Abstract
Targeting of the drugs administered systemically relies on the higher affinity of ligands for specific receptors to obtain selectivity in drug response. However, achieving the same goal inside the bladder is much easier with an intelligent pharmaceutical approach that restricts drug effects by exploiting the pelvic anatomical architecture of the human body. This regional therapy involves placement of drugs directly into the bladder through a urethral catheter. It is obvious that drug administration by this route holds advantage in chemotherapy of superficial bladder cancer and it has now become the most widely used treatment modality for this ailment. In recent years, the intravesical route has also been exploited either as an adjunct to an oral regimen or as a second-line treatment for neurogenic bladder 1, 2. Instillation of DNA via this route using different vectors has been able to restrict the transgene expression in organs other than bladder. The present review article will discuss the shortcomings of the current options available for intravesical drug delivery (IDD) and lay a perspective for future developments in this field.
Keywords: Intravesical Delivery, Liposomes, Bioadhesion, Hydrogel, Gene Therapy
Introduction
Systemically administered therapy for the bladder diseases often fails because only a small fraction of administered drugs reaches the desired site either due to poor absorption or due to losses from metabolism. IDD can avoid losses from first pass metabolism and allows the therapeutic effect of a drug to be localized at the desirable site with minimal systemic side effects. These characteristics can ensure potential and real benefits for patients having morbid adverse effects from oral administration 3. The pharmaceutical and biotechnology industries are continuously developing peptide, protein, biopolymer and macromolecular drugs for the treatment of diseases such as cancer. However, many of these new drugs have poor bioavailability when administered orally and often fail to induce a clinical response.
Fortunately, IDD can overcome intrinsic shortcomings of oral therapy such as drug or formulation specific vagaries in absorption, metabolism and renal excretion. For instance, the metabolite of orally administered Oxybutynin, N-desethyl-oxybutynin (DEO) is known to cause side effects in some patients 3. Interestingly, intravesical administration of Oxybutynin avoids its first pass effect and the amount of DEO in serum is drastically reduced. Similarly, patients with interstitial cystitis (IC) had to wait for 6 months to accrue any benefit from thrice daily oral administration of cytoprotective drug misoprostol 4. The need of a prolonged regimen for achieving efficacy can be traced to low amounts of drug excreted in the urine following oral administration. It is highly probable that IDD of a cytoprotective drug in cystitis patients would be able to reduce the duration of treatment. Besides, IDD will also be helpful in cases where resistant drug target in bladder forces the need of higher drug levels inside the bladder.
However, IDD has to overcome its own set of challenges and most prominent among them is the low residence time of a drug in the bladder that necessitates frequent instillation. Conventional vehicles used for the intravesical delivery fail to provide a sustained exposure of drug inside the bladder, which rarely lasts beyond the first voiding of urine after instillation. An important obstacle in the success of IDD arises from the low permeability of transitional epithelium of the bladder also known as urothelium. In the normal condition, the 6-7 cellular layer thick urothelium maintains high electrochemical gradients between urine and blood. In the healthy state it is almost impermeable to all the irritants present in urine. However, this tough barrier against IDD is somewhat compromised in the disease condition and even then passive diffusion is the only mode of membrane transport possible across urothelium. Subsequent to drug instillation into the bladder, the concentration of drug in the bladder tissue was linearly dependent on the concentration of drug in urine 5. Since passive diffusion is the sole driving force available for intravesical drug absorption, it is logical to expect an increase in trans-vesical (across the urothelium) drug transport coincident with improvement in the concentration gradient.
Conversely, trans-vesical transport can be adversely affected by dilution of instilled drug solution by residual urine in the bladder. Further decrements in the concentration gradient are also possible from steady accumulation of urine during the duration of instillation. This influence of the kidney on IDD can be mitigated by reducing the rate of urine production, which can be achieved with complete bladder emptying prior to dose administration and restricted fluid intake before and after instillation 6. This clever strategy was able to increase the urinary drug concentration and improve efficacy without a significant increase in toxicity 6. A phase III trial on IDD followed these techniques to enhance the penetration of mitomycin C across urothelium and nearly doubled the recurrence-free rate in superficial bladder cancer patients 7.
1.1. Bladder Permeability Barrier
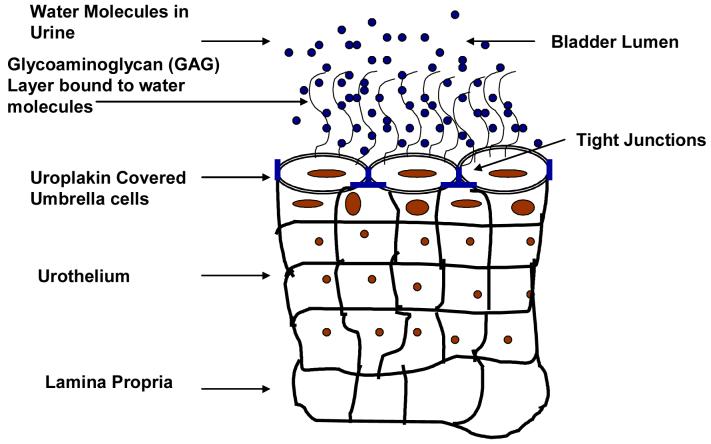
The water tight barrier between blood and urine formed by urothelium represents the toughest barrier to drug delivery known to man. The bladder permeability barrier (BPB) for IDD appears complementary to the impermeable endothelial interface separating blood from brain interstices known as the blood brain barrier (BBB) for drug delivery into brain. But, BPB may turn out to be tougher than the BBB because the former is made up of epithelial cells and the latter by a unique phenotype of endothelial cells lining the blood vessels of the brain. The main site of BPB is located at the superficial cells in transitional epithelium of bladder called umbrella cells named because of their characteristic shape (Fig.1) 8, 9. The umbrella cells erect a water tight barrier with the help of multiple rigid-looking plaques of its asymmetric unit membrane (AUM) in consort with tight junctions joining its apical surface 10. Nearly 90% of the apical surface of umbrella cells is covered by rigid plaques that are composed of four major uroplakins, UPIa (27 kDa), UPIb (28 kDa), UPII (15 kDa), and UPIII (47 kDa) 11. These uroplakins add to the strength of umbrella cells and to the BPB through the tight assembly of 16-nm particles into hexagonally packed 2D crystals (urothelial plaques). The crystalline lattice structure of uroplakin interact with specialized lipids of the apical membrane of umbrella cells to aid in the BPB.
Fig.1.
Illustration of bladder permeability barrier established by uroplakin covered umbrella cells of bladder epithelium (urothelium) and GAG layer that prevents adhesion.
The permeability of urothelium is further augmented by the mucin layer composed of glycosaminoglycans (GAG) present on the surface of umbrella cells. The hydrophilic nature of the GAG layer allows it to form a thin sheet of stationary aqueous layer on umbrella cells. The GAG layer acts as a major permeability barrier by physically blocking the instilled drug molecules from reaching the underlying tight junctions and cell membranes. Moreover, the apical membrane of umbrella cells (AUM) beneath the GAG layer further reinforces the BPB by its exceptionally low permeability 12-15. The antiadherence property of the GAG layer can block adhesion of foreign particles including an adenovirus, instilled as vectors of gene delivery. Parsons and coworkers hypothesized that symptoms of IC are related to changes in urothelial permeability arising from defects in the GAG layer 16, 17. Nevertheless, the impermeability of urothelium is frequently exploited for instilling potentially toxic agents into the bladder for achieving localized pharmacological effect.
2. Therapeutic Considerations for Instilling Potentially Toxic Agents for IC or Bladder Cancer
The drastic reduction in the incidence of systemic side effects by intravesical route allows the use of very toxic agents such as dimethylsulphoxide (DMSO) in the treatment of interstitial cystitis (IC) also known as painful bladder syndrome 18. The first report of IC treatment with intravesical instillation of DMSO came in 1967 19 and in 1978 it was FDA approved as a 50% solution (Rimso-50) with a primary indication of IC treatment 20. Another toxic agent frequently instilled into the bladder is a strain of Mycobacterium tuberculosis, Bacillus Calmette-Guerin (BCG). Instillation of BCG has now become an established therapy for recurrent superficial (papillary) bladder carcinoma and carcinoma in situ 21. BCG delays tumor progression and decreases the need for subsequent cystectomy with improved overall survival rates 22.
The mechanism of action for BCG is still unclear, although a greater rate of cell turnover has been postulated. BCG triggers a variety of local immune responses that appear to correlate with antitumor activity 23, 24. Immunomodulatory activity of BCG prompted its evaluation for immunotherapy of IC and it showed a favorable outcome in refractory IC patients 25. BCG instillation is now considered an alternative option for symptomatic treatment of IC 26. Inflammation is a major component of IC and agents or delivery systems that can modulate immune response may prove a viable option as an intravesical treatment option for IC treatment.
Newer forms of IC treatment aim to attack whatever is known for the pathogenesis of IC. The most consistent finding in most IC patients involves the dysfunction in extracellular matrix called the GAG layer and localization of a high number of activated mast cells in the bladder 27, 28. The glycosaminoglycans present on the bladder surface include chondroitin 4 and 6 sulfate, dermatan sulfate, heparan sulfate, and hyaluronic acid 29, 30. Treatment of IC by instilling sodium hyaluronate has been reported in IC patients for possible replenishment of defective GAG layer 31, 32. Heparin is another GAG effective as a treatment in approximately 50% of IC patients following its instillation 33.
Intravesical treatment of particularly severe chronic IC requires addressing the significant upregulation of afferents in the bladder 34. C-fiber afferents involved in aberrant micturition reflex of IC are believed to be silent under normal conditions, but are activated after bladder irritation and spinal cord injury 35, 36. Down-regulation of sensory nerves by using neurotoxins like capsaicin, resiniferatoxin RTX or botulinum toxin has proven itself a viable approach in urology 37, 38. The potent action of capsaicin is restricted only to the afferent fibers in the bladder wall and possible systemic neurotoxicity is also avoided with IDD 39. The hydrophobic nature of vanilloids necessitates the use of ethanol as a co-solvent with saline for the instillation into the bladder. Ethanol solution is well known to induce inflammation after topical application on tissues 40, 41 and a recent study demonstrated the superiority of nonalcoholic solvents for capsaicin when compared against RTX delivered in alcohol 42. Many other options have been tried to improve aqueous solubility of drugs administered systemically such as micelles and liposomes.
3. Liposomes & Nanotechnology
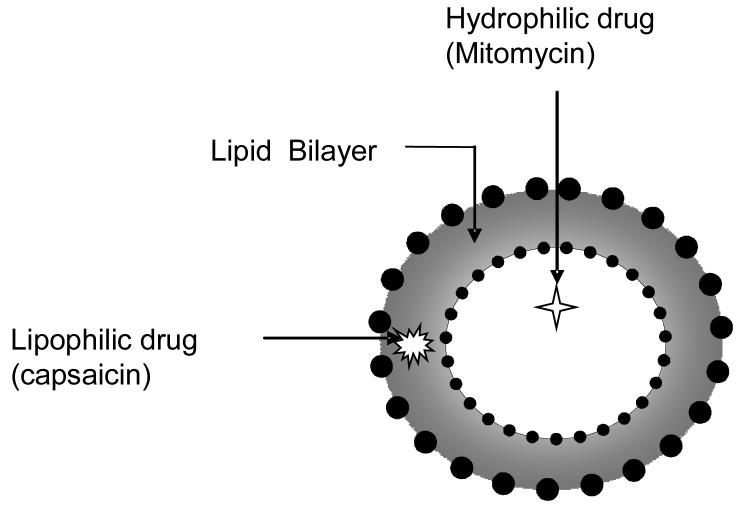
Liposomes are spherical vesicles consisting of an aqueous core enclosed in one or more phospholipid layers, that can be loaded with a great variety of molecules, such as small drug molecules, proteins, nucleotides and even plasmids 43-47, 48. Liposomes were first studied in 1961 by Bangham 49 and the flexibility in their compositions makes them versatile drug delivery vehicles (Fig.1). Liposomes are better suited than micelles for use as carriers of water insoluble drugs administered into the bladder, because instillation of micelles can have deleterious effects on urothelium 50, 51. Micelles formed by polyamide detergents such as Big CHAP (N,N-bis-(3-D-Gluconamidopropyl) cholamide) can remove lipids from the apical membranes of cells lining the bladder and make the urothelium more permeable for drug or gene transfer 52.
When used for intravenous route, liposomes were able to demonstrate improvement in aqueous solubility of hydrophobic drugs such as taxol and amphotericin 53. A recent study reported from our lab used liposomes as a vehicle for capsaicin and evaluated their potential as a vehicle for intravesical delivery in rats 54. Liposomes were able to deliver capsaicin with efficacy similar to ethanolic saline, but toxicity to the bladder was drastically reduced. A similar approach can prove useful in instillation of other vanilloids such as RTX and water insoluble drugs.
Instillation of liposome encapsulated radiolabeled IFN-α or radiolabeled liposomes into mouse bladder was able to achieve localized therapy with negligible penetration to other organs 55. Use of multilamellar liposomes was favored in cell culture studies and use of liposomes as a delivery vehicle improved the antiproliferative capacity of IFN-α in a resistant bladder cancer cell line 56. The in vivo effectiveness of liposomes in an orthotopic resistant bladder cancer model need to be examined in future studies to support the encouraging results obtained on cell lines.
Liposomes devoid of any drug were successful in promoting wound healing on skin perhaps by forming a film on the tissue surface 43, 44. Recent studies on non viral gene therapy of skin wounds confirmed the wound healing properties of liposomes 57, 58. Based on such encouraging reports, it was logical to expect that instillation of liposomes may be able to palliate IC symptoms in a rat model of bladder injury, because of the wound healing properties of liposomes 16, 17. Effect of liposomes alone in absence of any drug was studied in a rat model of bladder hyperactivity induced by breaching the GAG layer with protamine and thereafter irritating the bladder with KCl. Liposomes were able to partially reverse the high micturition frequency induced by protamine sulfate/KCl 59. These observations suggest that liposomes might enhance the barrier properties of a dysfunctional urothelium and increase its resistance against irritant penetration.
The apical surface of umbrella cells in urothelium facing urine posses a unique asymmetric unit membrane (AUM), whose protein component (uroplakins) have been well studied but information on its lipid component is largely missing 11, 60. The beneficial effect of liposomes on injured urothelium encourages investigation into lipid composition of urothelium and role of lipid signaling in normal and hyperactive bladder. Interestingly, all isoforms of a nuclear receptor family peroxisome proliferator-activated receptor (PPAR) implicated in the control of inflammatory responses have been localized in transistional epithelium of bladder and kidney. The functions of these isoforms in kidney have been well reported, where PPAR-α plays a major role in triggering fatty acid utilization, PPAR-β/δ contributes to survival of renal interstitial cell in medullary hyperosmality and PPAR-γ is involved in regulation of renal hemodynamic and water and sodium transport 61. Though, the functional role of PPAR isoforms in urothelium remains to be investigated. The link between the family of PPARs and lipid homeostasis in other tissues may suggest important implications for their role in permeability of urothelium in health and diseases such as IC.
A promising alternative to liposomes as drug carriers is slowly rising on the horizon from the field of nanoparticle delivery. Nanotechnology involves the creation and manipulation of colloidal particles that range from 10 to 1000 nm in size. Nanoparticles are produced by creating molecular nanostructures by either dissolving, adsorbing, entrapping, encapsulating drug molecules or by forming drug-polymer complexes. Nanoparticles with a well-defined particle size and shape can have immense potential for intravesical delivery as they can enhance the ability of drugs to cross the urothelium. Moreover, higher surface to volume ratio of nanoparticles can also be responsible for increase in transvesical absorption of encapsulated drugs. Lu et al designed a rapid releasing gelatin nanoparticles loaded with paclitaxel whose particle size ranged from 600 to 1,000 nm 62. The paclitaxel nanoparticles showed significant activity against human bladder cancer cell and resulted in higher tissue concentrations compared with paclitaxel formulated in the commercial cremophor emulsion 62. Nanotechnology holds tremendous promise for the future of intravesical delivery of drugs and genes into the bladder.
4. Gene Therapy of Bladder Cancer
Gene therapy can bring a radical change in the treatment of bladder cancer and other diseases affecting bladder. Intravesical administration of recombinant interferon (IFN) demonstrated only limited efficacy against superficial bladder cancer because of the inherent drawback of the intravesical route 63. Instillation of protein was only able to offer short term treatment of IFN because of the excretion of the instilled IFN with only a fraction of administered dose remaining after voiding. It was reasonable therefore to expect that delivery of IFN-α gene into the urothelium might improve this therapeutic modality and potentially provide a continuous secretion of IFN into the tumor microenvironment. Indeed, successful transgene expression into urothelium was able to sustain the production and secretion of the mature 165 amino acid human IFN-α2b protein inside the bladder 64. Moreover, in vivo gene transfer is a feasible approach against superficial bladder cancer because of its specific features such as easy external access for treatment through the urethra and lower risk of gene transfer to other organs 65. Recent developments such as generation of human superficial bladder tumor cells stably expressing green fluorescent protein after implantation in the mouse bladder have made it easier to evaluate efficacy of intravesical therapies and visualize the associated changes in cancer growth 66.
Harnessing the potential of sequence-specific recognition between complementary nucleic acid sequences (Watson-Crick base pairing) can also allow control of aberrant gene expression in the bladder. Overexpression of NGF mRNA in bladder is considered responsible for symptoms of IC and as expected, viral mediated expression of NGF gene in rat urothelium led to bladder hyperactivity 67. A short single-stranded oligo fragment can hybridize to its complementary mRNA target sequence and modify RNA processing either through steric blockade or by activating RNase H. Antisense approaches have been extensively used both for determining the function of specific mRNA and therapeutic purposes. It remains to be seen if specific degradation of target mRNA in bladder can also be achieved by using catalytic RNA (ribozymes) and DNA (DNAzymes) as similar tools have been successful in other tissues68,69.
RNA interference (siRNA) is an emerging frontier of gene therapy with immense therapeutic potential70. A recent study evaluated the potential of small interfering RNAs (siRNAs) in blocking mRNA for the enzyme Polo-like kinase-1 (PLK-1) involved in proliferation after intravesical administration71. PLK controls mitotic entry of proliferating cells and regulate many aspects of mitosis required for cytokinesis. Future studies for modulating gene expression may also employ the decoy approach for evaluating the potential of synthetic DNA fragments in competition against transcription factors. However, the latent potential of rational gene-based drug design remains unrealized for intravesical delivery largely due to lack of an adequate drug delivery system. A search is ongoing for suitable vectors for plasmid and oligos that can be used for intravesical delivery.
4.1. Viral Vectors
The adenovirus genome has been well-characterized and they have been successful in transducing exogenous DNA to both dividing and non-dividing cell types. Intravesical administration of replication-incompetent adenovirus was successful in restricting the transgene expression only to the bladder for two days after administration with several fold reduced expression by the 3rd day72. The luciferase gene expression was driven by the cytomegalovirus immediate early promoter in the expression cassette in place of the E1 region of the adenovirus vector.
Besides, adenoviral mediated delivery of the IFNα2b gene allowed detection of multiple-fold higher interferon concentration in bladder tissue and urine than those observed with instillation of the recombinant IFN protein itself 64. The concentration of IFN in urine followed the pattern of adenoviral mediated luciferase expression in tissue 72 with peak around 2 days and return to baseline by the 7th day. The gene transfer efficiency using viral vectors is known to be influenced by various physical parameters such as intravesical volume, instillation pressure and chemical treatment of urothelium prior to instillation 73. Instillation of higher volumes of viral vector was able to restrict significantly higher gene expression only to the bladder, but instillation at higher pressures resulted in higher transgene expression in other systemic organs. Recent studies employing the orthotopic murine bladder cancer model have demonstrated that gene transfer efficiency using attenuated vaccinia virus or canarypox virus is better than with adenovirus 74, 75.
The antiadherence property of the GAG layer present on the surface of urothelium acts as a nonspecific barrier in transduction of urothelium by adenovirus. Instillation of 22% ethanol in rodent bladder before administration of adenovirus was able to improve gene transfer because of disruption in the GAG layer 76. An even greater increase in gene transfer and expression was achieved by prior instillation of gene transfer-enhancing agent Syn3 64, 77. This polyamide compound was required to be instilled for an hour at 1 mg/ml concentration on two consecutive days prior to administration of adenovirus 77. However, a recent study reported by Connor et al 2005 showed that co-administration of Syn3 with adenovirus is also successful and infact Syn3 can be used as a vehicle for the adenovirus 64,66. Previously, a similar strategy of co administering adenovirus with a transduction-enhancing non-ionic polyamide detergent Big CHAP was used to improve the expression of p53 gene in a phase I study 52. Although, the use of Syn3 appears to be non-toxic to the bladder tissue, but the use of other transduction enhancing agents have been shown to produce adverse effects on the urothelium 50, 51,66.
A phase I gene therapy clinical trial based on IFN-α gene is proposed in superficial bladder cancer patients by using a study design already evaluated in a preclinical study 78. Single instillation of adenovirus containing IFN-α at doses of 1010-1011 particles/ml along with Syn3 was highly effective in reducing the size of human bladder tumors implanted orthotopically in nude mice 78. Urine levels of expressed IFN after treatment correlated with efficacy against bladder cancer model implanted in immunodeficient mice. Although the adenovirus vector appeared promising in preclinical study, its potential inflammatory toxicity could not be fully evaluated in an immunodeficient mouse model.
The dose-limiting toxicity of adenovirus vector containing p53 gene was evaluated in a phase I study on patients of locally advanced bladder cancer ineligible for cystectomy 79. The vectors showed no selective tropism for tumoral cells and the expression of specific transgene was transient as detected in bladder biopsy samples using Reverse-transcriptase polymerase chain reaction. Immunohistochemical analysis of bladder epithelium also did not reveal any changes in the protein levels of p53, p21waf1/cip1, or bax 79. Higher efficiency of virus mediated gene transfer will continue to hold interest for translating the success on murine models to clinical setup. However, the clinical toxicity of viral vectors observed following systemic administration will drive the search of nonviral vectors with higher efficiency of gene transfection. Development of efficient nonviral vectors will also serve the needs of intravesical delivery.
4.2. Nonviral Vectors
The potential of toxicity from inadvertent systemic absorption of viral vectors from the bladder has whetted interest in non-viral vectors. Among various approaches tried so far such as gene gun and electroporation, only the approach of lipoplexes has attracted considerable interest. Cationic liposomes have facilitated the delivery of DNA into mammalian cells through endocytosis of the complex followed by its escape into the cytosol from an endocytotic compartment 80, 81. Interestingly, endocytosis is also a critical process for the time-dependent changes in the area of bladder surface during voiding and urine storage 11, 82. Thus, it is possible that the entry of DNA/lipid complex into the urothelium cells is possibly mediated by endocytosis, which may be further tested using endocytosis inhibitors.
Compared to BCG therapy, IL-2 gene therapy using cytofectins, Dimyristoyl Rosenthal Inhibitor Ether DMRIE and dioleoylphosphatidylethanolamine DOPE proved better in improving survival rate of mice bearing orthotopic bladder cancer and inducing long-lasting tumor-specific immunologic memory 83. In a recent study, Esuvaranathan lab used a different vector based on cationic liposome, N-[1-(2,3-dioleoyloxyl)propyl]-N,N,N-trimethylammoniummethyl sulfate and methyl-beta-cyclodextrin-solubilized cholesterol to transfer plasmid containing IFN-α and GM-CSF into tumors implanted into mouse bladder 84. Cationic liposomes have also been used as a delivery vector for double-stranded siRNA into the bladder. The in vivo effectiveness of siRNA delivered using liposomes composed of lipid analogue 2-O-(2-DEAE)-carbamoyl-1,3-O-dioleoylglycerol and egg phosphatidylcholine was demonstrated in a murine orthotopic bladder cancer model 47.
The authors ruled out the contribution of innate immunity in the efficacy of siRNA against cancer by the failure of siRNA to induce transcription of IFN-ß gene in murine monocyte cell line RAW264.7. Therapy based on siRNA is relatively new and its comparative efficacy with respect to gene therapy in inhibiting bladder cancer needs to be determined in mouse models to make definitive conclusions about its effectiveness. Nonviral vectors has not been evaluated for gene transfer in the clinic and a comparative preclinical study on viral and non viral vectors for gene therapy of bladder cancer will be needed to justify their claim
5. Strategies for Improved Intravesical Therapy
The response of intravesical therapy in bladder cancer and cystitis is often incomplete and variable among patients from conventional formulations typically maintained in the bladder for only a short duration (i.e., 2 hours). Incomplete and variable response often seen with the use of conventional formulations for intravesical therapy can be partly attributed to resistant drug target or unsuccessful drug delivery to the diseased bladder tissue.
5.1. Improving the Permeability
Therapy by intravesical route can be further improved by helping drugs cross the permeability barrier of urothelium by physical (such as iontophoresis) and chemical (such as DMSO) enhancement methods.
5.1.1. Physical Approaches
The use of electromotive drug administration (EMDA) or iontophoresis for the enhancement of transdermal drug transport has a long tradition in medicine 85, 86. Using a very small electric current, iontophoresis increases the permeation of charged and neutral compounds through the process of electromigration and electro-osmosis. It is an active and potentially effective method for transporting drugs (such as mitomycin C, oxybutynin, and bethanechol) through the urothelium into deeper layers of the bladder 90. Response rate in high risk superficial bladder cancer was improved with increased bladder uptake of mitomycin C following intravesical electromotive administration 87, 88. In addition, several clinical reports demonstrate that intravesical EMDA of local anesthetics results in sufficient anesthesia for transurethral resection of bladder tumors, bladder neck incision and hydrodistension of the bladder 89-92. A different approach of local microwave-induced hyperthermia was used by Colombo, 2001 93 to enhance the efficacy of mitomycin C on small superficial tumor with minimal local side effect after intravesical administration.
In order to further increase transvesical drug permeation with minimal local side effect it would be interesting to see experiments conducted using a combination of iontophoresis with chemical enhancers. Electroporation uses higher voltage than that used for iontophoresis to increase the permeability of tissue under the influence of an electric field. It has been used for improving intravesical delivery of drugs in bladder carcinoma treatment 94. Voltage used in electroporation can be reduced to minimize tissue damage by combining electric current with low intensity ultra sound using the technique called sonophoresis95.
5.1.2. Chemical Approaches
Recently, certain peptides called “cell penetrating peptides” (CPP) or “protein transduction domains” (PTD) have been shown to be internalized in most cell types and, more importantly, allow the cellular delivery of conjugated biomolecules 96. They have been successful in crossing the blood brain barrier, but these peptides lack the ability to be cell selective and are therefore a poor choice for systemic drug targeting 97. However, instillation into bladder can overcome the poor selectivity of CPPs. Development of nucleic acid-based drugs is hindered by poor uptake into cells and their conjugation with CPPs could become an effective strategy for intravesical antisense therapeutics.
We examined the effect of using short length Trans-Activator of Transcription (TAT) peptide derived from human immunodeficiency virus for intravesical administration of large macromolecular drugs such as peptide nucleic acid (PNA). Other agents such as cationic lipids or polymers are ineffective in aiding translocation of PNA and its unassisted cellular uptake is very poor. PNA have been used for their antisense effect in various studies, because they form stable duplexes with the target mRNA and arrest translation of proteins 98. PNA has superior binding properties, and higher stability in biological media such as urine over a wide pH range, compared to traditional oligonucleotides and ribozymes 99. Eleven amino acid long TAT peptide was coupled to 18mer antisense PNA by Fmoc chemistry and similar chemistry was used to tag a fluorescent rhodamine probe. Translocation across rat urothelium was visualized by confocal microscopy of the red fluorescence of rhodamine in bladder sections (Tyagi et. al. unpublished observation). Using phage display technology, a peptide motif Ile/Leu-Ser-Gly-Leu that is selectively internalized in cultured urothelial cells was recently identified 100. It remains to be seen if conjugation of gene based drugs to this peptide motif can improve their permeability across the urothelium.
Currently, treatment of severe overactive bladder requires cystoscopic guided injections of botulinum toxin A (BOTOX) at 20 to 30 different sites of detrusor in bladder. Botox provides long lasting effect by blocking the release of acetylcholine from nerve endings to impair involuntary detrusor contractions 101. IDD of this high molecular weight protein toxin will require improvement in the permeability for its absorption. The absorption of this dangerous toxin botox has to be localized as any systemic absorption can prove fatal and therefore development of delivery techniques in the future has to take that into consideration. Pretreatment of urothelium with protamine sulfate was tried in rats to improve the permeability for botox 102, 103. The cationic nature of protamine sulfate allows a charge interaction with the anionically charged GAG layer leading to slight increases in permeability of urothelium 104, 105.
An exciting new approach for increasing permeability of instilled drugs across BPB may be the reversible opening of umbrella cell’s tight junctions. Encouraging results were obtained by increasing paracellular transport (through tight junctions) for intranasal delivery of peptides and proteins 106. Topical application of chitosan and cyclodextrins is thought to disrupt intercellular tight junctions and increase paracellular transport 107, 108. Recently developed quaternized chitosan derivatives such as triethyl chitosan and N-diethyl methyl chitosan might prove an efficient tool for improving paracellular transport of hydrophilic drugs in the bladder. These new positively charged chitosan derivatives could also interact with the tight junctions of colon epithelia 109.
Prior instillation of DMSO has also been reported to enhance the absorption of chemotherapeutic drug including paclitaxel and pirarubicin 110, 111. Sasaki reported that intravesical instillation of saponin before administration of anticancer drug (4′-O-tetrahydropyranyldoxorubicin, THP) can cause vacuolization and swelling of superficial cells, and the concentration of THP in bladder tissue was significantly higher than that of untreated animals, but no difference was revealed in plasma concentration 112, 113.
5.2. Increasing Residence Time in the Bladder
Sustained intravesical delivery of drugs can ensure continuous presence of drug in the bladder without the need for intermittent catheterization and drug concentration in the bladder would be constant without any peaks and valleys. It is also plausible to expect an increase in efficacy with increased duration of direct contact between the drug and the abnormal urothelium 55. A simple and sensible approach for sustained intravesical delivery is prolonged infusion into the bladder. This technique has often been applied for achieving slow and sustained release of drugs such as RTX and prostaglandins inside the bladder 114,115. RTX was infused at the flow rate of 25μl per hour for 10 days using a pigtail catheter inserted into the bladder through abdomen whereas PGE2 was infused transurethrally into the bladder for 3h in a 4 year old patient 115.
Forming a drug depot inside the bladder appears to be an attractive option over prolonged infusion. Aqueous solutions of poly (ethylene glycol-b-[DL-lactic acid-co-glycolic acid]-b-ethylene glycol) (PEG-PLGA-PEG) triblock copolymers form a free-flowing solution at room temperature and become a viscous gel at body temperature of 37°C 116. Its formulation does not require organic solvent and products from bioerosion of the biocompatible polymer are non-toxic PEG, glycolic acid and lactic acid 117. As such, a thermosensitive hydrogel formed by PEG-PLGA-PEG has been used for in situ gel formation for a depot of hydrophobic and hydrophilic drugs following subcutaneous administration in rats 118.
The triblock copolymer was used for sustaining the residence time of hydrophobic drugs in rat bladder after its instillation at room temperature. The temporal kinetics of drug excreted from hydrogel loaded with fluorescent probes can be studied by measurement of fluorescence in urine. Sustained delivery of misoprostol afforded by thermosensitive hydrogel was able to protect the rat bladder against cyclophosphamide induced cystitis 119. It would be interesting to discover the effect of loading wound healing agents such as growth factors into hydrogels instilled into damaged bladder and assess the speed of wound healing in urothelium.
5.2.1. Bioadhesion
Bioadhesion or mucoadhesion defines the interaction between a biological surface such as bladder mucosa (urothelium) and the polymer. The presence of a mucinglycocalyx domain in the bladder mucosa tempts its utilization for prolonging the residence time of drugs through bioadhesion 120. However, long before bioadhesion caught the fancy of drug delivery scientists; this powerful approach has been exploited by bacteria Escherichia coli for its adhesion to the bladder mucosa. As a matter of fact, the urinary tract infections are initiated by adhesion of uropathogenic E. coli to uroplakin receptors of umbrella cells through the FimH adhesin located at the tips of its type 1 pili 121. An IDD system based on bacterial adhesion factors could be a smart and efficient mechanism for increasing adhesion to epithelial surfaces 122.
Application of bioadhesion for IDD should be able to fulfill three main criteria; quick adhesion to the urothelium after instillation, should not bottleneck voiding of urine and should be retained in place for at least several hours. Adhesion between mucoadhesive polymers and extracellular matrix mucin found on the bladder surface is usually based on the attractive and repulsive forces operating at the molecular level. The efficiency of drug absorption is increased after coupling bioadhesion characteristics to microspheres, liposomes or nanoparticles because of improved intimate contact with the mucus layer. Microspheres based on chitosan are strongly mucoadhesive and their ocular instillation was able to increase the ocular residence time and decrease the frequency of administration for acyclovir 123.
Mucoadhesive materials are generally hydrophilic polymers that swell significantly in contact with water and eventually undergo complete dissolution. The molecules of hydrophilic polymers generally have hydroxyl, carboxyl or amine groups that favor adhesion to the mucosal surface upon wetting. The bioadhesive strength increases with an increase in molecular weight of polymer and these polymers can be categorized into following classes: anionic polymers such as sodium carboxymethyl cellulose and sodium alginate, cationic polymers such as chitosan and dextrans, nonionic polymers such as polyvinylpyrollidone, and cellulose derivatives such as hydroxypropylmethyl cellulose.
Post-operative chemotherapy in mice was successful with bioadhesive carriers based on polymers such as algin, chitosan and fibrinogen loaded with anticancer drugs 124. Bioadhesive microspheres poly(methylidene malonate-2.1.2) were able to release paclitaxel at the urothelium/urine interface of the mouse bladder for two days 120 and a gelatin based delivery system could release drugs for 12 days in the rabbit bladder 125. Another well known polymer is polyethylene glycol which improves bioadhesion by non-specific interpenetration of its polymer chains with mucus 126. Polymers such as chitosan and polycarbophil are ideal for a hydrophilic drug because they were able to retain good adhesion to isolated porcine urinary bladder after being fully hydrated 127, 128.
Temporal and spatial monitoring of instilled microparticles is possible with the technique of magnetic resonance imaging (MRI) 129. Polymeric microparticles were encapsulated with MRI contrast agent gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA) for measuring T1 relaxation rate of particles till 5 days after instillation. Partial clinical success has also been achieved through the use of mucoadhesion for IDD of oxybutynin in patients who suffer side effects from its metabolite N-desethyl-oxybutynin 130. Oxybutynin chloride was instilled twice daily at a dosage of 0.5 mg/ml in a case study involving 6 overactive bladder patients. The mucoadhesive solution of drug was prepared by adding 5% w/w hydroxypropylcellulose (HPC) to the oxybutynin solution to improve intravesical delivery of oxybutynin 130. The urodynamic studies performed on patients before starting the treatment and at 1 week and 3 years after the first instillation of oxybutynin revealed a significant increase in bladder capacity in 4 out of 6 patients.
Anchoring of lectins, bacterial adhesins and antibodies, etc. on the surface of the liposomes or microspheres can improve the therapeutic benefit after IDD of these carriers. Highly glycosylated proteins present at the bladder surface can specifically and non-covalently bind with lectins that are proteins of non-immune origin 131, 132. Therefore, attaching lectins to instilled drugs or delivery system can lend them bioadhesive characteristic for prolonged residence time 133, 134. Apart from lectin-mediated drug delivery systems, the carbohydrate specificity of mucus is also utilized by microorganisms to adhere to the gut or bladder mucosa 135.
6. Summary and Future directions
IDD can prove to be a sound alternative when disease has become refractory to treatment by drugs administered from other routes. If costs of treatment include physician visits, catheters, lubricant and man-hours required in getting the treatment are included then IDD can proved to be more expensive than oral drug administration. Besides, insertion of a catheter in the urethra can be a cause of discomfort in some patients and risk of UTI from IDD is possible if done carelessly.
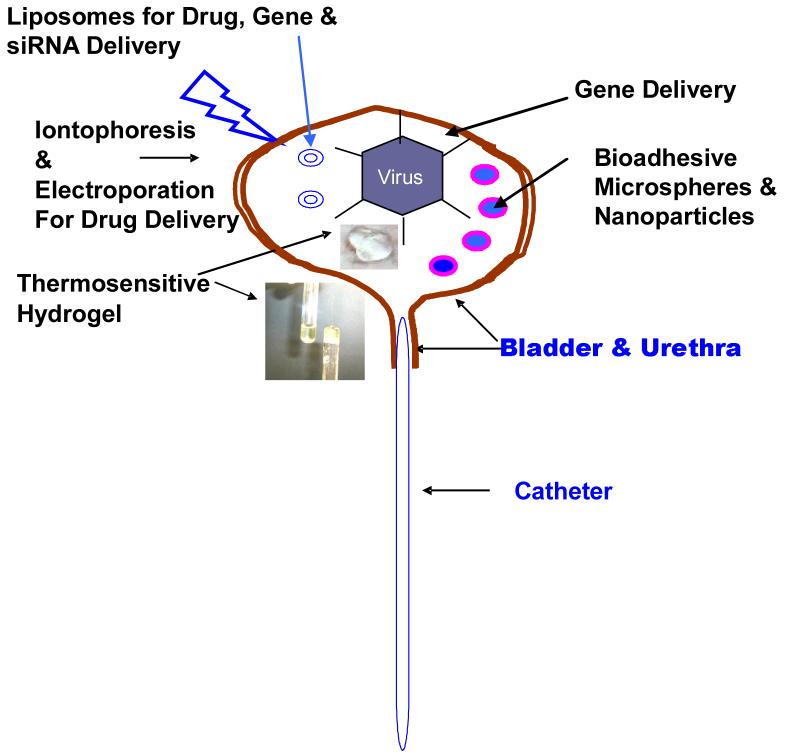
In summary, Table 1 shows the current methods of IDD and the practice of IDD using various physical and chemical agents is also illustrated in Fig.3. Among the IDD approaches for improving bladder permeability for instilled drugs, the physical approach of iontophoresis has gained wider acceptance in the clinic than the approach of using DMSO or protamine to chemically ablate the urothelium. The approach most effective for improving residence time inside the bladder so far is bioadhesion and its pre-clinical success has been reproduced in different labs. There is no doubt on the safety of liposomes as a carrier for drug or gene either systemically or via IDD. However, gene transfer efficiency of lipoplexes fall short of viral vectors and the higher gene transfer efficiency of adenovirus after co-administration with Syn3 justifies its clinical evaluation to realize the promise of gene therapy in bladder. The preclinical success of intravesical gene therapy in bladder cancer encourages its evaluation in treatment of IC. Unlike bladder cancer, the genes responsible for cystitis remain to be identified before therapy can be initiated. Further improvements in delivery systems would be necessary to optimize the delivery of gene, siRNA, antisense and proteins.
Table 1.
Use of delivery options in IDD for different drugs or therapeutic entities
| Advanced Delivery Options | Unique Advantage/Disadvantages | Drug/Therapeutic Entity | Clinical Testing | Authors |
|---|---|---|---|---|
| Iontophoresis & electroporation | Suited for Low Mol.wt. Drugs | Mitomycin C | Yes | Di Stasi, et al 2003 |
| DMSO & saponins | Cytotoxic and Poor Patient Acceptance | Paclitaxel | No | Chen et al, 2003 |
| Liposomes | Tissue Friendly Gene vector | Capsaicin, siRNA, IFNα, IL-2 | No |
Tyagi et al, 2004; Nogawa et al, 2005; Frangos et al, 1990 |
| Bioadhesive microspheres | Increased residence time | Paclitaxel, Mitomycin C, 5FU, Oxybutynin | Yes |
Le Visage et al, 2004 Ozturk et al ,2004 Singh et al, 1996 |
| Thermosensitive hydrogel | Higher Drug Loading capacity | Capsaicin & Misoprostol | No | Tyagi et al, 2004 |
| Adenovirus | Higher transduction efficiency | p53 gene, | Yes | Pagliaro et al, 2003 |
Fig. 3.
Schematic Diagram to Illustrate Advanced Delivery Options for Intravesical Drug & Gene Delivery.
These macromolecules are very expensive to produce and methods need to be developed to avoid the loss of instilled drugs in voided urine after administration. The search for an effective carrier that can ferry across GAG layer with minimum toxicity still continues for macromolecular based therapy. As illustrated in Fig.1, GAG layer is the primary barrier that retards the permeability of drugs across the bladder lumen. Agents such as protamine and transduction enhancing agents compromise the GAG layer for increased drug or gene delivery by IDD. The delivery systems using bioadhesion will continue to gain interest for extending the drug exposure in the bladder.
Future improvements in IDD are likely to gain from developments made in the field of nanotechnology. The techniques of bioluminenescence and fluorescence can be used to allow temporal and spatial monitoring of instilled drug carriers. It would be interesting to see how bioadhesive characteristics affect the behavior of liposomes and nanoparticles after instillation into the bladder. Moreover, since IDD is drug delivery across a mucosal surface and therefore future developments in this field can borrow from the developments made in drug delivery across other mucosal surfaces such as eye, colon, rectum and vagina.
Fig 2.
Schematic illustration of liposome and sites of drug entrapment.
Acknowledgement
The work in author’s lab (Leaf Huang) has been supported by a NIH grant DK 068556, and by a grant from the Fishbein Family Foundation. We will also like to thank Dr. Christian Coyle for assisting in the revision of this manuscript.
References
- (1).Lamm DL, Griffith JG. Semin Urol. 1992;10:39–44. [PubMed] [Google Scholar]
- (2).Igawa Y, Satoh T, Mizusawa H, Seki S, Kato H, Ishizuka O, Nishizawa O. BJU Int. 2003;91:637–641. doi: 10.1046/j.1464-410x.2003.04171.x. [DOI] [PubMed] [Google Scholar]
- (3).Buyse G, Waldeck K, Verpoorten C, Bjork H, Casaer P, Andersson KE. J Urol. 1998;160:892–896. doi: 10.1016/S0022-5347(01)62828-3. [DOI] [PubMed] [Google Scholar]
- (4).Kelly JD, Young MR, Johnston SR, Keane PF. Eur Urol. 1998;34:53–56. doi: 10.1159/000019679. [DOI] [PubMed] [Google Scholar]
- (5).Gao X, Au JL, Badalament RA, Wientjes MG. Clin Cancer Res. 1998;4:139–143. [PubMed] [Google Scholar]
- (6).Au JL, Badalament RA, Wientjes MG, Young DC, Warner JA, Venema PL, Pollifrone DL, Harbrecht JD, Chin JL, Lerner SP, Miles BJ. J Natl Cancer Inst. 2001;93:597–604. doi: 10.1093/jnci/93.8.597. [DOI] [PubMed] [Google Scholar]
- (7).Au JL, Jang SH, Wientjes MG. J Control Release. 2002;78:81–95. doi: 10.1016/s0168-3659(01)00488-6. [DOI] [PubMed] [Google Scholar]
- (8).Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML. Am J Physiol Renal Physiol. 1996;271:F886–894. doi: 10.1152/ajprenal.1996.271.4.F886. [DOI] [PubMed] [Google Scholar]
- (9).Min G, Stolz M, Zhou G, Liang F, Sebbel P, Stoffler D, Glockshuber R, Sun TT, Aebi U, Kong XP. J Mol Biol. 2002;317:697–706. doi: 10.1006/jmbi.2002.5442. [DOI] [PubMed] [Google Scholar]
- (10).Hohlbrugger G. J Urol. 1995;154:6–15. doi: 10.1016/s0022-5347(01)67208-2. [DOI] [PubMed] [Google Scholar]
- (11).Born M, Pahner I, Ahnert-Hilger G, Jons T. Eur J Cell Biol. 2003;82:343–350. doi: 10.1078/0171-9335-00326. [DOI] [PubMed] [Google Scholar]
- (12).Parsons CL. Urol Clin North Am. 1994;21:93–100. [PubMed] [Google Scholar]
- (13).Lande M, Donovan J, Zeidel M. J. Gen. Physiol. 1995;106:67–84. doi: 10.1085/jgp.106.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Staehelin LA, Chlapowski FJ, Bonneville MA. J. Cell Biol. 1972;53:73–91. doi: 10.1083/jcb.53.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Romih R, Jezernik K. Pflugers Arch. 1996;431:R241–242. doi: 10.1007/BF02346358. [DOI] [PubMed] [Google Scholar]
- (16).Parsons CL, Benson G, Childs SJ, Hanno P, Sant GR, Webster G. J Urol. 1993;150:845–848. doi: 10.1016/s0022-5347(17)35629-x. [DOI] [PubMed] [Google Scholar]
- (17).Parsons CL. World J Urol. 1994;12:38–42. doi: 10.1007/BF00182049. [DOI] [PubMed] [Google Scholar]
- (18).Parkin J, Shea C, Sant GR. Urology. 1997;49:105–107. doi: 10.1016/s0090-4295(97)00181-7. [DOI] [PubMed] [Google Scholar]
- (19).Stewart BH, Persky L, Kiser WS. J Urol. 1967;98:671–672. doi: 10.1016/S0022-5347(17)62954-9. [DOI] [PubMed] [Google Scholar]
- (20).Shirley SW, Stewart BH, Mirelman S. Urology. 1978;11:215–220. doi: 10.1016/0090-4295(78)90118-8. [DOI] [PubMed] [Google Scholar]
- (21).de Reijke TM, Kurth KH, Sylvester RJ, Hall RR, Brausi M, van de Beek K, Landsoght KE, Carpentier P. J Urol. 2005;173:405–409. doi: 10.1097/01.ju.0000150425.09317.67. [DOI] [PubMed] [Google Scholar]
- (22).Kassouf W, Kamat AM. Expert Rev Anticancer Ther. 2004;4:1037–1046. doi: 10.1586/14737140.4.6.1037. [DOI] [PubMed] [Google Scholar]
- (23).Poppas DP, Pavlovich CP, Folkman J, Voest EE, Chen X, Luster AD, O’Donnell MA. Urology. 1998;52:268–275. discussion 275-266. [PubMed] [Google Scholar]
- (24).Patard JJ, Saint F, Velotti F, Abbou CC, Chopin DK. Urol Res. 1998;26:155–159. doi: 10.1007/s002400050039. [DOI] [PubMed] [Google Scholar]
- (25).Zeidman EJ, Helfrick B, Pollard C, Thompson IM. Urology. 1994;43:121–124. doi: 10.1016/s0090-4295(94)80284-x. [DOI] [PubMed] [Google Scholar]
- (26).Lukban JC, Whitmore KE, Sant GR. Urol Clin North Am. 2002;29:649–660. doi: 10.1016/s0094-0143(02)00055-1. [DOI] [PubMed] [Google Scholar]
- (27).Parsons CL, Greene RA, Chung M, Stanford EJ, Singh G. J Urol. 2005;173:1182–1185. doi: 10.1097/01.ju.0000148361.82074.77. [DOI] [PubMed] [Google Scholar]
- (28).Theoharides TC, Sant GR. Int J Immunopathol Pharmacol. 2005;18:183–188. doi: 10.1177/039463200501800119. [DOI] [PubMed] [Google Scholar]
- (29).Tay H, Parsons CL, Stein PC. Urology. 1996;48:389–392. doi: 10.1016/s0090-4295(96)00209-9. [DOI] [PubMed] [Google Scholar]
- (30).de Deus JM, Girao MJ, Sartori MG, Baracat EC, Rodrigues de Lima G, Nader HB, Dietrich CP. Am J Obstet Gynecol. 2003;189:1654–1659. doi: 10.1016/s0002-9378(03)00867-6. [DOI] [PubMed] [Google Scholar]
- (31).Boucher WS, Letourneau R, Huang M, Kempuraj D, Green M, Sant GR, Theoharides TC. J Urol. 2002;167:380–384. [PubMed] [Google Scholar]
- (32).Nordling J, Jorgensen S, Kallestrup E. Urology. 2001;57:123. doi: 10.1016/s0090-4295(01)01079-2. [DOI] [PubMed] [Google Scholar]
- (33).Parsons CL. Expert Opin Pharmacother. 2004;5:287–293. doi: 10.1517/14656566.5.2.287. [DOI] [PubMed] [Google Scholar]
- (34).Dasgupta P, Chandiramani VA, Beckett A, Scaravilli F, Fowler CJ. BJU Int. 2000;85:238–245. doi: 10.1046/j.1464-410x.2000.00427.x. [DOI] [PubMed] [Google Scholar]
- (35).Cruz F. Urology. 2004;63:65–73. doi: 10.1016/j.urology.2003.11.001. [DOI] [PubMed] [Google Scholar]
- (36).Cruz F. Urology. 2002;59:51–60. doi: 10.1016/s0090-4295(01)01638-7. [DOI] [PubMed] [Google Scholar]
- (37).Cannon TW, Chancellor MB. Clin Obstet Gynecol. 2002;45:205–217. doi: 10.1097/00003081-200203000-00022. [DOI] [PubMed] [Google Scholar]
- (38).Chancellor MB, Yoshimura N. Urology. 2004;63:85–92. doi: 10.1016/j.urology.2003.10.034. [DOI] [PubMed] [Google Scholar]
- (39).Ritter S, Dinh TT. J Comp Neurol. 1992;318:103–116. doi: 10.1002/cne.903180108. [DOI] [PubMed] [Google Scholar]
- (40).Trevisani M, Gazzieri D, Benvenuti F, Campi B, Dinh QT, Groneberg DA, Rigoni M, Emonds-Alt X, Creminon C, Fischer A, Geppetti P, Harrison S. J Pharmacol Exp Ther. 2004;309:1167–1173. doi: 10.1124/jpet.103.064162. [DOI] [PubMed] [Google Scholar]
- (41).Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- (42).de Seze M, Wiart L, de Seze MP, Soyeur L, Dosque JP, Blajezewski S, Moore N, Brochet B, Mazaux JM, Barat M, Joseph PA. J Urol. 2004;171:251–255. doi: 10.1097/01.ju.0000100385.93801.d4. [DOI] [PubMed] [Google Scholar]
- (43).Reimer K, Fleischer W, Brogmann B, Schreier H, Burkhard P, Lanzendorfer A, Gumbel H, Hoekstra H, Behrens-Baumann W. Dermatology. 1997;195(Suppl 2):93–99. doi: 10.1159/000246039. [DOI] [PubMed] [Google Scholar]
- (44).Schafer-Korting M, Korting HC, Braun-Falco O. J Am Acad Dermatol. 1989;21:1271–1275. doi: 10.1016/s0190-9622(89)70342-x. [DOI] [PubMed] [Google Scholar]
- (45).Tsuruta T, Muraishi O, Katsuyama Y, Murata Y, Ichino M, Ogawa A. J Urol. 1997;157:1652–1654. [PubMed] [Google Scholar]
- (46).Hikosaka S, Hara I, Miyake H, Hara S, Kamidono S. Int J Urol. 2004;11:647–652. doi: 10.1111/j.1442-2042.2004.00855.x. [DOI] [PubMed] [Google Scholar]
- (47).Nogawa M, Yuasa T, Kimura S, Tanaka M, Kuroda J, Sato K, Yokota A, Segawa H, Toda Y, Kageyama S, Yoshiki T, Okada Y, Maekawa T. J Clin Invest. 2005;115:978–985. doi: 10.1172/JCI23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Zang Z, Mahendran R, Wu Q, Yong T, Esuvaranathan K. Int J Mol Med. 2004;14:713–717. [PubMed] [Google Scholar]
- (49).Bangham AD. Nature. 1961;192:1197–1198. doi: 10.1038/1921197a0. [DOI] [PubMed] [Google Scholar]
- (50).Connor RJ, Engler H, Machemer T, Philopena JM, Horn MT, Sutjipto S, Maneval DC, Youngster S, Chan T-M, Bausch J, McAuliffe JP, Hindsgaul O, Nagabhushan TL. Gene Therapy. 2001;8:41–48. doi: 10.1038/sj.gt.3301348. [DOI] [PubMed] [Google Scholar]
- (51).Ratliff TL. J Urol. 2005;173:2198–2199. [PubMed] [Google Scholar]
- (52).Kuball J, Wen SF, Leissner J, Atkins D, Meinhardt P, Quijano E, Engler H, Hutchins B, Maneval DC, Grace MJ, Fritz MA, Storkel S, Thuroff JW, Huber C, Schuler M. Journal of Clinical Oncology. 2002;20:957–965. doi: 10.1200/JCO.2002.20.4.957. [DOI] [PubMed] [Google Scholar]
- (53).Ng AW, Wasan KM, Lopez-Berestein G. Methods Enzymol. 2005;391:304–313. doi: 10.1016/S0076-6879(05)91017-3. [DOI] [PubMed] [Google Scholar]
- (54).Tyagi P, Chancellor MB, Li Z, De Groat WC, Yoshimura N, Fraser MO, Huang L. J Urol. 2004;171:483–489. doi: 10.1097/01.ju.0000102360.11785.d7. [DOI] [PubMed] [Google Scholar]
- (55).Frangos DN, Killion JJ, Fan D, Fishbeck R, von Eschenbach AC, Fidler IJ. J Urol. 1990;143:1252–1256. doi: 10.1016/s0022-5347(17)40248-5. [DOI] [PubMed] [Google Scholar]
- (56).Killion JJ, Fan D, Bucana CD, Frangos DN, Price JE, Fidler IJ. J Natl Cancer Inst. 1989;81:1387–1392. doi: 10.1093/jnci/81.18.1387. [DOI] [PubMed] [Google Scholar]
- (57).Lee JP, Jalili RB, Tredget EE, Demare JR, Ghahary A. J Interferon Cytokine Res. 2005;25:627–631. doi: 10.1089/jir.2005.25.627. [DOI] [PubMed] [Google Scholar]
- (58).Jeschke MG, Sandmann G, Finnerty CC, Herndon DN, Pereira CT, Schubert T, Klein D. Gene Ther. 2005;12:1718–1724. doi: 10.1038/sj.gt.3302582. [DOI] [PubMed] [Google Scholar]
- (59).Fraser MO, Chuang YC, Tyagi P, Yokoyama T, Yoshimura N, Huang L, De Groat WC, Chancellor MB. Urology. 2003;61:656–663. doi: 10.1016/s0090-4295(02)02281-1. [DOI] [PubMed] [Google Scholar]
- (60).Hu P, Meyers S, Liang F-X, Deng F-M, Kachar B, Zeidel ML, Sun T-T. Am J Physiol Renal Physiol. 2002;283:F1200–1207. doi: 10.1152/ajprenal.00043.2002. [DOI] [PubMed] [Google Scholar]
- (61).Sato K, Sugawara A, Kudo M, Uruno A, Ito S, Takeuchi K. Hypertens Res. 2004;27:417–425. doi: 10.1291/hypres.27.417. [DOI] [PubMed] [Google Scholar]
- (62).Lu Z, Yeh TK, Tsai M, Au JL, Wientjes MG. Clin Cancer Res. 2004;10:7677–7684. doi: 10.1158/1078-0432.CCR-04-1443. [DOI] [PubMed] [Google Scholar]
- (63).Malmstrom PU. BJU Int. 2002;89:681–686. doi: 10.1046/j.1464-410x.2002.02734.x. [DOI] [PubMed] [Google Scholar]
- (64).Connor RJ, Anderson JM, Machemer T, Maneval DC, Engler H. Urology. 2005;66:224–229. doi: 10.1016/j.urology.2005.02.015. [DOI] [PubMed] [Google Scholar]
- (65).Foresman WH, Messing EM. Seminars in Surgical Oncology. 1997;13:299–306. doi: 10.1002/(sici)1098-2388(199709/10)13:5<299::aid-ssu3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- (66).Benedict WF, Tao Z, Kim CS, Zhang X, Zhou JH, Adam L, McConkey DJ, Papageorgiou A, Munsell M, Philopena J, Engler H, Demers W, Maneval DC, Dinney CP, Connor RJ. Mol Ther. 2004;10:525–532. doi: 10.1016/j.ymthe.2004.05.027. [DOI] [PubMed] [Google Scholar]
- (67).Lamb K, Gebhart GF, Bielefeldt K. J Pain. 2004;5:150–156. doi: 10.1016/j.jpain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- (68).Liu J, Timmers AM, Lewin AS, Hauswirth WW. Invest Ophthalmol Vis Sci. 2005;46:3836–3844. doi: 10.1167/iovs.04-1283. [DOI] [PubMed] [Google Scholar]
- (69).Khan A, Benboubetra M, Sayyed PZ, Ng KW, Fox S, Beck G, Benter IF, Akhtar S. J Drug Target. 2004;12:393–404. doi: 10.1080/10611860400003858. [DOI] [PubMed] [Google Scholar]
- (70).Bartz S, Jackson AL. Sci STKE. 2005;2005:pe39. doi: 10.1126/stke.2952005pe39. [DOI] [PubMed] [Google Scholar]
- (71).Tsvetkov LM, Tsekova RT, Xu X, Stern DF. Cell Cycle. 2005;4:609–617. [PubMed] [Google Scholar]
- (72).Wood M, Perrotte P, Onishi E, Harper ME, Dinney C, Pagliaro L, Wilson DR. Cancer Gene Ther. 1999;6:367–372. doi: 10.1038/sj.cgt.7700090. [DOI] [PubMed] [Google Scholar]
- (73).Siemens DR, Austin JC, See WA, Tartaglia J, Ratliff TL. J Urol. 2001;165:667–671. doi: 10.1097/00005392-200102000-00091. [DOI] [PubMed] [Google Scholar]
- (74).Siemens DR, Crist S, Austin JC, Tartaglia J, Ratliff TL. J Urol. 2003;170:979–984. doi: 10.1097/01.ju.0000070925.10039.23. [DOI] [PubMed] [Google Scholar]
- (75).Fodor I, Timiryasova T, Denes B, Yoshida J, Ruckle H, Lilly M. J Urol. 2005;173:604–609. doi: 10.1097/01.ju.0000143196.37008.2c. [DOI] [PubMed] [Google Scholar]
- (76).Engler H, Anderson SC, Machemer TR, Philopena JM, Connor RJ, Wen S-F, Maneval DC. Urology. 1999;53:1049–1053. doi: 10.1016/s0090-4295(98)00641-4. [DOI] [PubMed] [Google Scholar]
- (77).Yamashita M, Rosser CJ, Zhou JH, Zhang XQ, Connor RJ, Engler H, Maneval DC, Karashima T, Czerniak BA, Dinney CP, Benedict WF. Cancer Gene Ther. 2002;9:687–691. doi: 10.1038/sj.cgt.7700488. [DOI] [PubMed] [Google Scholar]
- (78).Tao Z, Connor RJ, Ashoori F, Dinney CP, Munsell M, Philopena JA, Benedict WF. Cancer Gene Ther. 2006;13:125–130. doi: 10.1038/sj.cgt.7700865. [DOI] [PubMed] [Google Scholar]
- (79).Pagliaro LC, Keyhani A, Williams D, Woods D, Liu B, Perrotte P, Slaton JW, Merritt JA, Grossman HB, Dinney CP. J Clin Oncol. 2003;21:2247–2253. doi: 10.1200/JCO.2003.09.138. [DOI] [PubMed] [Google Scholar]
- (80).Gao X, Huang L. Biochem Biophys Res Commun. 1991;179:280–285. doi: 10.1016/0006-291x(91)91366-k. [DOI] [PubMed] [Google Scholar]
- (81).Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. J Biol Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- (82).Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G. Mol Biol Cell. 2002;13:830–846. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Horinaga M, Harsch KM, Fukuyama R, Heston W, Larchian W. Urology. 2005 doi: 10.1016/j.urology.2005.03.052. [DOI] [PubMed] [Google Scholar]
- (84).Wu Q, Mahendran R, Esuvaranathan K. Clin Cancer Res. 2003;9:4522–4528. [PubMed] [Google Scholar]
- (85).Schuetz YB, Naik A, Guy RH, Kalia YN. Expert Opin Drug Deliv. 2005;2:533–548. doi: 10.1517/17425247.2.3.533. [DOI] [PubMed] [Google Scholar]
- (86).Dyson C. Arch Ophthal. 1949;42:416–421. doi: 10.1001/archopht.1949.00900050424007. [DOI] [PubMed] [Google Scholar]
- (87).Di Stasi SM, Giannantoni A, Stephen RL, Capelli G, Navarra P, Massoud R, Vespasiani G. J Urol. 2003;170:777–782. doi: 10.1097/01.ju.0000080568.91703.18. [DOI] [PubMed] [Google Scholar]
- (88).Riedl CR, Stephen RL, Daha LK, Knoll M, Plas E, Pfluger H. J Urol. 2000;164:2108–2111. [PubMed] [Google Scholar]
- (89).Jewett MA, Valiquette L, Sampson HA, Katz J, Fradet Y, Redelmeier DA. J Urol. 1999;161:482–485. [PubMed] [Google Scholar]
- (90).Rosamilia A, Dwyer PL, Gibson J. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:142–145. doi: 10.1007/BF02764846. [DOI] [PubMed] [Google Scholar]
- (91).Fontanella UA, Rossi CA, Stephen RL. Br J Urol. 1997;79:414–420. doi: 10.1046/j.1464-410x.1997.32419.x. [DOI] [PubMed] [Google Scholar]
- (92).Gurpinar T, Truong LD, Wong HY, Griffith DP. J Urol. 1996;156:1496–1501. [PubMed] [Google Scholar]
- (93).Colombo R, Brausi M, Da Pozzo L, Salonia A, Montorsi F, Scattoni V, Roscigno M, Rigatti P. Eur Urol. 2001;39:95–100. doi: 10.1159/000052419. [DOI] [PubMed] [Google Scholar]
- (94).Lee CF, Chang SY, Hsieh DS, Yu DS. Cancer Gene Ther. 2004;11:194–207. doi: 10.1038/sj.cgt.7700658. [DOI] [PubMed] [Google Scholar]
- (95).Ogawa R, Kagiya G, Feril LB, Jr., Nakaya N, Nozaki T, Fuse H, Kondo T. J Urol. 2004;172:1469–1473. doi: 10.1097/01.ju.0000139589.52415.3d. [DOI] [PubMed] [Google Scholar]
- (96).Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- (97).Astriab-Fisher A, Sergueev D, Fisher M, Shaw BR, Juliano RL. Pharm Res. 2002;19:744–754. doi: 10.1023/a:1016136328329. [DOI] [PubMed] [Google Scholar]
- (98).Nielsen PE. Methods Mol Biol. 2005;288:343–358. doi: 10.1385/1-59259-823-4:343. [DOI] [PubMed] [Google Scholar]
- (99).Pooga M, Land T, Bartfai T, Langel U. Biomol Eng. 2001;17:183–192. doi: 10.1016/s1389-0344(01)00075-2. [DOI] [PubMed] [Google Scholar]
- (100).Ardelt PU, Wood CG, Chen L, Mintz PJ, Moya C, Arap MA, Wright KC, Pasqualini R, Arap W. J Urol. 2003;169:1535–1540. doi: 10.1097/01.ju.0000055477.37115.66. [DOI] [PubMed] [Google Scholar]
- (101).Chancellor MB. J Urol. 2005;174:818. doi: 10.1097/01.ju.0000175099.01082.d0. [DOI] [PubMed] [Google Scholar]
- (102).Vemulakonda VM, Somogyi GT, Kiss S, Salas NA, Boone TB, Smith CP. J Urol. 2005;173:621–624. doi: 10.1097/01.ju.0000143189.19835.f3. [DOI] [PubMed] [Google Scholar]
- (103).Khera M, Somogyi GT, Salas NA, Kiss S, Boone TB, Smith CP. Urology. 2005;66:208–212. doi: 10.1016/j.urology.2005.01.055. [DOI] [PubMed] [Google Scholar]
- (104).Tzan CJ, Berg JR, Lewis SA. Am J Physiol. 1994;267:C1013–1026. doi: 10.1152/ajpcell.1994.267.4.C1013. [DOI] [PubMed] [Google Scholar]
- (105).Cetinel S, Ercan F, Sirvanci S, Sehirli O, Ersoy Y, San T, Sener G. J Urol. 2003;169:1564–1568. doi: 10.1097/01.ju.0000049649.80549.17. [DOI] [PubMed] [Google Scholar]
- (106).Johnson PH, Quay SC. Expert Opin Drug Deliv. 2005;2:281–298. doi: 10.1517/17425247.2.2.281. [DOI] [PubMed] [Google Scholar]
- (107).Smith JM, Dornish M, Wood EJ. Biomaterials. 2005;26:3269–3276. doi: 10.1016/j.biomaterials.2004.06.020. [DOI] [PubMed] [Google Scholar]
- (108).Yang T, Hussain A, Paulson J, Abbruscato TJ, Ahsan F. Pharm Res. 2004;21:1127–1136. doi: 10.1023/b:pham.0000032998.84488.7a. [DOI] [PubMed] [Google Scholar]
- (109).Avadi MR, Jalali A, Sadeghi AM, Shamimi K, Bayati KH, Nahid E, Dehpour AR, Rafiee-Tehrani M. Int J Pharm. 2005;293:83–89. doi: 10.1016/j.ijpharm.2004.12.016. [DOI] [PubMed] [Google Scholar]
- (110).Chen D, Song D, Wientjes MG, Au JL. Clin Cancer Res. 2003;9:363–369. [PubMed] [Google Scholar]
- (111).Hashimoto H, Tokunaka S, Sasaki M, Nishihara M, Yachiku S. Urol Res. 1992;20:233–236. doi: 10.1007/BF00299723. [DOI] [PubMed] [Google Scholar]
- (112).Sasaki M, Hashimoto H, Yachiku S. Nippon Hinyokika Gakkai Zasshi. 1994;85:1353–1362. doi: 10.5980/jpnjurol1989.85.1353. [DOI] [PubMed] [Google Scholar]
- (113).Hashimoto H, Yachiku S, Watabe Y, Niibori D, Yamauchi K, Osanai H, Ohashi K, Wakabayashi A, Fujisawa M. Gan To Kagaku Ryoho. 1994;21:833–838. [PubMed] [Google Scholar]
- (114).Lazzeri M, Spinelli M, Beneforti P, Malaguti S, Giardiello G, Turini D. Eur Urol. 2004;45:98–102. doi: 10.1016/s0302-2838(03)00418-4. [DOI] [PubMed] [Google Scholar]
- (115).Miller LJ, Chandler SW, Ippoliti CM. Ann Pharmacother. 1994;28:590–594. doi: 10.1177/106002809402800508. [DOI] [PubMed] [Google Scholar]
- (116).Jeong B, Bae YH, Kim SW. J Control Release. 2000;63:155–163. doi: 10.1016/s0168-3659(99)00194-7. [DOI] [PubMed] [Google Scholar]
- (117).Ronneberger B, Kao WJ, Anderson JM, Kissel T. J Biomed Mater Res. 1996;30:31–40. doi: 10.1002/(SICI)1097-4636(199601)30:1<31::AID-JBM5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- (118).Jeong B, Bae YH, Kim SW. J Biomed Mater Res. 2000;50:171–177. doi: 10.1002/(sici)1097-4636(200005)50:2<171::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- (119).Tyagi P, Li Z, Chancellor M, De Groat WC, Yoshimura N, Huang L. Pharm Res. 2004;21:832–837. doi: 10.1023/b:pham.0000026436.62869.9c. [DOI] [PubMed] [Google Scholar]
- (120).Le Visage C, Rioux-Leclercq N, Haller M, Breton P, Malavaud B, Leong K. J Urol. 2004;171:1324–1329. doi: 10.1097/01.ju.0000103922.12319.59. [DOI] [PubMed] [Google Scholar]
- (121).Bouckaert J, Berglund J, Schembri M, De Genst E, Cools L, Wuhrer M, Hung CS, Pinkner J, Slattegard R, Zavialov A, Choudhury D, Langermann S, Hultgren SJ, Wyns L, Klemm P, Oscarson S, Knight SD, De Greve H. Mol Microbiol. 2005;55:441–455. doi: 10.1111/j.1365-2958.2004.04415.x. [DOI] [PubMed] [Google Scholar]
- (122).Jin Whan Lee JHP, Robinson Joseph R. Journal of Pharmaceutical Sciences. 2000;89:850–866. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- (123).Genta I, Conti B, Perugini P, Pavanetto F, Spadaro A, Puglisi G. Journal of Pharmacy and Pharmacology. 1997;49:737–742. doi: 10.1111/j.2042-7158.1997.tb06103.x. [DOI] [PubMed] [Google Scholar]
- (124).Ozturk E, Eroglu M, Ozdemir N, Denkbas EB. Adv Exp Med Biol. 2004;553:231–242. [PubMed] [Google Scholar]
- (125).Ye Z, Chen J, Zhang X, Li J, Zhou S, Yang W, Zhang Y. J Tongji Med Univ. 2001;21:145–148. doi: 10.1007/BF02888081. [DOI] [PubMed] [Google Scholar]
- (126).Lele BS, Hoffman AS. Journal of Controlled Release. 2000;69:237–248. doi: 10.1016/s0168-3659(00)00303-5. [DOI] [PubMed] [Google Scholar]
- (127).Lehr CM. Crit Rev Ther Drug Carrier Syst. 1994;11:119–160. [PubMed] [Google Scholar]
- (128).Lehr CM, Bouwstra JA, Bodde HE, Junginger HE. Pharm Res. 1992;9:70–75. doi: 10.1023/a:1018931811189. [DOI] [PubMed] [Google Scholar]
- (129).Chen HH, Le Visage C, Qiu B, Du X, Ouwerkerk R, Leong KW, Yang X. Magn Reson Med. 2005;53:614–620. doi: 10.1002/mrm.20395. [DOI] [PubMed] [Google Scholar]
- (130).Saito M, Watanabe T, Tabuchi F, Otsubo K, Satoh K, Miyagawa I. Int J Urol. 2004;11:592–596. doi: 10.1111/j.1442-2042.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- (131).Robinson MA, Charlton ST, Garnier P, Wang XT, Davis SS, Perkins AC, Frier M, Duncan R, Savage TJ, Wyatt DA, Watson SA, Davis BG. Proc Natl Acad Sci U S A. 2004;101:14527–14532. doi: 10.1073/pnas.0303574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Haas J, Lehr C-M. Expert Opinion on Biological Therapy. 2002;2:287–298. doi: 10.1517/14712598.2.3.287. [DOI] [PubMed] [Google Scholar]
- (133).Bhavanandan VP, Puch S, Guo X, Jiang W. Adv Exp Med Biol. 2001;491:95–108. doi: 10.1007/978-1-4615-1267-7_7. [DOI] [PubMed] [Google Scholar]
- (134).Kajiwara H, Yasuda M, Kumaki N, Shibayama T, Osamura Y. Tokai J Exp Clin Med. 2005;30:177–182. [PubMed] [Google Scholar]
- (135).Ishikawa K, Sundelin B, Mollby R, Normark S, Winberg J. Scand J Urol Nephrol. 2004;38:3–14. doi: 10.1080/00365590410031021. [DOI] [PubMed] [Google Scholar]