Abstract
Background and aim: Hemodialysis (HD) patients are exposed to persistent inflammatory state. Erythropoietin resistance is known to be strongly associated with chronic inflammation. Aim of the study was to analyze the effect of elevated inflammatory markers on hemoglobin levels and rhEPO requirements in stable patients of our hemodialysis center.
Patients and methods: The study population consisted of 42 patients, 19F/23M, mean age 54.5±12.0 years. C-reactive protein (CRP), interleukin-6 (IL-6), hemoglobin (Hb), ferritin and left ventricular mass index (LVMi) were recorded. Group 1 consisted of 10 patients with Hb ≤ 10 g/dl, mean 8.3±1.2 g/dl and Group 2, of 10 patients with Hb ≥ 10 g/dl, mean 12.6±1.91 g/dl. None of these 20 patients had been previously treated with rhEPO. Group 3 consisted of 22 patients with mean Hb 10.1±1.5 g/dl after treatment with rhEPO.
Results: Group 1 patients had significanty higher IL-6 concentrations than Group 2 (5.21±3.94 vs 3.03±3.64, p < 0.03). Group 3, treated with rhEPO had IL-6 concentrations significantly lower compared to Group 1 (1.15±3.81 vs 3.03±3.64, p < 0.05). HD pts in Group 1 presented significantly higher CRP concentrations compated to pts of Group 2 and 3 (23.1±9.1 vs 7.02±8.7 and 7.89±9.6 respectivly, p < 0.05). A negative correlation was demonstrated between IL-6 and Hb level (r: 0.33 p < 0.05).
Conclusions: A better management of anemia might improve inflammatory state and survival in this population.
Keywords: ESRD, inflammation, anemia, rhEPO requirements
Patients with chronic renal failure (CRF) undergoing hemodialysis (HD) are exposed to persistent inflammatory state, as shown by elevated interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) plasma concentrations. Erythropoietin resistance is known to be strongly associated with chronic inflammation.
Malnutrition and inflammation are non-traditional risk factors for the increased incidence of cardiovascular disease in hemodialysis patients. Anemia is a major risk factor in patients with CRF and correction of renal anemia decreases cardiovascular mortality and co-morbidity. In patients with CRF, the compensatory mechanism that increases the generation of erythrocytes is impaired because the control of endogenous erythropoietin production in the kidney appears to be impared. Other factors causing anemia in hemodialysis petients are chronic blood loss, iron deficiency, a reduction in erythrocyte survival time and impaired erythropoiesis1–3.
Coexistence of renal and cardiac disease is associated with high morbidity and mortality. This pathophysiological condition, in which combined cardiac and renal dysfunction amplifies a progression to the failure of the individual organ, has been denoted as cardio- renal anemia syndrome. The chronic inflammatory state present in both, chronic renal failure and cardiac failure, can cause oxidative stress and renin secretion which is stimulated by cytokines3.
Aim of this study was to analyze the effects of inflammatory markers (IL-6, TNF-α and CRP) and rhEPO requirements in hemodialysis patients.
Patients and methods
The study population consisted of 42 patients, 19F/23M, age 54.5 ± 12 years, dialysis duration 59.6 ± 42 months. Patients were included last 6 months, laboratory values: CRP, IL-6, TNF-α, ferritin, intact parathyroid hormone (iPTH), calcium (Ca++), phosphate (P), albumin, hemoglobin (Hb), lipid parameters (triglyceride, total cholesterol, HDL-cholesterol, LDL- cholesterol), creatinine, and clinical findings: body mass index-BMI, systolic blood pressure (SBP), diastolic blood pressure(DBP), left ventricular mass index (LVMI) were recorded and analyzed retrospectively. Group 1 consisted of 10 patients with Hb ≤ 10g/dl (mean Hb 8.3 ± 1.2 g/dl) and Group 2 of 10 patients with Hb ≥ 10g/dl (mean Hb 12.6 ± 1.9 g/dl). None of these 20 patients had been previously treated with rhEPO. Group 3 consisted of 22 patients with mean Hb 10.1 ± 1.5 g/dl after treatment with rhEPO. These patients had been on rhEPO treatment for > four months. All patients were dialyzed with bicarbonate solution. Dialysis was performed three times weekly for 3-4 hours.
The patients were dialyzed with different membranes, (mainly cuprophane or polysulfone). Blood samples for the biochemical evaluation were drawn in the predialysis time. Serum iPTH was measured on automatic Bayer luminiscens system Model ACS180, by "two-site sandwich immuno-assay". The normal range of values considered to be 10 to 65 ng/L. Plasma concentrations of IL-6 and TNF-α were measured in duplicate by Immunotech immunoassays ( IM1120, IM11120 Beckman Coulter TM) in serum samples. This ELISA is a one immunological step sandwich type assay. Plasma concentration of hsCRP was measured by Olympus (Latex) assay on the Olympus AU400 analyzer. Serum calcium, phosphate, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides were measured by routine assay using an autoanalyzer. BMI was expressed as kg/m2. SBP and DBP were measured at the beginning of each dialysis session and their values reported as monthly mean. We used standard Doppler echo examinations to determine of left ventricular mass index (LVMI).
Statistical Analysis
Descriptive statistics are expressed as mean ± SD. Statistical analysis was performed using the Student's t test for paired data and Pearson's correlation test. Differences were considered significant when p < 0.05.
Results
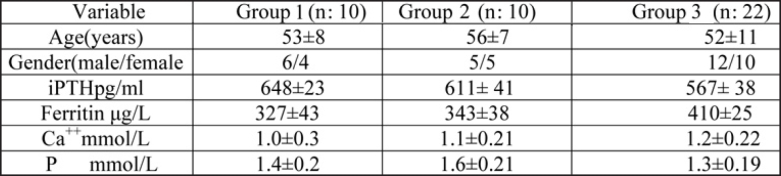
Table 1 shows baseline characteristics of the participants. There were no significant differences in regard to gender and iPTH, ferritin, Ca++, and P among the three groups.
Table 1. Baseline characteristics and some biochemical parameters of HD patients.
Values are presented as mean±SD.
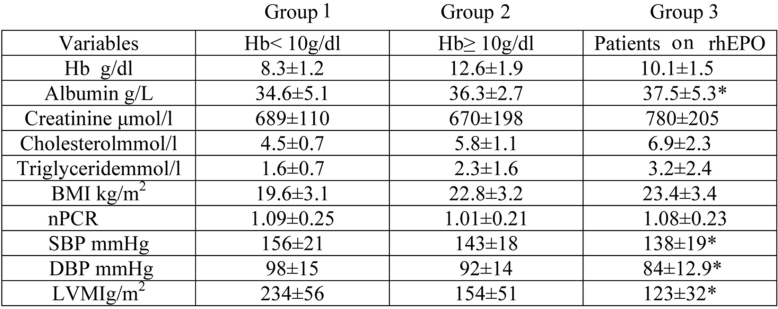
The other biochemical and cardiovascular parameters are shown in Table 2. Patients in the Group 3 had a significantly lower blood pressure (both systolic and diastolic), and LVMI compared with the patients in the Group 1. In addition, albumin level was lower in the Group 1 compared with the patients in the Group 3.
Table 2. Comparison of biochemical and nutritional parameters in hemodialysis patients.
Values are presented as mean±SD
*p < 0.05 compared with group I.
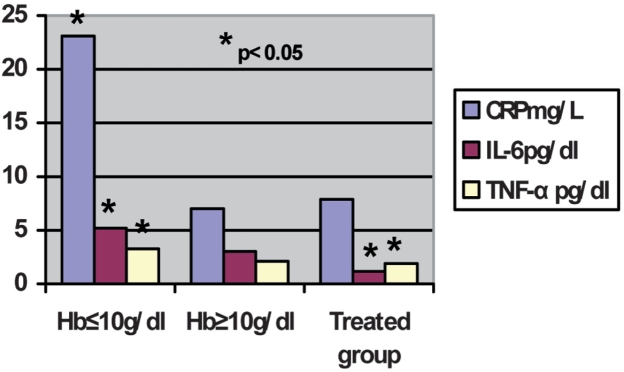
Figure 1 shows the plasma concentrations of the inflammatory markers. Plasma level of IL-6 in all patients studied was 4.25± 3.85 pg/dl. In Group 1, with Hb < 10g/dl, without rhEPO therapy, patients had an IL-6 concentration of 5.21 ± 3.94 pg/dl. This was significantly higher (p: 0.03) than in untreated patients with Hb ≥ 10g/dl (3.03 ± 3.64 pg/dl). In Group 3, treated with rhEPO patients had IL-6 plasma concentration of 1.16±3.82 pg/dl, which was significantly lower (p < 0.05) than in Group 1 (Hb < 10 g/dl) without rhEPO therapy, but not significantly different from Group 2 (Hb ≥ 10 g/dl) without rhEPO therapy. The TNF-α plasma concentration in all patients studied was 2.17 ± 1.58 pg/dl. Group 1, patients showed no difference in TNF-α plasma concentration (3.26 ± 1.63 pg/dl) in comparison with Group 2 ( 2.12 ± 2.04 pg/dl) (Figure 1). The Group 3 patients, treated with rhEPO, had an TNF-α plasma concentration of 1.89 ± 1.98 pg/dl which differed significantly from that of Group 1 (p < 0.04) but not Group 2. Comparison between the three groups showed that the hemodialysis patients with Hb < 10 g/dl had significantly higher plasma concentrations of CRP (mean 23.1 ± 9.1 mg/L) than other hemodialysis patients with Hb ≥ 10 g/dl or hemodialysis patients treated with rhEPO therapy (mean 7.02 ± 8.7 mg/L vs mean 7.89 ± 9.6 mg/L, p < 0.05).
Figure 1. Inflammatory parameters in hemodialysis patients.
An inverse correlation was also demonstrated between both, plasma concentration of IL-6 and TNF-α and Hb (r: - 0.62 p = 0.01).There was also an inverse correlation between LVMI (r: - 0.33 p < 0.05) and Hb level.
Discussion
The present data supports the hypothesis that inflammation is presented in the majority of HD patients. This study confirms that inflammation is associated with an increased LVMI and decreased Hb level in ESRD patients. We have also shown that the patients with higher inflammatory markers did differ significantly for nutritional (albumin level) and cardiovascular parameters (SBP, DBP and LVMI). Patients with higher levels of inflammatory markers had lower Hb level.
Several studies demonstrated protection against inflammation by Epo administration. Animal studies showed that Epo administration decreased the infiltration of inflammatory cells after spinal cord compression5. Several clinical studies have demonstrated the protective effects of Epo on cardiac function6. Regression of left ventricular hypertrophy in anemic CRF patients treated with Epo has been observed7.
It is evident that most of the classical risk factors associated with a tendency to atherosclerotic disease in the general population also play a role in HD patients. Two factors (malnutrition and inflammation) were identified in many clinical studies. It is well recognized that malnutrition and hypoalbuminemia are important predictive factors of mortality in patients with ESRD. In addition, high concentrations of the acute-phase protein CRP are strongly associated with death within 1 year in pre-dialysis patients and in dialysis as well8,9. Left ventricular hypertrophy is a major cardiovascular complication and an important predictor of mortality in ESRD patients. Inflammatory markers and LVMI are interrelated and combine adversely to enhance the mortality and cardiovascular death risk of ESRD patients10,11.
Nutritional parameters were better in group with rhEPO therapy. Similar results have been reported by other investigators. Albumin, a classical marker of nutritional status, also reflects systemic inflammation. Our results show that patients with havier anemia had higher levels of IL-6 and TNF-α. Those patients were malnourished and their nutritional parameters were bad. High blood pressure and cardiovascular complications were frequently seen in malnourished HD patients12–15.
It follows that a substantial part of the inflammatory state is due to renal anemia. Total correction of anemia led to significant decreases in the plasma concentrations of the inflammatory cytokines, IL-6 and TNF-α. Gouva et al showed that treating anemia in early renal failure stage slows progression of renal failure16.
It is now known that chronic inflammation increases the risk of premature atherosclerosis and cardiovascular disease in patients with CKD4. Correction of anemia in HD patients and some nutritional parameters improve inflammatory state and decrease plasma concentration of inflammatory cytokines and other parameters which contribute of cardiovascular disease9,16–18.
The present study suggests that the increase of inflammation in HD patients could be associated with anemia, where the nutritional status plays a role as one of the components of cardiorenal anemia syndrome.
Anemia and epoetin therapy is influenced by a variety of factors such as inflammatory markers, nutritional status, and cardiovascular parameters. Patients who had higher plasma levels of inflammatory markers showed lower hemoglobin levels than those with lower level of inflammation11,12.
Moreover, elevated inflammatory cytokines and acutephase proteins, resulting from malnutrition-inflammation complex, may contribute to the maintenance of anemia in hemodialysis patients. Malnutrition and inflammation may also limit erythropoesis in patients receiving rhEPO for the correction of anemia. In conclusion, therapy with rhEPO has led to improvements in cardiovascular function and reduced LVMI in HD patients.
References
- 1.Hampl H, Riedel E, Scigalla P, Stabell U, Wendel G. Erythropoiesis and erythrocyte age distribution in hemodialysis patients undergoing erythropoietin therapy. Blood Purif. 1990;8:117–125. doi: 10.1159/000169954. [DOI] [PubMed] [Google Scholar]
- 2.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 3.Sommerburg O, Grune T, Hampl H, Riedel E, Ehrich JH, Siems WG. Does treatment of renal anemia with recombinant erythropietin influence oxidative stress in hemodialysis patients? Clin Nephrol. 2000;53:S23–S29. [PubMed] [Google Scholar]
- 4.Jie KE, Verhaar MC, Cramer MJ, et al. Erythropoetin and the cardiorenal syndrome: cellular mechanisms on the cardiorenal connectors. Am J Physiol Renal Physiol. 2006;291:F932–F944. doi: 10.1152/ajprenal.00200.2006. [DOI] [PubMed] [Google Scholar]
- 5.Gorio A, Gokmen N, Erbayraktar S, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci USA. 2002;99:9450–9455. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van der Meer P, Voors AA, Lipsic E, Smilde TD, van Gilst WH, van Veldhuisen DJ. Prognostic value of plasma erythropoietin on mortality in patients with chronic heart failure. J Am Coll Cardiol. 2004;44:63–67. doi: 10.1016/j.jacc.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 7.Portoles J, Torralbo A, Martin P, Rodrigo J, Herrero JA, Barrientos A. Cardiovascular effects of recombinant human erythropoietin in predialysis patients. Am J Kidney Dis. 1997;29:541–548. doi: 10.1016/s0272-6386(97)90335-8. [DOI] [PubMed] [Google Scholar]
- 8.Stenvinkel P, Heimburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 10.Amann K, Rychlik I, Miltenberger-Milteny G, Ritz E. Left ventricular hyperthrophy in renal failure. Kidney Int. 1998;68(Suppl):S78–S85. doi: 10.1046/j.1523-1755.1998.06818.x. [DOI] [PubMed] [Google Scholar]
- 11.Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 12.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17:432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 13.Stenvinkel P, Barany P, Heimburger O, Pecoits-Filho R, Lindholm B. Mortality, malnutrition, and atherosclerosis in ESRD: what is the role of interleukin-6? Kidney Int Suppl. 2002;80:103–108. doi: 10.1046/j.1523-1755.61.s80.19.x. [DOI] [PubMed] [Google Scholar]
- 14.Stenvinkel P, Alvestrand A. Inflammation in end-stage renal disease: sources, consenquences, and therapy. Semin Dial. 2002;15:329–337. doi: 10.1046/j.1525-139x.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 15.Rasic-Milutinovic Z, Perunicic G, Pljesa S, Gluvic Z, Ilic M, Stokic E. Metabolic syndrome in HD patients: association with body composition, nutritional status, inflammation and serum iron. Intern Med. 2007;46:945–951. doi: 10.2169/internalmedicine.46.0092. [DOI] [PubMed] [Google Scholar]
- 16.Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int. 2004;66:753–760. doi: 10.1111/j.1523-1755.2004.00797.x. [DOI] [PubMed] [Google Scholar]
- 17.Perunicic-Pekovic GB, Rasic ZR, Pljesa SI, et al. Effect of n-3 fatty acids on nutritional status and inflammatory markers in haemodialysis patients. Nephrology. 2007;12:331–336. doi: 10.1111/j.1440-1797.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 18.Perunicic-Pekovic GB, Pljesa SI, Rasic ZR, et al. Inflammatory cytokines and malnutrition as related to risk for coronary heart disease in hemodialysis patients. Bantao Journal. 2007;5:13–15. doi: 10.1139/Y08-018. [DOI] [PubMed] [Google Scholar]