Abstract
Background and aim: The synchronous and consecutive (metachronous) development of two or more primary adenocarcinomas accounts for 3 to 5 % of cases of colorectal cancer. Aim of this study is to review our experience in the management of patients with synchronous and metachronous lesions, and reach conclusions regarding their optimal diagnosis, treatment and follow-up.
Patients and methods: Between 1987 and 2004, 12 patients (seven men and five women, mean age 67.5 years, range 47-83 years) with synchronous (three patients) and metachronous (nine patients) lesions were treated, comprising 4.3% of all patients submitted to surgery for colorectal cancer. The diagnosis lag for metachronous lesions ranged from 1.5 to 14 years. All three patients with synchronous cancers had two lesions.
Results: Staging colonoscopy and abdominal CT was conducted in 10 patients while the remaining two underwent only abdominal CT due to their critical condition at presentation. Surgery had curative intent in 10 patients and palliative in two. The mean postoperative hospital stay was 21 days (10 - 49 days). The postoperative mortality was zero. Patients survival after curative procedures was 80% for the first year, 60% for the third and 50% for the fifth year. After palliative surgery, survival was 50% for the first year, and zero for the third.
Conclusions: Patients with colorectal cancer must be followed up regularly after surgery. Follow up aims at early diagnosis and treatment of metachronous lesions that can appear many years after diagnosis of the primary lesion. Preoperative colonoscopy is an invaluable diagnostic (biopsy) and staging (exclusion of synchronous lesions, localization of the primary) modality, dictating the surgical approach. Additionally, it contributes to cancer prevention allowing the discovery and removal of small polyps before their transformation.
Keywords: colorectal cancer, synchronous carcinomas, metachronous carcinomas
Multiple primary carcinomas often occur in the rectum and colon. The time lag between the first and second malignant transformation is variable. Two or more primary carcinomas can coexist at the time of diagnosis (synchronous), or develop consequently (metachronous), sometimes years after resection of the first primary.
According to Cunliffe et al1, synchronous adenocarcinomas can be two or more in number, detected either pre / intraoperatively, or in a 6 month period postoperatively. They should be distinctly separate by at least 4 cm distance and they should not consist of submucosal spread or a satellite lesion of each other. In any other case they are considered as regional spread or metastatic lesions. In contrast, metachronous carcinomas can be defined as those diagnosed 6 months after the operation for the primary lesion, and located in a different part of the large intestine, so as to not represent a recurrence.
It is well known that hereditary colorectal cancer syndromes, such as familial adenomatous polyposis (FAP), and inflammatory bowel disease (ulcerative colitis - UC) predispose to the development of multiple colorectal carcinomas. These patients, as well as patients with carcinoma in situ were excluded from this study. In all studied patients. Factors contributing to the development of synchronous and metachronous lesions were investigated in all patients studied.
Patients and methods
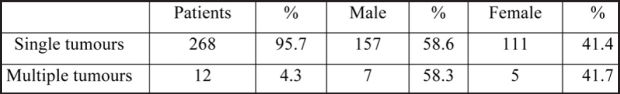
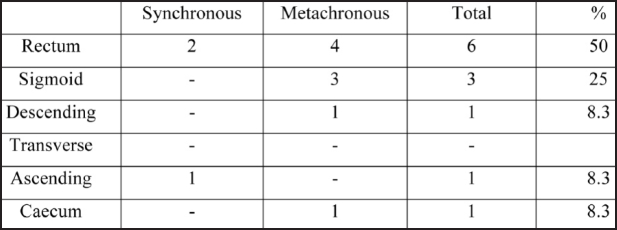
Twenty hundred and eighty patients operated for colorectal cancer during the period 1987-2004 were retrospectively studied. Two hunded and sixty eight patients (95.7%) had single and 12 (4.3%) multiple primary cancers (Table 1). Patients with synchronous or metachronous lesions comprised of seven men (58.3%) and five women (41.7%). Their mean age was 67.5 years (range 47-83). The tumours were synchronous in three patients (25%, two male and one female) and metachronous in nine (75%, five male and four female). Metachronous adenocarcinomas were identified 18 months to 14 years after excision of the primary lesion. The tumours were located more frequently in the rectum, followed by the sigmoid colon (Table 2).
Table 1. Distribution of carcinomas according to patients' sex.
Table 2. Distribution of tumours to the different parts of the large intestine.
In two patients with synchronous carcinomas the diagnosis was suspected on barium enema and confirmed with colonoscopy and biopsy. In the third patient the diagnosis was made intra-operatively, since he was operated urgently because of bowel obstruction and perforation. A barium enema was performed in all patients with metachronous lesions. The examination was diagnostic in all patients; but in 8 of them the diagnosis was confirmed with colonoscopy and biopsy. Colonoscopy was helpful in the discovery and removal of adenomatous polyps in three patients (a patient with synchronous and two with metachronous carcinomas). These polyps were benign on histological examination. An abdominal CT scan was performed in all patients for staging purposes.
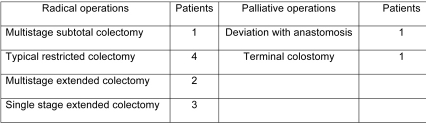
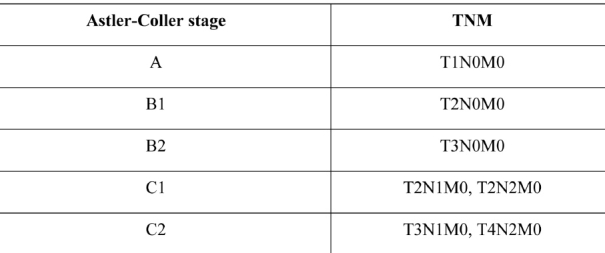
All patients underwent surgery. Three patients with synchronous tumours had two primary lesions that were removed with extensive colectomy. Nine patients with metachronous lesions underwent consecutive operations for tumour removal. Operations were radical and considered curative in 10 patients. Two patients underwent a palliative resection because of liver metastases (a patient with metachronous carcinoma), and locally extensive disease (a patient with synchronous carcinomas, Table 3). Two patients who underwent curatively intended surgery had synchronous carcinomas. Histological examination lesions, according to the Astler-Coller classification showed stage B2 lessions while the remaining eight patients belonged to the metachronous carcinoma group. In two, it was impossible to locate the histological results of the primary cancers because they were operated in a different hospital, and more than 10 years passed before the metachronous lesion diagnosis. Tumour stage (of the metachronous lesions) was B2 according to the Astler-Coller classification. In the remaining patients the tumours were of stages A, B1, B2 and C2 (Astler-Coller classification, Table 4).
Table 3. Types of surgical operations performed.
Table 4. Colorectal cancer staging (Astler Coller and TNM classifications).
Results
As observed in this series, there is a higher incidence of multiple adenocarcinomas in male patients (41.7%). Only 10 patients had adequate postoperative follow up (from six months to 15 years). Two patients with curatively curatively intended resections were lost to follow up. Postoperative mortality was zero. Survival rate after radical excisions was 80% in 1 year, 60% in 3 years and 50% in 5 years. Fifty percent of patients who underwent palliative procedures survived for 1 year, and none for three.
Discussion
Multiple primary adenocarcinomas of the large intestine were described for the first time by Czerny in 18801. The reported incidence rates vary from 2 to 8%2–5. The actual percentage is probably higher, considering the fact that not all tumours are discovered (diagnostic difficulties) and because some patients only undergo palliative operations. The incidence of multiple primary adenocarcinomas in our study was 4.3%.
The preoperative diagnosis of multiple synchronous colorectal carcinomas remains difficult. The results are still not satisfactory and the second location is often missed, although there has been a major improvement in the available techniques. The identification of a tumour that justifies the clinical symptoms usually suspends further investigation. Thus, persistence in diagnostic approach is necessary4,6,7.
Kaibara et al4 reported a 60% frequency of preoperative diagnosis by double contrast barium examination (DCBE) and/or colonoscopy, whereas Takeuchi et al8 reported that using both methods resulted in an overall preoperative accuracy of 77.8%. In our series of three patients with synchronous colorectal carcinoma, two were diagnosed preoperatively. In the remaining patient, the synchronous tumour was discovered intraoperatively (emergency operation for bowel obstruction).
A predisposition for multiple primary colorectal carcinomas in patients with longstanding ulcerative colitis (18%) and familial adenomatous polyposis (21%)9 is well known. A smaller risk is estimated for patients with family history of colorectal cancer10. Three patients with multiple colorectal cancer and FAP were excluded from our study. One of our patients had a family history of hereditary, non - polyposis colorectal cancer (HNPCC or Lynch syndrome), an autosomal dominant disease characterised by development of synchronous or metachronous colorectal cancers, usually at a young age. The associated genetic defect lies at the mismatch repair genes, responsible for the correction of DNA bases mismatch. The coexistence of adenomatous polyps is also considered a risk factor for the development of metachronous lesions8,11–13. In our study three out of 12 patients with multiple primary colorectal cancers had coexisting adenomatous polyps.
Several lines of evidence point out that multiple carcinomas of the large intestine are more frequent in octogenarians, whereas the first primaries occur in 60 to 70 years old patients1,9. In our series, single carcinomas were diagnosed in older patients (average 60.3 years), whereas patients with multiple carcinomas were younger (average 55.6 years old). Thus, detailed and accurate preoperative staging and intraoperative examination to identify synchronous colorectal carcinomas are also necessary in younger patients14.
As seen in our series, multiple primary colorectal carcinomas, as well as single ones, are located with a higher incidence in the rectum and sigmoid colon1,4,15.
The type of the initial operative procedure for multiple colorectal carcinomas remains controversial. Some authors propose radical operations, such as total colectomy with ileorectal anastomosis16,17, in order to remove coexisting synchronous tumours and polyps that were misdiagnosed, to prevent future development of metachronous tumours. On the other hand, others1,18–20 recommend a more conservative approach for older patients and radical procedures for younger than 60 year old patients with regionally confined non-metastatic disease, especially for those with concurrent adenomatous polyps. We agree with the second opinion because patients with colorectal carcinomas are usually old, and are characterised by increased comorbidity making them poor candidates for major surgery. Regular postoperative follow up of patients with colorectal cancer will reduce the risk of metachronous carcinoma. In accordance with our series, several studies showed no significant difference in survival between multiple and single colorectal cancers4,6.
No operation for colorectal cancer should be done without thorough evaluation of the disease characteristics in each particular patient, so as to lead to a beneficial operative strategy. The specimen should include the regional lymph nodes, in order to treat the local spread, and enough intestine length to prevent recurrence or subsequent malignant change.
References
- 1.Cunliffe WJ, Hasieton PS, Tweedie DE, Schofield PF. Incidence of synchronous and metachronous colorectal carcinoma. Br J Surg. 1984;71:941–943. doi: 10.1002/bjs.1800711210. [DOI] [PubMed] [Google Scholar]
- 2.Moertel GG, Bargen JA, Dockerty MB. Multiple carcinomas of the large intestine: a review of the literature and a study of 261 cases. Gastroenterology. 1958;34:85–98. [PubMed] [Google Scholar]
- 3.Polk HC, Jr, Spratt JS, Jr, Butcher HR., Jr Frequency of multiple primary malignant neoplasms associated with colorectal carcinoma. Am J Surg. 1965;109:71–75. doi: 10.1016/s0002-9610(65)80106-4. [DOI] [PubMed] [Google Scholar]
- 4.Kaibara N, Koga S, Jinnal D. Synchronous and metachronous malignancies of the colon and rectum in Japan with special reference to a coexisting early cancer. Cancer. 1984;54:1870–1874. doi: 10.1002/1097-0142(19841101)54:9<1870::aid-cncr2820540917>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos V, Michalopoulos A, Basdanis G, et al. Synchronous and metachronous colorectal carcinoma. Tech Coloproctol. 2004;8(Suppl 1):s97–s100. doi: 10.1007/s10151-004-0124-y. [DOI] [PubMed] [Google Scholar]
- 6.Agrez MV, Ready R, Ilstrup D, Beart RW. Metachronous colorectal malignancies. Dis Colon Rec. 1982;25:569–574. doi: 10.1007/BF02564169. [DOI] [PubMed] [Google Scholar]
- 7.Kiefer PJ, Thorson AG, Christensen MA. Metachronous colorectal cancer. Time interval to presentation of a metachronous cancer. Dis Colon Rect. 1986;29:378–382. doi: 10.1007/BF02555051. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi H, Toda T, Nagasaki S, et al. Synchronous multiple colorectal adenomas. J Surg Oncol. 1997;64:304–307. doi: 10.1002/(sici)1096-9098(199704)64:4<304::aid-jso10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Greenstein AJ, Heimann TM, Sachar DB, Slater G, Aufsen AH ., Jr A comparison of multiple synchronous colorectal cancer in ulcerative colitis, familiar polyposis colon and de novo cancer. Ann Surg. 1986;203:123–128. doi: 10.1097/00000658-198602000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinol V, Andreu M, Castells A, Paya A, Bessa X, Jover R. Synchronous colorectal neoplasms in patients with colorectal cancer: predisposing individual and familial factors. Dis Colon Rectum. 2004;47:1192–1200. doi: 10.1007/s10350-004-0562-7. [DOI] [PubMed] [Google Scholar]
- 11.Bussey HJR, Wallace MH, Morson BC. Metachronous carcinoma of the large intestine and intestinal polyps. Proc R Soc Med. 1967;60:208–210. [PMC free article] [PubMed] [Google Scholar]
- 12.Heald RJ, Bussey HJ. Clinical experiences at St Mark's Hospital with multiple synchronous cancer of the colon and rectum. Dis Colon Rectum. 1975;18:6–10. doi: 10.1007/BF02587230. [DOI] [PubMed] [Google Scholar]
- 13.Rickert RR, Auerbach D, Garfinkel L, et al. Adenomatous lesions of the large bowel and autopsy survey. Cancer. 1979;43:1845–1857. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Chen HS, Sheen-Chen SM. Synchronous and "early" metachronous colorectal adenocarcinoma: analysis of prognosis and current trends. Dis Colon Rectum. 2000;43:1093–1099. doi: 10.1007/BF02236556. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda Y, Saku M, Kawanaka H, et al. Distribution of synchronous and metachronous multiple colorectal cancers. Hepatogastroenterology. 2004;51:443–446. [PubMed] [Google Scholar]
- 16.Lillehei RC, Wangesteen OH. Bowel function after colectomy for cancer, polyps and diverticulitis. JAMA. 1955;159:163–170. doi: 10.1001/jama.1955.02960200009003. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal I, Baronofsky ID. Prognostic and therapeutic implications of polyps in metachronous colic carcinoma. JAMA. 1960;172:37–41. doi: 10.1001/jama.1960.03020010094010. [DOI] [PubMed] [Google Scholar]
- 18.Wright HK, Thomas WH, Cleveland JC. The low recurrence rate of colonic carcinoma in ileocolic anastomoses. Surg Gynecol Obstet. 1969;129:960–962. [PubMed] [Google Scholar]
- 19.Aderson BB. Synchronous cancer of the colon of a case for more definitive resection in colon cancer. J Natl Med Assoc. 1978;70:583–585. [PMC free article] [PubMed] [Google Scholar]
- 20.Tsantilas D, Ntinas A, Petras P, et al. Metachronous colorectal adenocarcinomas. Tech Coloproctol. 2004;8(Suppl1):s202–s204. doi: 10.1007/s10151-004-0157-2. [DOI] [PubMed] [Google Scholar]