Abstract
Context
Medication treatment of alcoholism is presently not particularly robust. Neuroimaging techniques might predict which medications could be useful in the treatment of alcohol dependence.
Objective
To explore the effect of naltrexone, ondansetron, or the combination of these medications on cue-induced craving and ventral striatum activation.
Design, Setting, Participants
Functional brain imaging (Phillips 1.5T scanner) was conducted during alcohol cue presentation in 90 non-treatment seeking alcohol-dependent (by DSM-IV criteria) and 17 social drinking (less than 14 drinks per week) paid volunteers recruited through advertisements at an academic center.
Interventions
A taste of alcohol and a series of alcohol related pictures, neutral beverage pictures and visual control images were provided to volunteers after seven days of double blind randomly assigned daily dosing with 50mg naltrexone (n=23), 0.50mg ondansetron (n=23), the combination of the two medications (n=20), or matching placebos (n=24).
Main Outcome Measures
Difference in brain blood oxygen level-dependent (BOLD) magnetic resonance when viewing alcohol pictures versus neutral beverage pictures with a particular focus on ventral striatum activity comparison across medication groups. Self-ratings of alcohol craving.
Results
The combination treatment decreased craving for alcohol. Naltrexone with (p=0.02) or without (p=0.049) ondansetron decreased alcohol cue-induced activation of the ventral striatum. Ondansetron by itself was similar to naltrexone and the combination in the overall analysis but intermediate in a regions specific analysis.
Conclusions
Consistent with animal data suggesting that both naltrexone and ondansetron reduce alcohol-stimulated dopamine output in the ventral striatum, the current study found evidence that these medications, alone or in combination, could decrease alcohol cue-induced activation of the ventral striatum, consistent with their putative treatment efficacy.
INTRODUCTION
There is considerable data supporting the use of the opiate antagonist naltrexone in the treatment of alcohol dependence.1-3 Naltrexone is FDA-approved for the treatment of alcoholism and has been shown to either reduce the priming effect or reward (stimulation) effect of alcohol.4-8 Also, in clinical treatment studies, naltrexone has been found to enhance abstinence 9 to reduce drinks per drinking day, 1-2 to reduce craving 3 and to enhance resistance (reduce urge and impulse) to drink.1,10 Unfortunately, not all studies with naltrexone have been positive. 11-12 In addition, a meta-analysis of 27 randomized controlled trials of naltrexone reported that while short-term treatment with naltrexone decreased relapse, the number of patients needed to be treated in order to achieve a better outcome over placebo response was 7.13 This number needed to treat for a positive effect of naltrexone over placebo was recently confirmed in a large multi-site trail, the COMBINE Study.14 This evidence suggests that not everyone responds to treatment with naltrexone. It is not clear whether naltrexone works by blocking cue-induced reinforcement as suggested by some animal 15-16 and human 17-18 studies, or if it works primarily on blocking alcohol's pharmacological reward properties. 4, 19-21
The relative lack of robust data in regards to the medication treatment of alcohol dependence has led to the idea of combining medications to improve treatment outcomes. The rationale is to utilize medications that target multiple neurotransmitter systems thought to be involved in alcoholism. One such study was the above-mentioned COMBINE Study in which naltrexone alone, acamprosate alone, or the combination of the two medications was evaluated.14 Unfortunately, while there was no increased efficacy from the combination of the medications in this study, at least one smaller study suggested efficacy of combined naltrexone/acamprosate. 22
While not FDA-approved, there is evidence for the potential clinical utility of 5HT3 antagonist drugs in the treatment of alcoholism. 23-24 Ondansetron is a 5HT3 antagonist that has been found to have potential clinical utility in terms of animal studies 25 and human clinical laboratory paradigms. 24,26 In clinical trials, Sellers et al. 27 reported a greater reduction in drinks per drinking day in a subgroup of subjects treated with low dose ondansetron (0.25mg BID) compared to placebo or high dose ondansetron (2.0mg BID) and Johnson et al. 28 found that ondansetron (4 ug/kg) reduced drinks per drinking day and increased abstinent days in early onset alcoholics but not in late onset alcoholics.
Secondary to the possible synergistic mechanisms on decreasing alcohol use, the combination of naltrexone and ondansetron has been studied in preclinical and clinical studies. Both rats and mice evaluated in a limited access paradigm had a greater reduction in alcohol intake when both medications were given together versus either medication alone. 29 In addition, an 8-week study in 20 early onset alcoholics showed a significant difference in drinks per day between subjects who received naltrexone in combination with ondansetron and those who received placebo. 30
It has been thought that human alcohol-cue based laboratory paradigms might provide useful transitional data between animal laboratory support for potential alcohol treatment medications and clinical trials. 4,19,21 However, the study of medication effects on alcohol-cue response in the clinical laboratory is difficult secondary to the variability of subjective response (e.g. craving) to cues, and the variability in objective peripheral measures of autonomic arousal and response such as heart rate changes, salivary output, etc. 31 In addition, these subjective and peripheral measures provide more distal and only correlative information as to what might be happening in the brain of the addicted/dependent individual. This has lead us and others to seek a more proximal brain signal of alcohol-cue induced urges to drink and reward salience in which to explore medication effects as a potential predictor of treatment utility.
Several brain-imaging technologies have been refined and applied to the study of brain activation during presentation of drug-related cues. Recent studies have indicated that similar findings may be emerging during the presentation of alcohol cues.32-37 While imaging studies have begun to shed light on the areas of the brain involved in alcohol craving, data regarding the impact of drug treatments on these structures are lacking.
An area of cue-stimulated activation noted by our group has been the ventral striatum. 35 The mesolimbic dopamine pathway that projects from the ventral tegmental area (VTA) to a structure within the ventral striatum, the nucleus accumbens (Nac), has been implicated as a major site for the reinforcing actions of many addictive drugs including ethanol 38-42 and that naltrexone has been shown to block this effect. 15-16,43 Therefore, the goal of the current study was to 1) replicate our previous findings that alcoholics have differential brain activation to alcohol cues compared to social drinkers, especially in the ventral striatum and 2) explore, in a double-blind, placebo-controlled fashion, the effect of naltrexone, ondansetron, or the combination of the medications on cue-induced craving and ventral striatum activation. A priori hypotheses were that participants treated with naltrexone or ondansetron would have lower ventral striatum activation to alcohol cues compared to placebo-treated participants and that the combination of naltrexone and ondansetron would have a greater reduction in cue-induced craving and ventral striatum activation as compared to participants treated with naltrexone or ondansetron alone.
METHODS
Participants
Non-treatment seeking individuals (n=125) meeting criteria for alcohol dependence participated in a larger protocol that included a limited-access, bar-lab paradigm whose general methods have been previously described.4 From this larger study, 100 participants agreed to take part in a brain imaging study. Of these 100 participants, 10 subjects were excluded: head movement (2), artifact (1), mechanical problems (1), incomplete craving ratings in the scanner (5) or a positive pre-scan breath alcohol level (1). Therefore, 90 non-treatment seeking individuals were evaluable in the analysis. Non-treatment seeking alcoholics, after baseline evaluation, were assigned through urn randomization (using a double dummy placebo controlled design) to one of four experimental groups: naltrexone, ondansetron, naltrexone and ondansetron or placebo. Participants received study drugs for 8 days (days 1−5 being a natural observation period). On day 7, after a minimum of 24 hours of abstinence, participants in the current study underwent a functional MRI brain scan with cue stimulation. The bar-lab study took place on day 8. A smaller group of social drinker controls (N=17) who were recruited and randomly assigned to the same medication groups and protocol were used as procedure controls as a comparison/contrast group for the brain imaging sub-study
Potential participants, recruited through newspaper and community ads based on drinking at least 20 drinks per week, were told that the study was investigating effects of medications that may have beneficial effects for alcoholics in treatment. All participants met DSM-IV criteria for alcohol dependence (APA, 1994), including loss of control drinking or an inability to cut down or quit, but they denied any active involvement in, or desire for, alcohol treatment. Exclusion criteria for all participants were as follows: current DSM-IV criteria for drug dependence by verbal report and urine drug screens, other major DSM-IV Axis I disorders, psychoactive medication or substance use (except marijuana) in the past 30 days or a positive urine drug screen, current suicidal or homicidal ideation, past history of alcohol-related medical illness, liver enzymes ≥ 2.5 times above normal, or significant health problems. Participants who smoked greater than 10 cigarettes per day were also excluded. All participants were screened for DSM-IV criteria using the entire Structured Clinical Interview for all the DSM-IV Axis 1 Disorders (SCID). 44
Procedures
Upon arrival for the first session, the study was described in detail to the participant and Informed Consent was obtained using a form and procedures approved by the Investigational Review Board at our institution. Each participant was then evaluated with a number of standard interview, questionnaire, and medical diagnostic procedures similar to those in other studies reported by our group. 4,19 Interview procedures included a demographic form, the alcohol and drug section of the SCID administered by a trained physician, and a timeline follow-back interview to quantify drinking during the preceding 90 days.45 The Obsessive-Compulsive Drinking Scale (OCDS) 46, the Self-Administered Alcohol Screening Test (SAAST) 47, and the Alcohol Dependence Scale (ADS)48 were administered. Finally, a urine specimen was collected to screen for abused drugs, and a blood sample collected for liver function and general health screening. Additional assessments were conducted at a second session (conducted within one week of the first session) including psychiatric sections of the SCID. In addition, a physical exam was conducted by a physician assistant and reviewed by a physician.
Participants who passed all screening and eligibility criteria were randomly assigned to receive naltrexone 50mg, ondansetron 0.25mg BID, naltrexone 50mg and ondansetron 0.25mg BID or matching placebos. The medication regimen was for eight days. Medication ingestion was witnessed on Days 1, 6, and 7 by research staff. All medications, including inactive placebo, were blister packed and administered in standard gel caps with 25 mg riboflavin added to assess for compliance via a laboratory based urinary fluorescence assay. Urine samples were obtained and assessed for riboflavin at baseline and Day 7. Samples showing greater than 1500 ng/ml of riboflavin were considered compliant. 49
Participants were given no explicit instructions regarding use of alcohol or modification of their drinking behavior for Days 1 − 5. However, they were required to abstain completely from drinking on Day 6 and Day 7. On Day 6, several assessments were completed. Participants were clinically evaluated for alcohol withdrawal using the Clinical Institute Withdrawal Assessment for Alcohol - Revised (CIWA-Ar).50 A 6-Day version of the timeline follow-back interview (in which they reported their alcohol consumption since the outset of the medication period) was also completed. The symptom checklist and the OCDS were repeated. Participants were instructed to return the next day for the imaging session.
The cue-induced MRI scanning procedures are similar to those used in prior work by our group. 35 Briefly, on the day of the imaging session, participants completed assessment questionnaires (TLFB, OCDS, CIWA-Ar), were breathalyzed, and a rapid urine drug screen obtained. No participant had evidence of alcohol use or a positive urine drug screen prior to the imaging session. They were then fitted with 3-D MR compatible goggles (Magnetic Resonance Technology, Northridge CA) and a 2-D trackball was placed under their dominant hand. After positioning in the scanner, participants were checked to ensure that they could view the cues comfortably while wearing the goggles and were trained to rate their “urge to consume alcohol” by moving the track-ball along a 100 mm analog scale anchored on one end as “not at all” and the other as “maximum possible”. During initial scanner tuning and structural scanning (T1 weighted 3-D volume and T1-weighted structural scan in the functional scan plane), participants were shown relaxation pictures. They were given a sip of preferred spirits in non-carbonated juice through a straw placed in their mouth, and then shown the 12 min 48 sec of alternating stimuli with BOLD image acquisition. Subjects self-rated their craving in real-time (after each picture block) using the track-ball during the picture viewing and brain image acquisition. After the imaging session, they were escorted out of the scanner room, rinsed their mouths with water, and given a breathalyzer test (the sip of alcohol does not produce measurable breath alcohol readings). They were then given instructions not to drink that evening, reminded of the next day's breathalyzer measurement and experiment and sent home, either with a friend or family member or by taxi.
Alcohol Cues
These cue-induced MRI scanning procedures are similar to those used in our prior work. 35 Briefly, alcohol and non-alcohol beverage picture cues were selected primarily from the Normative Appetitive Picture System (NAPS n=38) but were supplemented with 22 additional cues selected from advertisements to avoid repeating the same stimuli during the scanning sequence. Visual control pictures match the alcohol cues in color and hue but lack any object recognition. A sequence for stimulus presentation has been created consisting of six, 90-second, epochs. Each epoch contains three 24-second blocks (1 block each of alcohol, non-alcohol beverage and visual control pictures) and one 24-second rest (cross-hair). Each 24-second block is made up of 5 individual pictures, each displayed for approximately 4.8 seconds. The alcohol blocks are specific to a beverage type (beer, wine or liquor), with two blocks per type. In order to control for time and order effects across subjects, the order of the individual pictures, the blocks within the epoch, and the epochs are all randomly presented. After each 24 second block, subjects were asked to rate their “urge to consume alcohol”.
MRI Image Acquisition
Participants wore earplugs and head movement was restricted using cushions surrounding the head. MRI scans were performed in a Philips 1.5 T MR scanner with actively shielded magnet and high-performance gradients (27 mT/m, 72 T/m-sec). An initial high resolution 142 slice 1 mm thick sagittal T1 weighted scan was obtained for later volumetric and co-registration analysis and to ensure there is was no significant anatomical brain pathology. A structural scan was then obtained consisting of 25 coplanar coronal slices (5 mm thick/0 mm gap) covering the entire brain and positioned using a sagittal scout image. Following another manual tuning for echoplanar imaging, the cue-induction paradigm was performed while also acquiring Blood Oxygen Level Dependent (BOLD) weighted coronal scans in the exact plane as before using a gradient echo, echo-planar (EPI) fMRI sequence (tip angle=90°, TE=27.0 ms, TR=3000ms, FOV=27.0 cm, 25, 5 mm thick, slices, gap = 0.0 mm, with frequency selective fat suppression).
Statistical Analysis
Baseline Characteristics
Analyses of baseline drinking and demographics were performed with either ANOVA (continuous variables) or Chi-square (categorical variables).
fMRI Data Analyses
MR scans were transferred into ANALYZE format and then further processed on Sun workstations (Sun Microsystems, Palo Alto, CA), using Matlab 6.1 (Mathworks, Sherborn, MA) with Statistical Parametric Mapping software 2 (SPM2, The Wellcome Department of Cognitive Neurology, London, http://www.fil.ion.bpmf.ac.uk). Default settings were used unless indicated otherwise. All volumes were realigned to the first volume. After realignment (including the adjustment for sampling errors), for all subjects, movement across the entire scan was less than 1 mm in 3 axes and less than 1 degree in 3 orientations. Then, the images were stereotactically normalized into a standard space with a resolution of 3×3×3 mm voxels using the averaged functional EPI image -the Montreal Neurological Institute (MNI) EPI template in SPM2. Subsequently, the data were smoothed with an anisotropic 8×8×8 mm Gaussian kernel and high-pass filtered (cut-off period=240s). This first level of statistical analysis used a boxcar function convolved with the modeled hemodynamic response function as the basic function for the general linear model. Contrast-maps were obtained of the difference between alcohol minus beverage, alcohol minus visual control, alcohol minus rest, beverage minus visual control, and visual control minus rest for each patient individually with the six head movement parameters included as covariates. The subject-specific contrasts were then entered into a second-level analysis, to obtain a random effect analysis of activation effects in the entire group. The combined group t maps were thresholded using puncorrected ≤ .001 and cluster statistical weight (spatial extent threshold) of 15 voxels.
The fMRI data were analyzed without knowledge of specific medication group assignment. The individual data were divided into 5 groups (corresponding to 4 medication and one social drinker group without specific identification of treatments applied). In order to identify activity in the ventral striatum among all subjects, conjunction analysis51 was preformed with the Multiple Regression (no constant term) in Basic Models. The voxel location of the highest t valve (uncorrected p < .001) was used to create a mask for time course extraction. A small volume of 6 mm radius spherical regions of interest (ROI) was used to create a mask within the ventral striatum that was centered at the location (MNI coordinates) of the right nucleus accumbens (9, 6, −8). With the mask, averaged time courses of multi voxels were generated from each individual data.
Specific Effects of Medication on Alcohol Cue Induced Ventral Striatum Activation
Analysis of the ventral striatum data was performed as a three-level hierarchical linear regression (HLM 6.04) with time (level 1) nested within condition (level2) nested within subject (level 3). Level 2 predictors, the dummy coded variables representing the contrasts of beverage relative to each of the other conditions (cross hair, visual control, neutral pictures), were analyzed as random variates. Level three predictors were those distinguishing between subjects, i.e. drug group and any constant, subject-level covariate, e.g. age. This a priori defined analysis focused on the differential effects of each of the between-subject variables (different medication conditions) on the difference in ventral striatum activation generated during neutral-beverage versus alcohol visual stimuli (the dependent variable of interest). The medication conditions were represented with indicator coded dummy variables using the placebo group as a reference. The analysis further allowed overall tests of whether the beverage to alcohol ventral striatum activation difference varied across the sequential alcohol picture blocks of the experimental protocol (block by alcohol interaction).
Although preliminary analyses revealed a modest increase in ventral striatum activation (bold response) across blocks (time) (t(98)=2.2,p=.03), this was the same for all picture stimuli contrasts (p's for all block by condition interactions>.65) and neither the block (time) effect nor interactions varied with medication group (all p's > .25). Furthermore, in no case was the pattern of significant contrast effects altered by the inclusion of any block (time) related variables. In summary, although there was individual variation in low frequency drift, the group relationships stayed constant across the experimental session and are reported below.
Craving Analysis
Analysis of craving scores was performed in a hierarchical linear regression similar to that used for ventral striatum activation, but was only a two level model, stimulus condition nested within subject. The primary analysis was directed at estimating the difference in craving during the beverage versus alcohol conditions. Estimates of the alcohol minus beverage contrast for ventral striatum activation were calculated from the Bayesian residuals of the overall hierarchical model and were compared (using standard regression techniques) with the overall craving experienced by the participants while in the scanner.
RESULTS
Demographics and Subjective Ratings
As can be seen in Table 1, there were no significant baseline differences in demographics or alcohol use parameters between the medication groups. However, there was, as designed, a significant difference between the medication groups and social drinker groups in drinking parameters (p<0.01). There was no evidence of alcohol withdrawal symptoms in any group as the CIWA-Ar scores were zero. In addition, urine drug screens obtained prior to the scanning session were negative. Medication compliance in all groups was greater than 90% by urinary riboflavin and pill counts. Two items in the symptom check-list discriminated between the treatment groups. “Nausea-vomiting” and “dizziness” were significantly higher in participants receiving naltrexone than in subjects receiving ondansetron alone or placebo (overall group effect: χ2 (3)=16.9, p=.001;and χ2 (3)=12.1, p=.007 for nausea and dizziness, respectively). Both were largely absent in the ondansetron alone group (2 of 23 and 0 of 23 for the two side-effects respectively) and the incidence did not differ from placebo (p>.5). Neither significantly predicted ventral striatum activation when entered as a covariate (t(90)=1.15, p=.25 and t(90)=1.4 p=.17) or significantly changed the group relationship in the analysis.
Table 1.
Demographics and Drinking History
| |
Naltrexone N=23 |
Ondansetron N=23 |
Nal + Ond N=20 |
Placebo N=24 |
Control N=17 |
|---|---|---|---|---|---|
| Age | 27.22 ±8.84 | 25.13 ±6.88 | 26.15 ±8.82 | 24.75 ±5.74 | 25.18 ±4.00 |
| Gender (%Male) | 74% | 65% | 70% | 75% | 82% |
| Race (%Caucasian) | 87% | 96% | 85% | 88% | 94% |
| Education (yrs.) | 14.65 ±1.87 | 14.43 ±1.47 | 15.40 ±1.14 | 13.25 ±2.07 | 16.24 ±1.30 |
| Drinks/Drinking Day | 11.33 ±6.39 | 9.64 ±5.43 | 8.31 ±3.68 | 9.19 ±3.90 | 3.18 ±1.82 |
| Drinks/Past Month | 205.92 ±88.78 | 227.48 ±76.95 | 202.26 ±88.69 | 196.74 ±84.67 | 24.96 ±18.35 |
| OCDS Total | 17.52 ±6.70 | 18.64 ±6.43 | 16.65 ±5.74 | 19.42 ±7.42 | 2.94 ±1.89 |
| SAAST Total | 3.22 ±1.24 | 3.52 ±1.24 | 3.50 ±1.47 | 3.48 ±1.72 | 2.13 ±0.04 |
| ADS Total | 14.09 ±6.39 | 13.70 ±6.95 | 14.05 ±5.36 | 16.10 ±6.95 | 1.57 ±2.21 |
Differences between treatment conditions were non-significant. Drinking history and drinking measure (OCDS, SAAST, ADS) scores in the control group were significantly different than treatment groups (p<.01).
Craving
As can be seen in Figure 1, there were significant differences between the groups with regards to the craving ratings during visual presentation within the scanner. As expected, social drinkers had reduced craving as compared to the placebo-treated non-treatment seeking alcoholics (p=.001). Non-treatment seeking alcoholics treated with the combination of naltrexone/ondansetron had significantly reduced craving while viewing the alcohol pictures within the scanner as compared to participants treated with placebo (p=.035) There were no significant differences in craving scores between the placebo and naltexone groups (p=.2) or placebo and ondansetron groups (p=.56).
Figure 1.
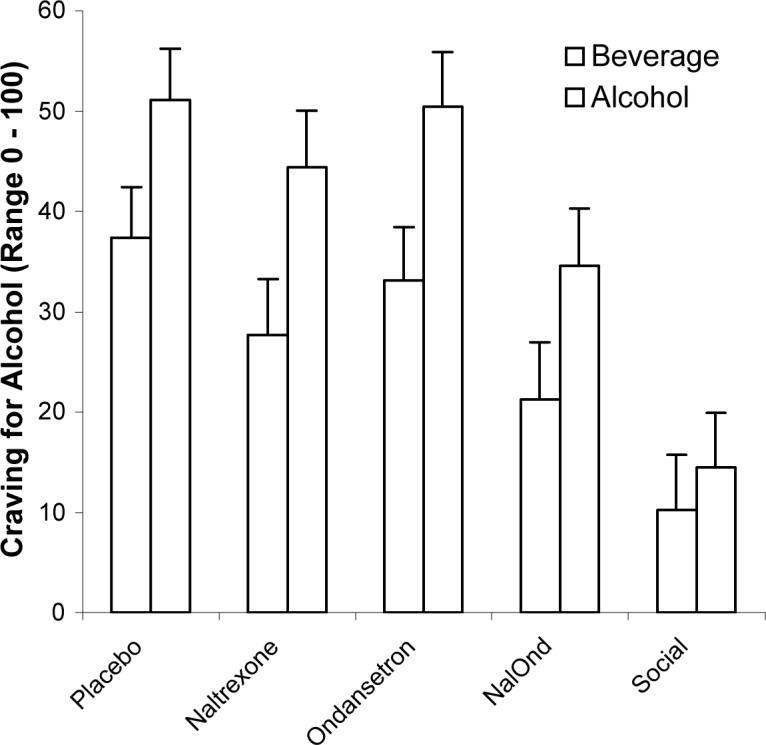
Subjective craving for alcohol and beverage cues were rated within the scanner. Subjects treated with the combination of naltrexone/ondansetron had significantly less craving for alcohol as compared to placebo-treated subjects (p=.035). In addition, social drinking controls had less craving for alcohol as compared to placebo-treated subjects (p=.001).
Comparison of Alcohol Cues with Beverage Cues
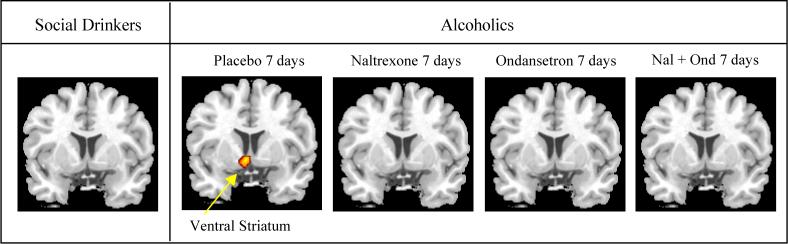
The brain areas that significantly activated within each group during the comparison of alcohol cues and beverage cues by SPM2 analysis are summarized in Table 2 and depicted in Figure 2. Consistent with our previous study, 35 the placebo-treated non-treatment seeking alcoholics had activation in prefrontal and limbic regions, areas not activated in social drinkers. Confirming our a priori hypothesis, alcoholics treated with naltrexone, either alone or in combination with ondansetron, did not experience the ventral striatum activation seen in the placebo-treated alcoholics. Additionally, ventral striatum activation was not detected in the ondansetron-treated alcoholics in this analysis.
Table 2.
Brain Areas Activated by Comparison
|
Alcohol-Beverage Comparison |
|
|
|
|
|
|
|---|---|---|---|---|---|---|
| Group | Side | X | Y | Z | t score | Regions |
| Naltrexone | L | −8 | 43 | 53 | 4.44 | superior medial frontal |
| R | 3 | −92 | 16 | 5.27 | cuneus | |
| R | 4 | −95 | 12 | 7.83 | calcarine | |
| Ondansetron | R | 29 | −88 | 24 | 4.29 | occipital |
| R | 8 | −68 | 3 | 4.07 | lingual | |
| Nal + Ond | R | 5 | 62 | 31 | 6.05 | superior medial frontal |
| L | −1 | 26 | 31 | 4.37 | ant-cingular | |
| L | −45 | −77 | 24 | 5.08 | middle occipital | |
| R | 34 | −81 | 27 | 4.22 | middle occipital | |
| R | 4 | −91 | 21 | 4.51 | cuneus | |
| L | −1 | −61 | 17 | 8.01 | calcarine | |
| L | −39 | 27 | −14 | 6.54 | inferior orbital frontal | |
| R | 3 | −79 | −16 | 4.43 | cerebelum | |
| Placebo | L | −18 | 41 | 40 | 5.49 | superior frontal |
| L | −2 | −37 | 35 | 4.56 | mid-cingulate | |
| R | 1 | 90 | 20 | 5.29 | cuneus | |
| L | −3 | −57 | 16 | 4.93 | calcarine | |
| R | 5 | 63 | 18 | 5.53 | superior medial frontal | |
| R | 17 | −18 | 1 | 4.13 | thalamus | |
| R | 1 | 59 | −3 | 4.03 | med-frontal | |
| R | 8 | 7 | −8 | 5.01 | ventral striatum | |
| L | −21 | −23 | −14 | 4.25 | parahippocampus | |
| R | 14 | 69 | −15 | 4.93 | cerebelum | |
| R | 29 | −42 | −18 | 4.77 | fusiform | |
| Social Drinking | L | −7 | −99 | 9 | 3.99 | calcarine |
| Alcohol-Visual Control Comparison | ||||||
|---|---|---|---|---|---|---|
| Naltrexone | L | −9 | 63 | 30 | 4.26 | superior medial frontal |
| R | 4 | −94 | 7 | 4.96 | calcarine | |
| L | −24 | −93 | −15 | 7.52 | lingual | |
| R | 25 | −78 | −18 | 5.85 | cerebelum | |
| Ondansetron | L | −13 | 39 | 51 | 4.87 | superior frontal |
| L | −25 | −76 | 43 | 4.23 | occipital | |
| R | 1 | 3 | 35 | 4.17 | anti-cingular | |
| L | −7 | 60 | 32 | 4.15 | superior medial frontal | |
| R | 32 | −90 | 20 | 5.52 | occipital | |
| L | −1 | −69 | 12 | 6.44 | calcarine | |
| R | 23 | −25 | −11 | 4.91 | hippocampus | |
| L | −23 | −32 | −12 | 4.87 | parahippocampus | |
| Nal + Ond | L | −10 | 50 | 46 | 4.64 | superior medial frontal |
| R | 17 | 48 | 45 | 4.39 | superior frontal | |
| R | 11 | −94 | 20 | 4.72 | occipital | |
| R | 2 | −91 | 7 | 6.42 | calcarine | |
| L | −23 | −28 | −10 | 4.51 | hippocampus | |
| Placebo | L | −2 | 36 | 51 | 5.51 | superior medial frontal |
| L | −20 | −81 | 45 | 4.76 | cuneus | |
| L | −17 | 47 | 38 | 4.67 | superior frontal | |
| R | 1 | −91 | 17 | 4.94 | cuneus | |
| L | −8 | 53 | 9 | 4.31 | anti-cingular | |
| L | −15 | 4 | 3 | 5.07 | pallidum | |
| R | 1 | −82 | −2 | 7.65 | calcarine | |
| R | 9 | 6 | −8 | 4.78 | ventral striatum | |
| L | −26 | −29 | −12 | 4.49 | parahippocampus | |
| R | 25 | −30 | −14 | 4.67 | parahippocampus | |
| L | −35 | 30 | −18 | 4.85 | inferior orbital frontal | |
| R | 39 | −69 | −22 | 5.25 | cerebelum | |
| L | −31 | −49 | −22 | 5.46 | cerebelum | |
| Social Drinking | L | −19 | −100 | −10 | 4.16 | lingual |
| Beverage-Visual Control Comparison | ||||||
|---|---|---|---|---|---|---|
| Naltrexone | No activation | |||||
| Ondansetron | R | 17 | −18 | 70 | 5.65 | precentral gyrus |
| Nal + Ond | R | 28 | −96 | −4 | 4.56 | occipital |
| L | −15 | −101 | −8 | 5.83 | lingual | |
| Placebo | L | −14 | −100 | −10 | 5.41 | lingual |
| R | 27 | −82 | −13 | 4.4 | lingual | |
| R | 30 | −82 | −20 | 4.82 | cerebelum | |
| Social Drinking | L | 24 | −69 | −19 | 6.09 | cerebelum |
| R | 21 | −30 | −12 | 4.02 | parahippocampus | |
| L | −21 | −95 | −10 | 5.56 | lingual | |
| R | 18 | −93 | −6 | 7.68 | occipital |
Figure 2.
Brain regions with significantly increased activation in one task (alcohol) compared with another (beverage) are depicted in color on coronal structural magnetic resonance imaging scans (p ≤.001).
Comparisons of Other Cues
The brain areas significantly activated during the comparison of alcohol cues with visual control cues are summarized in Table 2. There was quite a bit of similarity to areas activated in the Alcohol Cue/Beverage Cue comparison in non-treatment seeking alcoholics and different from social drinkers who had minimal salient alcohol-cue activations.
Ventral Striatum Activation
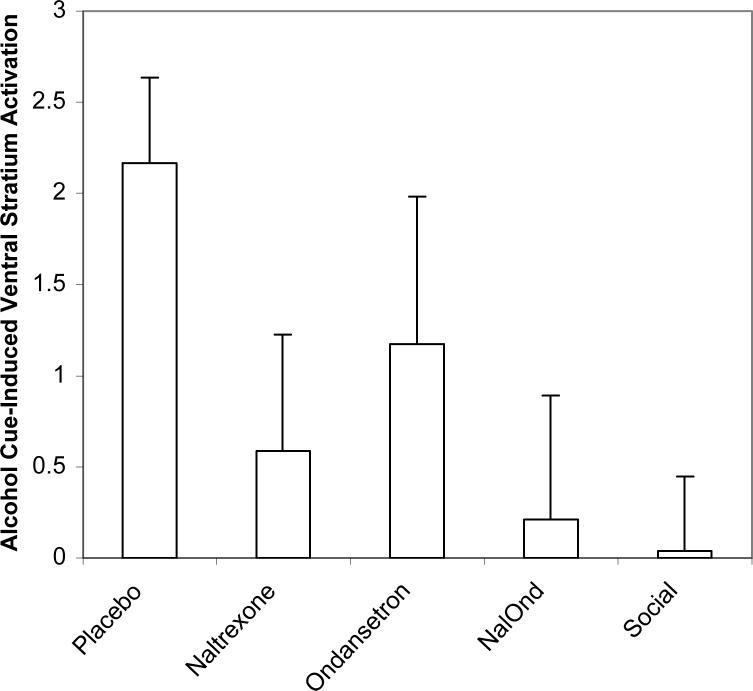
Activation in the ventral striatum as defined by the ROI HLM analysis in the various medication and social drinking control participants is shown in Figure 3. There was a significant difference between the placebo-treated alcoholics and social drinkers (p=.001). Also, within alcoholics, those that received placebo had significantly more alcohol cue-induced ventral striatum activation than those treated with naltrexone (p=.049), or naltrexone/ondansetron (p=.02). However, those treated with ondansetron were intermediate between the placebo and other drug groups, being non-significantly lower than placebo but not as low as the groups taking naltrexone with, or without, ondansetron. Thus while this between-medication group HLM analysis of ventral striatum activation showed medication effects in the same direction as in the within-medication group SPM2 analysis, a more powerful effect for naltrexone than for ondansetron emerged in the HLM analysis.
Figure 3.
Ventral striatum activation (contrast of alcohol-cue activation minus beverage-cue activation) was significantly decreased in the combination naltrexone/ondansetron group (p=.02), the naltrexone alone (p=.049), and the social drinking controls (p=.001) as compared to placebo treated subjects.
Relationship of Craving with Ventral Striatum Activation
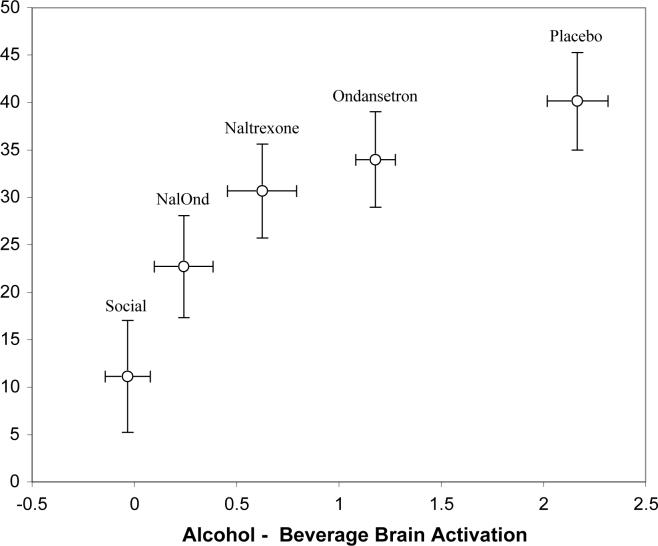
There is a strong curvolinear relationship across groups between the mean craving for alcohol during the scanning session and the mean of the alcohol minus beverage comparison (Figure 4). Linear regression of craving scores against the log of the alcohol/beverage ventral striatum activation was highly significant (B=.04, Se=.0048, p= 002), with mean activation explaining over 95% of the variance in the craving group means.
Figure 4.
There is a strong curvolinear relationship across groups between the mean craving for alcohol during the scanning session and the mean of the alcohol minus beverage comparison (B=.04, Se=.0048, p= 002) in the ventral striatum.
COMMENT
While others have utilized fMRI neuroimaging technology to evaluate medication effects in alcoholics,52 to our knowledge, this is the first study utilizing fMRI to evaluate alcohol cue-induced changes in regional brain activity, along with subjective reports of craving, during double-blind medication treatment. The results indicate that participants treated with the combination of naltrexone and ondansetron had significantly less craving while viewing alcohol cues within the MRI scanner as compared to placebo-treated participants. Unexpectedly, there was no difference in craving while viewing the alcohol cues within the scanner between placebo and naltrexone alone or placebo and ondansetron alone groups.
Our a priori hypothesis was that alcohol cue-induction would result in activation of the ventral striatum in the placebo group in contrast to social drinkers. Importantly, consistent with our previous report 35 there was a significant difference in ventral striatum activation between placebo treatment and social drinking controls (p=.001). Furthermore, we hypothesized that there would be reduction in this ventral striatum activation by both naltrexone and ondansetron and a greater reduction in ventral striatum activation in the combination treatment group than in either medication group alone. Consistent with that hypothesis, in our region of interest analysis, the most significant decrease in alcohol cue-induced ventral striatum activation as compared to placebo treatment was observed in the naltrexone/ondansetron group (p=.02). While naltrexone alone did suppress activation more than placebo treatment, the difference was less robust (p=.049) and ondansetron alone caused a non-significant lower activation than placebo.
The ventral striatum contains the nucleus accumbens (Nac) which is considered one of the primary neural substrates mediating addiction.53-54 It has been implicated in the rewarding properties of reinforcing behaviors and substances of abuse and has extensive cortical and subcortical connections. Systemic and oral ethanol administration increases the dopamine concentration in the Nac.55-60 Dopaminergic projections from the ventral tegmental area (VTA) to the Nac fire in response to presentation of reward cues and reward anticipation 41,61-63 and human PET imaging studies have implicated striatal dopamine systems in alcohol effects.64-66
Importantly, alcohol-associated cues (light or environmental) have signaled an increase in Nac dopamine output prior to actual alcohol consumption in animals.16,67 Since participants in our cue paradigm do not attain a measurable blood alcohol level, the alcohol taste and visual cue activation of the ventral striatum is consistent with alcohol-cue stimulation of Nac dopamine release in animals. Other fMRI studies in humans found that anticipation of increasing monetary rewards in healthy volunteers yielded increasing Nac activation 68 and that memory of rewarding stimuli was preceded by differential activation of the VTA and Nac 69, both consistent with our finding of increased salience of alcohol-cue activation of these areas in alcohol dependent individuals. Of interest, naltrexone has been found to block the Nac activated dopamine release in anticipation of drinking and actual alcohol consumption.15,16,43 Taken together, these data would suggest that naltrexone might be capable of disrupting “alcohol-induced reward memory” in alcoholics leading to reduced cue-responsiveness, craving and relapse. The addition of ondansetron to alcohol might enhance this effect.
It is thought that the 5HT3 receptor interacts with dopamine cells in the VTA-Nac reward pathway. 5HT3 agonists can stimulate dopamine release in the Nac and also augment the ethanol-induced release of dopamine.58,70 This effect is blocked by 5HT3 antagonists.58,70,71 The effects of the 5HT3 antagonists are similar to those of naltrexone in these models 43 suggesting that the two drugs may have synergistic actions.72
Of note, the findings in the placebo group are consistent with our previous work 33,35 and other published cue-induced imaging studies involving substances of abuse. Regions activated include both limbic and cortical areas. These include various portions of the cingulate gyrus,73-80 the orbital cortex 77,79,81, and the ventral striatum.33,73,78 While in our hands the ventral striatum seems to be most affected by alcohol cue-induced activation, these others areas might play a significant role in reinforced memories, subjective desire to drink, and perhaps attempts to resist urges and thoughts of drinking. These issues require further exploration.
In summary, the current study provides further evidence of the utility of neuroimaging techniques to not only further our understanding of the neurobiological basis of alcoholism, but also as a tool to provide crucial information regarding therapeutic manipulations of these underlying substrates of addiction. As such, neuroimaging can provide a bridge between preclinical and clinical work. Consistent with animal data suggesting that both naltrexone and ondansetron reduce alcohol-stimulated dopamine output in the ventral striatum, the current study found evidence that these medications could decrease cue-induced activation of the ventral striatum. The relationship between this deactivation, craving, alcohol consumption and relapse drinking during treatment all require further exploration. In addition, individual differences in medication effects on alcohol-cue brain deactivation, such as genetic makeup, age of onset of alcohol drinking and dependence, severity of dependence, gender, and racial/ethnic differences are all worthy of future study.
ACKNOWLEDGEMENTS
Supported by grants P50 AA010761 and K23 AA00314
Presented in part as an oral presentation at the 2006 Research Society on Alcoholism Meeting
This study was funded by the National Institute of Alcohol Abuse and Alcoholism P50 AA010761. Dr. Myrick was also funded through NIAAA K23 AA00314 and the VA Research and Development Service.
Footnotes
Presented in part as an oral presentation at the 2006 Research Society on Alcoholism Meeting in Baltimore, MD.
Access to data: The principal investigator (Hugh Myrick, MD.) takes full responsibility for the integrity of the data and the accuracy of the data analysis. All authors have had full access to all the data in the study.
REFERENCES
- 1.Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics. American Journal of Psychiatry. 1999;156(11):1758–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- 2.O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE. Rounsaville B Naltrexone and coping skills therapy for alcohol dependence. Archives of General Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 3.Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Archives of General Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 4.Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology. 2004;173(1−2):32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- 5.Davidson D, Swift R, Fitz E. Naltrexone increases the latency to drink alcohol in social drinkers. Alcoholism: Clinical and Experimental Research. 1996;20:732–739. doi: 10.1111/j.1530-0277.1996.tb01679.x. [DOI] [PubMed] [Google Scholar]
- 6.Davidson D, Palfai T, Bird C, Swift R. Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcoholism: Clinical and Experimental Research. 1999;23(2):195–203. [PubMed] [Google Scholar]
- 7.O'Malley S, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone augments neuroendocrine responses to ethanol in alcohol dependent subjects. Alcoholism: Clinical and Experimental Research. 1999;23(5):121A. Abstract. [Google Scholar]
- 8.Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H. Naltrexone-induced alterations in human ethanol intoxication. American Journal of Psychiatry. 1994;151:1463–1467. doi: 10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- 9.O'Malley SS, Croop RS, Wroblewski JM, Labriola DF, Volpicelli JR. Naltrexone in the treatment of alcohol dependence: a combined analysis of two trials. Psychiatric Annals. 1995;25(11):681–688. [Google Scholar]
- 10.Roberts JS, Anton RF, Latham PK, Moak DH. Factor structure and predictive validity of the Obsessive Compulsive Drinking Scale. Alcoholism: Clinical and Experimental Research. 1999;23:1484–1491. [PubMed] [Google Scholar]
- 11.Gastpar M, Bonnet U, Boning J, Mann K, Schmidt LG, Soyka M, Wetterling T, Kielstein V, Labriola D, Croop R. Lack of efficacy of naltrexone in the prevention of alcohol relapse: results from a German multicenter study. J Clin Psychopharmacol. 2002;22(6):592–298. doi: 10.1097/00004714-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. New England Journal of Medicine. 2001;345(24):1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- 13.Srisuraponont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8(2):267–280. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- 14.Anton RF, O'Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–71. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middaugh LD, Szumlinski KK, Patten YV, Marlowe A-LB, Kalivas PW. Chronic ethanol consumption by C57BL/6 mice promotes tolerance to its interoceptive cues and increases extracellular dopamine, an effect blocked by naltrexone. Alcohol Clin Exp Res. 2003;27(12):1892–1900. doi: 10.1097/01.ALC.0000099264.36220.48. [DOI] [PubMed] [Google Scholar]
- 17.Palfai T, Davidson D, Swift R. Influence of naltrexone on cue-elicited craving among hazardous drinkers: the moderational role of positive outcome expectancies. Exp Clin Psychopharmacol. 1999;7(3):266–273. doi: 10.1037//1064-1297.7.3.266. [DOI] [PubMed] [Google Scholar]
- 18.Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB. Naltrexone's effects on reactivity to alcohol cues among alcoholic men. J Abnorm Psychol. 2000;109(4):738–42. [PubMed] [Google Scholar]
- 19.Drobes DJ, Anton RF, Thomas SE, Voronin K. A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: naltrexone and nalmefene. Neuropsychopharmacology. 2003;28:755–764. doi: 10.1038/sj.npp.1300101. [DOI] [PubMed] [Google Scholar]
- 20.McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology. 2000;22(5):480–492. doi: 10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]
- 21.O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;160(1):19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- 22.Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, Kampf P, Stracke R, Baehr M, Naber D, Wiedemann K. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60(1):92–99. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- 23.Grant KA. The role of 5-HT3 receptors in drug dependence. Drug and Alcohol Dependence. 1995;38:155–171. doi: 10.1016/0376-8716(95)01120-n. [DOI] [PubMed] [Google Scholar]
- 24.Johnson BA, Cowen PJ. Alcohol-induced reinforcement: dopamine and 5-HT3 receptor interactions in animals and humans. Drug Development Research. 1993;30:153–169. [Google Scholar]
- 25.Tomkins DM, Le AD, Sellers EM. Effect of the 5-HT3 antagonist ondansetron on voluntary ethanol intake in rats and mice maintained on a limited access procedure. Psychopharmacology. 1995;117(4):479–485. doi: 10.1007/BF02246222. [DOI] [PubMed] [Google Scholar]
- 26.Swift RM, Davidson D, Whelihan W, Kuznetsov O. Ondansetron alters human alcohol intoxication. Biological Psychiatry. 1996;40:514–521. doi: 10.1016/0006-3223(95)00432-7. [DOI] [PubMed] [Google Scholar]
- 27.Sellers EM, Toneatto T, Romach MK, Somer GR, Sobell LC, Sobell MB. Clinical efficacy of the 5-HT3 antagonist ondansetron in alcohol abuse and dependence. Alcoholism: Clinical and Experimental Research. 1994;18(4):879–885. doi: 10.1111/j.1530-0277.1994.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson BA, Roache JD, DiClemente CC, Prihoda TJ, Tiouririne NA, Javors MA, Bordnick PS. Ondansetron and alcohol consumption: preliminary analysis of a double-blind trial. Alcoholism: Clinical and Experimental Research. 1999;23(5):81A. [Google Scholar]
- 29.Le AD, Sellers EM. Interaction between opiate and 5-HT3 receptor antagonists in the regulation of alcohol intake. Alcohol and Alcoholism. 1994;S2:545–549. [PubMed] [Google Scholar]
- 30.Ait-Daoud N, Johnson BA, Prihoda TJ, Hargita ID. Combining ondansetron and naltrexone reduces craving among biologically predisposed alcoholics: preliminary clinical evidence. Psychopharmacology. 2001;154(1):23–27. doi: 10.1007/s002130000607. [DOI] [PubMed] [Google Scholar]
- 31.Drobes DJ, Thomas SE. Assessing craving for alcohol. Alcohol Res Health. 1999;23(3):179–186. [PMC free article] [PubMed] [Google Scholar]
- 32.Braus DF, Wrase J, Grusser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. Journal of Neural Transmission. 2001;108(7):887–94. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- 33.George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58(4):345–52. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- 34.Kareken DA, Sabri M, Radnovich AJ, Claus E, Foresman B, Hector D, Hutchins GD. Olfactory system activation from sniffing: effects in piriform and orbitofrontal cortex. Neuroimage. 2004;22(1):456–65. doi: 10.1016/j.neuroimage.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29(2):393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 36.Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. American Journal of Psychiatry. 2001;158(7):1075–83. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- 37.Wrase J, Grusser SM, Klein S, Diener C, Hermann D, Flor H, Mann K, Braus DF, Heinz A. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. European Psychiatry: the Journal of the Association of European Psychiatrists. 2002;17(5):287–91. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- 38.Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38(2):95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- 39.Katner SN, Weiss F. Neurochemical characteristics associated with ethanol preference in selected alcohol-preferring and non-preferring rats: a quantitative microdialysis study. Alcohol Clin Exp Res. 2001;25(2):198–205. [PubMed] [Google Scholar]
- 40.Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- 41.Melendez RI, Rodd-Henricks ZA, Engleman EA, Li TK, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol preferring rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002;26(3):318–325. [PubMed] [Google Scholar]
- 42.Wise RA. Opiate reward: sites and substrates. Neurosci Biobehav Rev. 1989;13(2−3):129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- 43.Benjamin D, Grant ER, Pohorecky LA. Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Research. 1993;621:137–140. doi: 10.1016/0006-8993(93)90309-b. [DOI] [PubMed] [Google Scholar]
- 44.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (clinical version) American Psychiatric Publishing, Inc.; Washington, DC: 1997. [Google Scholar]
- 45.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: Assessing normal drinkers' reports of recent drinking and a comparative evaluative across several populations. Br J Addict. 1988;83(4):393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 46.Anton RF, Moak DH, Latham PK. The Obsessive Compulsive Drinking Scale. A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53:225–231. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- 47.Davis LJ, Hurt RD, Morse RM, O'Brien PC. Discriminant analysis of the Self-Administered Alcoholism Screening Test. Alcohol Clin Exp Res. 1987;11(3):269–273. doi: 10.1111/j.1530-0277.1987.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 48.Skinner HA, Allen BA. Alcohol dependence syndrome: Measurement and validation. Journal of Abnormal Psychology. 1982;91(3):199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- 49.Anton RF. New methodologies for pharmacologic treatment trials for alcohol dependence. Alcohol Clin Exp Res. 1996;20(Suppl 7):3A–9A. doi: 10.1111/j.1530-0277.1996.tb01183.x. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). British Journal of Addiction. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 51.Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10(4):385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 52.Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Flor H, Mann K, Heinz A. Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res. 2006;30(8):1349–1354. doi: 10.1111/j.1530-0277.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 53.Pointieri F, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increased extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Nat Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koob GF, Le Moal M. Drug abuse: hedonic hemostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 55.Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- 56.Mocsary Z, Bradberry C. Effect of ethanol on extracellular dopamine in nucleus accumbens: comparison between Lewis and Fisher 344 rat strains. Brain Res. 1996;706:194–198. doi: 10.1016/0006-8993(95)01200-1. [DOI] [PubMed] [Google Scholar]
- 57.Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellullar dopamine concentration in rat nucleus accumbes by microdialysis. Alcohol Clin Exp Res. 1998;22:367–374. [PubMed] [Google Scholar]
- 58.Yoshimoto K, Mcbride W, Lumeng L. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1991;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- 59.Weiss F, Mitchiner M, Bloom FE, Koob GF. Free-choice responding for ethanol versus water in alcohol preferring (P) and unselected wistar rats is differentially modified by naloxone, bromocriptine, and methysergide. Psychopharmacology (Berl) 1990;101:178–186. doi: 10.1007/BF02244123. [DOI] [PubMed] [Google Scholar]
- 60.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- 61.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 62.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 63.Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J neurosci. 1992;12(12):4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schredkenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DAPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162(8):1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- 65.Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58(10):779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 66.Yoder KK, Kareken DA, Seyoum RA, O'Connor SJ, Wang C, Zheng QH, Mock B, Morris ED. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29(6):965–970. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- 67.Katner SN, Kerr TM, Weiss F. Ethanol anticipation enhances dopamine efflux in the nucleus accumbens of alcohol-preferring (P) but not Wistar rats. Behav Pharmacol. 1996;7(7):669–674. [PubMed] [Google Scholar]
- 68.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 70.Campbell AD, McBride WJ. Serotonin-3 receptor and ethanol-stimulated dopamine release in the nucleus accumbens. Pharmacology, Biochemistry and Behavior. 1995;51(4):835–842. doi: 10.1016/0091-3057(95)00050-7. [DOI] [PubMed] [Google Scholar]
- 71.Wozniak KM, Pert A, Linnolia M. Antagonism of 5-HT3 receptors attenuates the effects of ethanol on extracellular dopamine. Eur J Pharmacol. 1990;198(2):287–289. doi: 10.1016/0014-2999(90)90015-x. [DOI] [PubMed] [Google Scholar]
- 72.Le AD, Tomkins DM, Sellers EM. Use of serotonin (5-HT) and opiate-based drugs in the pharmacotherapy of alcohol dependence: an overview of the preclinical data. Alcohol Alcohol Suppl. 1996;1:27–32. [PubMed] [Google Scholar]
- 73.Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 74.Brody AL, Mandelkern MA, London ED, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Madsen D, Jarvick ME. Brain metabolic changes during cigarette craving. Arch Gen Psychaitry. 2002;59(12):1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 75.Childress AR, Mozley PD, McElgin W, Fitzgerald J. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156(1):11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelly D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 77.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58(4):334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 79.Mass LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rodgers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- 80.Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158(1):86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- 81.Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64(9):775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]