplicate by Immunotech IL-6 immunoassays and Immunotech TNF-alpha immunoassays (Beckman CounterTM). Insulin resistance index was calculated from fasting insulin and glucose concentration by using Homeostatic Model Assessment score (HOMA-IR formula: glucose x insulin/22.5)10.
Statistical analysis
Appropriate descriptive statistical parameters were used to describe observed patients. Normally distributed data are shown as the mean ± SD, whereas other as the median and interquartile range (difference between 25th and 75th percentile numbers). Comparisons between groups were made using two-tailed Student's t test or the Mann- Whitney U test. Categorical variables were compared using Fisher's exact test. Plasma cytokines, ferritin, insulin and HOMA-IR were positively skewed and log-transformed to normal distribution to calculate Pearson ® correlation coefficients, in order to explore the relations between changes in HOMA-IR and demographic and metabolic characteristics respectively. Multivariate regression analysis was performed to determine potentially independent predictors of HOMA-IR changes after Epo treatment.
Results
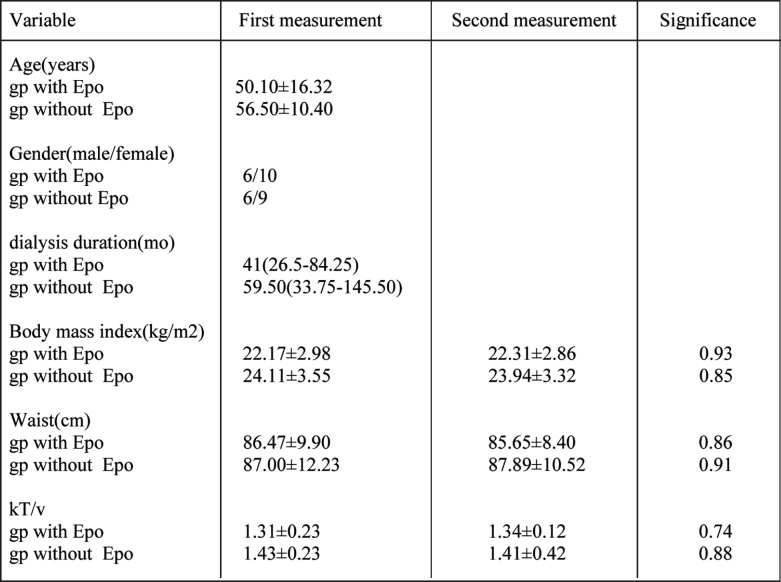
Patients demographic as well as clinical and laboratory data before and 6 months after initial visit are shown in Table 1 and Table 2. Baseline demographic were similar in both groups.
Table 1. Demographic data of HD patients at the beginning and at the end of study.
Data are presented as the mean ± SD and as the median and interquartiles range, for variables with the skewed distribution gp: group.
Table 2. Clinical data of study HD patients on the beginning and at the end of study period with and without Epo treatment.
Data are presented as the mean ± SD and as the median and interquartiles range, for variables with the skewed distribution. gp: group.
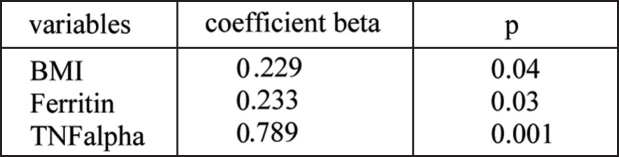
Treatment of anemia with Epo led to a significant increase of Hb and Hct as well as significant decrease of insulin levels. HOMA-IR, inflammatory cytokines, TNF-alpha, and IL-6. There were no significant changes regarding Hb, Hct, iron, TSAT, ferittin, fasting glucose, fasting insulin, HOMA-IR, TNF-alpha and IL-6 in patients with no Epo therapy. To determine the potential independent factors contributing to insulin resistance decrease after Epo treatment, multivariate linear regression analysis were performed. The variable changes that correlated with change of HOMA-IR were also included. In a model that explained 96% variation of the HOMA-IR, the changes of BMI (β: 0.229, p: 0.04), serum ferritin (β: 0.233, p: 0.03), and TNF-alpha (β: 0.789, p: 0.001) were independent factors in the prediction of HOMA-IR changes in Epo treated HD patients after adjustment for age, sex and Kt/v (Table 3). The changes of HOMA-IR were not significant in the patients with no Epo treatment.
Table 3. Multivariate linear regression analysis between HOMA-IR and variables of anemia, iron status and inflammatory markers after the study period and Epo treatment.
R2: 0.96, p: 0.001.
Discussion
Insulin resistance is presented in the vast majority of our non-diabetic patients on maintenance HD5. Many factors have been implicated in the pathogenesis of insulin resistance in ESRD patients11,12. In our previous study we found that nutritional status and serum iron determined the level of calculated index of insulin resistance in HD patients8. It has been reported that the improvement of anemia by Epo reversed insulin resistance in uremic patients12,13. Concern has arisen about administration of large doses of parenteral iron that may be associated with morbidity and mortality, particularly from infection14. This can be explained via the wellknown role of iron as a growth factor for bacteria and supposed inhibitory role on neutrophil function15. Additionally, growing evidences pointed out to pro-oxidant properties of iron therapy and subsequently higher oxidative stress in HD patients which contributed to higher risk of cardiovascular morbidity and mortality16.
There is no enough data available at present about the effect of iron overload and Epo administration on insulin resistance in ESRD patients. Mak et al11 demonstrated that Epo administration could repair insulin sensitivity in HD patients preferably by correction of anemia than an iron overload. Tuzcu et al17 confirmed that treatment with Epo was associated with improvement of anemia and insulin sensitivity in uremic patients, but without suggestion about its potential mechanism. Spaia et al18 demonstrated the beneficial effect of Epo treatment on insulin resistance in HD patients besides the improvement of anemia. Our findings are in concordance with that statement, because our Epo-treated patients showed the trend toward reduction of iron overload with significant improvement of inflammatory status and insulin resistance. Furthermore, the reduction of ferritin and the levels of inflammatory cytokines were independent predictor of insulin resistance improvement. Correction of anemia did not correlate with change of insulin resistance in treated group.
In the present study we showed significant insulin resistance improvement (33%) after 6 month Epo treatment. Conversely, that was not the case with the Epo untreated group. The recorded high serum ferritin level resulted from ineffective utilization of iron stores, because the patients were out the regular recombinant human Epo treatment. It is well-known that chronic inflammatory diseases, particularly ESRD, are associated with an increase of iron stores or ferritin levels12, 14, 19. Inflammation may not have an effect on serum ferritin, unless there is enough iron stores in the body so that serum ferritin is somewhat increased20. Liver dysfunction and inflammatory factors may interfere with the synthesis and clearance of ferritin, thereby increasing serum ferritin levels not related to iron metabolism. In the present study, there was a significant correlation between serum iron, TSAT and ferritin level, but not between ferritin and TNF-alpha and IL-6 respectively. This finding possibly indicates the ferritin as a marker of iron stores rather than an inflammatory marker. However, Rogers et al20, showed that IL-1β induces ferritin gene expression via translational control of its mRNA. This inflammatory induction of ferritin synthesis is Epo and insulin resistant in HD patients different from iron dependent ferritin gene expression. In recent years, a large body of evidence showed that uremia itself is a state of some level inflammation as reflected by elevation of the classical inflammatory biomarkers, TNF-alpha, IL-6 and CRP21. To assess the potential role TNF-alpha and IL-6 as inflammatory cytokines in mediating insulin resistance in our patients, we measured their plasma concentrations before and after the treatment. A significant reduction of TNF alpha and IL-6 levels was observed after the Epo treatment, despite their higher levels before the treatment. Multivariate regression analysis revealed that the change in TNFalpha level was significant predictive factor for the insulin resistance improvement in Epo treated subjects. Moreover, we could speculate that Epo treatment indirectly improved insulin resistance in patients on maintenance HD. Recent studies suggested that insulin resistance may be a central mechanism for uremic malnutrition22. Therefore, insulin resistance may contribute to malnutrition-inflammation-atherosclerotic (MIA) syndrome23.
TNF-alpha induces insulin resistance in experimental animal models by mechanisms that involve serine phosphorylation of the insulin receptor substrate 1 (IRS-1)24–26, decreasing its tyrosine phosphorylation by the insulin receptor (IR) kinase. The activated kinases phosphorylate serine residues on IRS-1 and inhibit insulin-induced PI 3-kinase activity, resulting in reduced insulin-stimulated AKT2 activity. Lowered AKT2 activity fails to activate GLUT4 translocation, and other downstream AKT2-dependent events, and consequently insulin-induced glucose uptake is reduced27,28. In vitro and in vivo studies show that TNF-α inhibition of insulin action is, at least, antagonized by thiazolidinediones, further supporting the role of TNF-alpha in insulin resistance. In addition, several other mechanisms could account for the effect of TNF-alpha on obesity-related insulin resistance - increased lipolysis in adipocytes with an increased release of free fatty acids (FFA) and reduced synthesis of adiponectin. During recent years, various and controversial data were reported about the effects of Epo treatment on inflammation in ESRD patients. In a cross-sectional analysis of 339 haemodialysis patients, Kalantar-Zadeh et al29 found a positive correlation between CRP levels and EPO dose at linear regression analysis. Agulirea et al30 confirmed that the effect of Epo treatment is paradigmatic, because Epo is able to decrease or increase some pro- and anti-inflammatory cytokines in ESRD patients, depending on the type of tissue. Increased level of TNF-alpha was explained with improved immune status.
Significant reduction of inflammatory cytokines, TNF-alpha and IL-6, and improvement of anemia as well as insulin resistance, was registered just in Epo study-treated patients. The mechanisms responsible for improved insulin resistance might be related to reduction of chronic inflammation, or reduced over-expression of gene for NF-kB in skeletal muscle31. On the other hand, insulin resistance of ESRD patients might also be due to a defect in mitochondrial oxidative phosphorylation28, but there is no enough data in accordance with that statement. Improved intramuscular fatty acid metabolism accompanied with repair of insulin resistance without significant association with inflammatory status will be confirmation for that statement.
In conclusion, our study demonstrated that improvement of insulin resistance in patients with ESRD on maintenance HD with Epo was independent of anemia correction. These data also identified that repair of chronic inflammation, with reduced level of inflammatory cytokines, particularly TNF-alpha, and iron overload or ferritin level, were siginifanct predictive factors for an improvement of insulin resistance. Nutritional status, estimated by serum albumin, was also significant predictor of insulin resistance. Our data revealed the necessity of lower iron dose to be administered in Epo-treated patients on HD to achieve better insulin sensitivity.
References
- 1.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 2.Yao Q, Lindholm, B, Stenvinkel P. Inflammation as a cause of malnutrition, atherosclerotic cardiovascular disease, and poor outcome in hemodialysis patients. Hemodialysis International. 2004;8:118–129. doi: 10.1111/j.1492-7535.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- 3.Frank H, Heusser K, Höffken B, Huber P, Schmieder RE, Schobel HP. Effect of erythropoietin on cardiovascular prognosis parameters in hemodialysis patients. Kidney Int. 2004;66:832–840. doi: 10.1111/j.1523-1755.2004.00810.x. [DOI] [PubMed] [Google Scholar]
- 4.Mak RH. 1,25-Dihydroxyvitamin D3 corrects insulin and lipid abnormalities in uremia. Kidney Int. 1998;53:1353–1357. doi: 10.1046/j.1523-1755.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 5.Rasic-Milutinovic Z, Peruničić-Peković G, Plješa S, et al. Impaired insulin sensitivity and insulin secretion in hemodialysis patients with and without secondary hyperparathyroidism. Malta Medical Journal. 2004;16:18–22. [Google Scholar]
- 6.Stenvinkel P, Ottosson-Seeberger A, Alvestrand A. Renal hemodynamics and sodium handling in moderate renal insufficiency: the role of insulin resistance and dyslipidemia. J Am Soc Nephrol. 1995;5:1751–1760. doi: 10.1681/ASN.V5101751. [DOI] [PubMed] [Google Scholar]
- 7.Rasic-Milutinovic Z, Peruničić G, Plješa S, et al. Is insulin independent predictor of mortality in hemodialysis patients. Yugoslav Medical Biochemistry. 2004;23:43–49. [Google Scholar]
- 8.Rasic-Milutinovic Z, Perunicic G, Pljesa S, Gluvic Z, Ilic M, Stokić E. Metabolyc syndrome in HD patients: association with body composition, nutritional status, inflammation and serum iron. Intern Med. 2007;46:945–951. doi: 10.2169/internalmedicine.46.0092. [DOI] [PubMed] [Google Scholar]
- 9.Drueke T, Witko-Sarsat V, Massy Z, et al. Iron therapy, advanced oxidation protein products, and carotid artery intimamedia thickness in end-stage renal disease. Circulation. 2002;106:2212–2217. doi: 10.1161/01.cir.0000035250.66458.67. [DOI] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Mak RH, DeFronzo RA. Glucose and insulin metabolism in uremia. Nephron. 1992;61:377–382. doi: 10.1159/000186953. [DOI] [PubMed] [Google Scholar]
- 12.Mak RH. Correction of anemia by erythropoietin reverses insulin resistance and hyperinsulinemia in uremia. Am J Physiol. 1996;270:F839–F844. doi: 10.1152/ajprenal.1996.270.5.F839. [DOI] [PubMed] [Google Scholar]
- 13.Borissova AM, Djambazova A, Todorov K, Dakovska L, Tankova T, Kirilov G. Effect of erythropoietin on the metabolic state and peripheral insulin sensitivity in diabetic patients on hemodialysis. Nephrol Dial Transplant. 1993;8:93–95. doi: 10.1093/oxfordjournals.ndt.a092282. [DOI] [PubMed] [Google Scholar]
- 14.Feldman HI, Santanna J, Guo W, et al. Iron administration and clinical outcome in hemodialysis patients. J Am Soc Nephrol. 2002;13:734–744. doi: 10.1681/ASN.V133734. [DOI] [PubMed] [Google Scholar]
- 15.Boelaert JR, Daneels RF, Schurgers ML, Matthys EG, Gordts BZ, Van Landuyt HW. Iron overload in haemodialysis patients increases the risk of bacteraemia: A prospective study. Nephrol Dial Transplant. 1990;5:130–134. doi: 10.1093/ndt/5.2.130. [DOI] [PubMed] [Google Scholar]
- 16.Bayes B, Pastor MC, Bonal J, Foraster A, Romero R. Oxidative stress, inflammation and cardiovascular mortality in haemodialysis - role of seniority and intravenous ferrotherapy: analysis at 4 years of follow-up. Nephol Dial Transplant. 2006;21:984–890. doi: 10.1093/ndt/gfi294. [DOI] [PubMed] [Google Scholar]
- 17.Tuzcu A, Bahceci M, Yilmaz E, Bahceci S, Tuzcu S. The comparison of insulin sensitivity in non-diabetic hemodialysis patients treated with and without recombinant human erythropoietin. Horm Metab Res. 2004;36:716–720. doi: 10.1055/s-2004-826021. [DOI] [PubMed] [Google Scholar]
- 18.Spaia S, Pangalos M, Askepidis N, et al. Effect of short-term rHuEPO treatment on insulin resistance in haemodialysis patients. Nephron. 2000;84:320–325. doi: 10.1159/000045606. [DOI] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, Rodriguez RA, Humphreys MH. Association between serum ferritin and measures of inflammation, nutrition and iron in hemodialysis patients. Nephrol Dial Transplant. 2004;19:141–149. doi: 10.1093/ndt/gfg493. [DOI] [PubMed] [Google Scholar]
- 20.Rogers JT, Bridges KR, Durmowicz GP, Glass J, Auron PE, Munro HN. Translational control during the acute phase response. Ferritin synthesis in response to interleukin-1. J Biol Chem. 1990;265:14572–14578. [PubMed] [Google Scholar]
- 21.Zoccali C, Mallamaci F, Tripepi G. Inflammatory proteins as predictors of cardiovascular disease in patients with end-stage renal disease. Nephrol Dial Transplan. 2004;19(Suppl 5):67–72. doi: 10.1093/ndt/gfh1059. [DOI] [PubMed] [Google Scholar]
- 22.Lee SW, Park GH, Lee SW, Song JH, Hong KC, Kim MJ. Insulin resistance and muscle wasting in non-diabetic end-stage renal disease patients. Nephrol Dial Transplant. 2007;22:2554–2562. doi: 10.1093/ndt/gfm204. [DOI] [PubMed] [Google Scholar]
- 23.Shoji T, Nishizawa Y. Chronic kidney disease as a metabolic syndrome with malnutrition-need for strict control of risk factors. Intern Med. 2005;44:179–187. doi: 10.2169/internalmedicine.44.179. [DOI] [PubMed] [Google Scholar]
- 24.Hotamisligil GS. Mechanisms of TNF-alpha-induced insulin resistance. Exp Clin Endocrinol Diabetes. 1999;107:119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 25.Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor alpha-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J Biol Chem. 1995;270:23780–23784. doi: 10.1074/jbc.270.40.23780. [DOI] [PubMed] [Google Scholar]
- 26.Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol. 2006;17:1388–1394. doi: 10.1681/ASN.2004100842. [DOI] [PubMed] [Google Scholar]
- 27.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 28.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 30.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Lee GH, Nissenson AR, Kopple JD. Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. Am J Kidney Dis. 2003;42:761–773. doi: 10.1016/s0272-6386(03)00915-6. [DOI] [PubMed] [Google Scholar]
- 31.Aguilera A, Bajo MA, Diez JJ, et al. Effects of human recombinant erythropoietin on inflammatory status in peritoneal dialysis patients. Adv Perit Dial. 2002;18:200–205. [PubMed] [Google Scholar]