Summary
The mammalian cilium protrudes from the apical/lumenal surface of polarized cells, and acts as a sensor of environmental cues. Numerous developmental disorders and pathological conditions have been shown to arise from defects in cilia-associated signaling proteins. Despite mounting evidence that cilia are essential sites for coordination of cell signaling, little is known about the cellular mechanisms controlling their formation and disassembly. Here we show that define interactions between the pro-metastatic scaffolding protein HEF1/Cas-L/NEDD9 and the oncogenic Aurora A (AurA) kinase at the basal body of cilia causes phosphorylation and activation of HDAC6, a tubulin deacetylase, promoting ciliary disassembly. We show that this pathway is both necessary and sufficient for ciliary resorption, and constitutes an unexpected non-mitotic activity of AurA in vertebrates. Moreover, we demonstrate that small molecule inhibitors of AurA and HDAC6 selectively stabilize cilia from regulated resorption cues, suggesting a novel mode of action for these clinical agents.
Introduction
In polycystic kidney disease (PKD), Bardet-Biedl Syndrome (BBS), and other disorders, mutations in cilia-associated structural or signaling proteins cause insensitivity to external mechanical and diffusible signaling cues, resulting in disorganized, hyperplastic cell growth (Benzing and Walz, 2006; Pan et al., 2005; Singla and Reiter, 2006). On the organismal level, ciliary defects produce renal cysts, infertility, respiratory disorders, situs inversus, and predisposition to obesity, diabetes, and hypertension. Notably, recent studies have shown that the Hedgehog, Wnt, PDGFαα, and other signaling cascades are coordinated at cilia (Cano et al., 2004; Huangfu and Anderson, 2005; Liu et al., 2005; Schneider et al., 2005; Simons et al., 2005; Tanaka et al., 2005). The frequent deregulation of these pathways during cell transformation, together with the common disappearance of cilia in transformed cells, raises the possibility that defective ciliary signaling may promote cancer.
Although an increasing number of proteins are being defined as ciliary structural components or cilia-associated signaling proteins, very little is currently known about the cellular machinery controlling the formation and resorption of cilia. It has long been known that cilia are regulated dynamically throughout the cell cycle. In many cells, resorption occurs at mitotic entry, and reappearance after progression into G1. However, resorption is not solely linked to mitotic entry, with some cells undergoing waves of resorption at different points in cell cycle: for example, Tucker et al. have noted ciliary resorption as cells emerge from quiescence, prior to S-phase (Quarmby and Parker, 2005; Rieder et al., 1979; Tucker et al., 1979). Given their increasingly apparent role in detecting and transmitting extracellular signals, regulated formation, disassembly, or shortening of cilia may play an important role in cellular growth controls, serving as a rheostat to limit response to overly persistent or abnormal cell growth cues in the extracellular environment.
A cilium arises from a basal body, a structure that differentiates from one of the centrioles in the centrosome in non-proliferating cells and organizes the microtubule bundles that constitute the ciliary axoneme. Cilia are evolutionarily related to the motile flagella of lower eukaryotes, such as the green algae Chlamydomonas. Genetic studies in Chlamydomonas have recently begun to dissect the process of flagellar resorption (Bradley and Quarmby, 2005; Marshall et al., 2005; Pan and Snell, 2005; Quarmby, 2004). These studies have identified altered functionality of the intraflagellar transport (IFT) machinery and destabilization of the axoneme as hallmarks of disassembly, and implicated CALK and other kinases as regulators of disassembly. The means by which CALK becomes activated at initiation of disassembly and the critical CALK effectors in the disassembly process remain unknown, as does the relevance of these observations to higher eukaryotes.
CALK is very distantly related to the human Aurora A (AurA) kinase, with 55% similarity centered on the protein catalytic domain. In humans, Aurora A (AurA) is a centrosomal kinase that regulates mitotic entry through activation of Cdk1-cyclin B and other substrates that organize the mitotic spindle (Bischoff et al., 1998; Marumoto et al., 2005). AurA amplification or activation is common in many cancers characterized by centrosomal amplification and genomic instability (Anand et al., 2003; Goepfert et al., 2002; Gritsko et al., 2003). In the past year, upregulation of the HEF1 (Law et al., 1996; O’Neill et al., 2000) scaffolding protein by amplification or epigenetic means has recently been identified as part of a pro-metastatic signature in breast cancer (Minn et al., 2005), shown to contribute to the aggressiveness of glioblastomas (Natarajan et al., 2006), and found to be critical for progression to metastasis in melanomas (Kim et al., 2006). Although HEF1 is best known as a transducer of integrin-initiated attachment, migration, and anti-apoptotic signals at focal adhesions (O’Neill et al., 2000), we have recently documented interactions between HEF1 and AurA at the centrosome that are necessary for cellular progression through mitosis (Pugacheva and Golemis, 2005; Pugacheva and Golemis, 2006).
In this study, we demonstrate that an association between AurA and HEF1 at cilia in response to extracellular cues is required for ciliary disassembly. We also show that AurA activation is independently sufficient to induce rapid ciliary resorption, and that AurA acts in this process through phosphorylating HDAC6, thus stimulating HDAC6-dependent tubulin deacetylation (Hubbert et al., 2002) and destabilizing the ciliary axoneme. Importantly, our identification of a spatiotemporally restricted action of AurA at the ciliary basal body in cells emerging from G0 demonstrates an unexpected non-mitotic activity for AurA in vertebrate cells. We also determine that small molecule inhibitors of AurA and HDAC6 reduce regulated disassembly of cilia, which may have important implications for the action of these drugs in the clinic. Together, these data reveal important activities for HEF1, AurA, and HDAC6 in regulation of ciliary resorption, which should also inform the actions of these proteins in cell cycle and cancer (Hideshima et al., 2005; Kim et al., 2006; Marumoto et al., 2005; Pugacheva and Golemis, 2005).
Results
A system for regulated ciliary assembly and disassembly
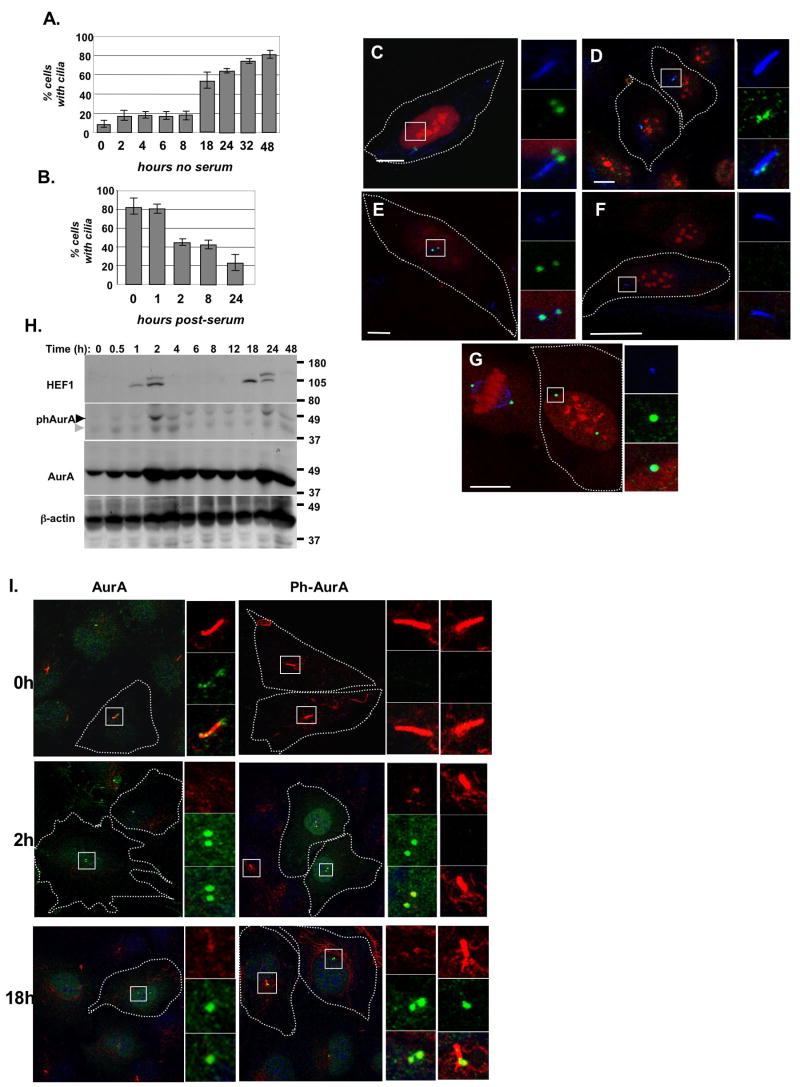
We established a system to study ciliary dynamics in the hTERT-RPE1 cell line. 48 hours after plating cells at 50-70% confluence in Opti-MEM medium without serum, >80% of hTERT-RPE1 cells had clearly visible cilia (Figure 1A). Cilia were typically of 3-4 μm length, with an acetylated α-tubulin-marked axoneme adjacent to two γ-tubulin-positive structures reflecting the basal body and the second cellular centriole (Supplemental Figure S1A). Treatment of these ciliated cells with medium containing 10% fetal bovine serum (FBS) caused ciliary disassembly over the following 24 hours (Figure 1B). This disassembly occurred in two waves, with the first occurring 1-2 hours after serum stimulation, and the second after 18-24 hours. FACS analysis, BrDU staining, and observation of condensed DNA and mitotic figures indicated that cells remained predominantly in G1 phase at 2 hours post-serum addition, while during the 18-24 hour disassembly wave, most cells were entering mitosis (Figures S1B, S1C). This disassembly behavior was not unique to hTERT-RPE1 cells, as we observed a comparable biphasic resorption profile in the IMCD-3 murine and Caki-1 human renal cell lines (Figures S1D, S1E). To begin to assess serum components that might regulate ciliary disassembly, we have assessed PDGF, TGF-β, and EGF (Figure S2). Of these, only PDGF elicited a partial response. Full disassembly likely requires the combined input of several distinct serum components.
Figure 1. Activation of AurA at the basal body occurs during ciliary disassembly.
A. Assembly of cilia. An average of 200 cells were counted in two independent experiments B. Disassembly of cilia induced by serum stimulation. An average of 150 cells were counted in each of 4 experiments. C. Immunofluorescence of quiescent cells with antibody to AurA (green), acetylated α-tubulin (blue), and DNA (red). Scale bar 10 μm. In this and subsequent panels, boxes in main image indicate structures shown at high magnification to right. D. Immunofluorescence of quiescent cells with polyclonal rabbit antibody to HEF1 (green), also visualizing acetylated α-tubulin (blue), and DNA (red); compare also to E. Scale bar 10 μm. E. Immunofluorescence of quiescent cells with monoclonal antibody to HEF1 (green), also visualizing γ-tubulin (blue) and DNA (red). Scale bar 5 μm. See also Supplemental Figure S3A. F. Immunofluorescence of quiescent cells with antibody to phospho-Aura (green), acetylated α-tubulin (blue), and DNA (red). Scale bar 12.5 μm. G. Immunofluorescence of serum-stimulated cells with antibody to phospho-Aura (green), acetylated α-tubulin (blue), and DNA (red). Scale bar 5 μm. H. Western analysis of AurA and HEF1 in hTERT-RPE1 cells after serum stimulation. Western blots shown represent strips and reprobes of a single gel. Higher molecular weight HEF1 band reflects hyperphosphorylation, and coincides with AurA activation and ciliary disassembly at 2 and 24 hours after serum addition (at time point 0). Light gray arrow indicates cross-reactivity of phospho-AurA directed antibody with total AurA; black arrow indicates phospho-AurA. See also Supplemental Figure 1H. I. Immunofluorescence depicting AurA activation in serum-stimulated cells during disassembly of cilia. All images are merged panels of acetylated α-tubulin (red), phospho-AurA or total AurA (green) and DNA (blue).
Dynamic regulation of HEF1 and AurA at the basal body during ciliary disassembly
AurA (Figure 1C) and HEF1 (Figures 1D,E) localized to the basal body and the second centriole in quiescent, ciliated hTERT-RPE1 cells. In contrast, activated (T288-phosphorylated) AurA was not detected at basal bodies of cilia in quiescent cells (Figure 1F, 1I (0h)) under fixation conditions at which it was clearly evident in mitotic cells (Figure 1G).
If AurA were functionally important for ciliary disassembly, we would expect changes in the activity of AurA 1-2 hours after serum treatment, potentially accompanied by changes in the AurA activator HEF1. Indeed, HEF1 expression increased at 1-2 hours after serum stimulation, dropped, and peaked again at ~18-24 hours after serum stimulation (Figure 1H). HEF1 initially appeared as a faster migrating 105 kD species, with a slower migrating 115 kD species appearing later. This 115 kD species represents S/T-phosphorylated HEF1, is most abundant during the G2/M compartment in actively cycling cells, and is associated with AurA activation (Law et al., 1998; Pugacheva and Golemis, 2005). Total AurA levels sometimes increased slightly at 2 hours post-serum stimulation, but were largely unaffected (Figures 1H). In contrast, peaks of phospho-T288-AurA appeared precisely at each of the two waves of ciliary disassembly (Figures 1H, I). Strikingly, phospho-T288-AurA was almost never detected at a basal body near a well-formed cilium. Although phospho-T288-AurA invariably co-localized with both γ-tubulin-marked basal bodies/centrioles and with total AurA, in 85-90% of cells with phospho-T288-AurA, centrioles had no accompanying cilium. In 10-15% of cells with phospho-T288-AurA, centrioles with adjacent acetylated α-tubulin-marked cilia were observed, but these cilia were significantly shortened (~1-2 versus 3.5 μm) (Figure 1I). Similar profiles of HEF1 and AurA expression and activation were observed in serum-treated IMCD3 and Caki-1 cells, and PDGF-treated hTERT-RPE1 cells (Figures S2B,C,F,G). The simplest interpretation of these results is that activation of AurA at the basal body immediately precedes the rapid disassembly of cilia.
HEF1-dependent activation of AurA induces ciliary disassembly
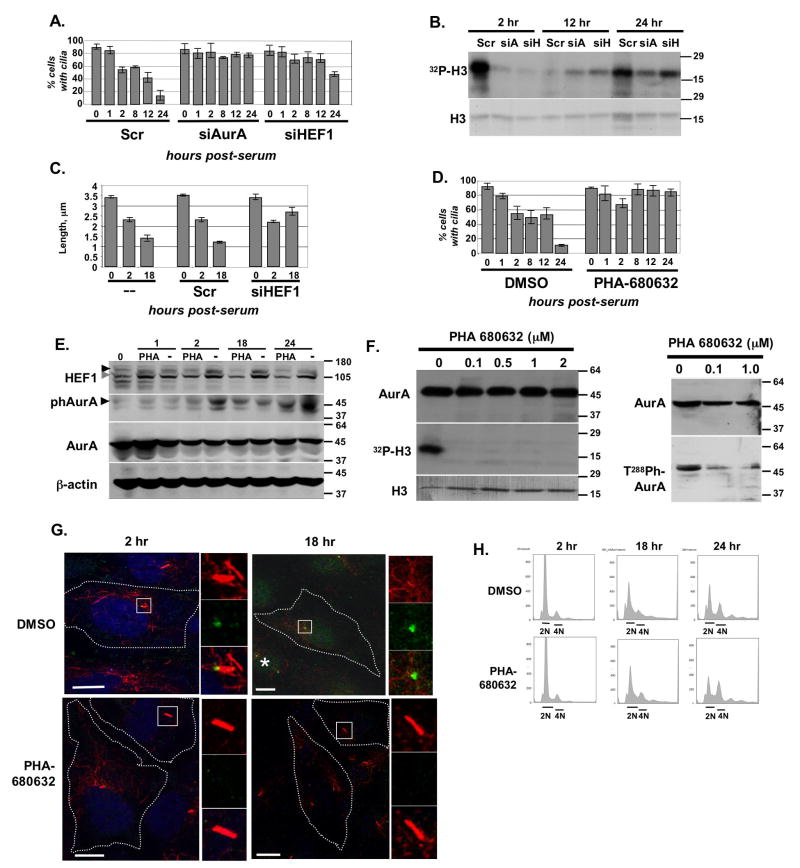
We used two complementary approaches to establish that AurA activation is necessary and sufficient for induction of ciliary disassembly, and that HEF1 is likely to contribute to this process. First, exponentially growing hTERT-RPE1 cells were treated with siRNA targeting AurA or HEF1, or with control siRNA, plated for 2 days in OptiMEM to allow cilia formation, then treated with serum to induce ciliary disassembly. Immunoblotting confirmed siRNA treatment efficiently depleted AurA and HEF1 (Figure S4A). AurA depletion blocked and HEF1 depletion greatly limited serum-induced disassembly (Figure 2A). AurA activation was substantially reduced in cells treated with siRNA to HEF1 (Figure 2B); this correlated with reduced levels of AurA in HEF1-depleted cells (Figure S4B), implying HEF1 contributes to AurA stabilization as well as activation. Particularly at the second wave of ciliary disassembly, the residual cilia in HEF1-depleted cells were significantly longer than those in control cells (Figure 2C), implying that HEF1 modulates the disassembly process. Importantly, cells treated with siRNA to AurA or HEF1, or with control siRNA, were all ~ 80% ciliated before addition of serum, leading us to conclude that the predominant role for HEF1 and AurA is at the time of disassembly, i.e., these proteins are not required to form cilia.
Figure 2. Activation of AurA is necessary for ciliary resorption.
A. Disassembly of cilia in cells treated with siRNA to AurA or HEF1, or with Scrambled (Scr) control siRNA, for 0 to 24 hours after serum addition. Assay performed 3 times, with an average of 100 cells counted/experiment by acetylated tubulin staining. Results were confirmed using a second antibody (anti-glutamylated tubulin) to independently score cilia following depletion (Supplemental Figures S4D, S4E). B. Ciliary disassembly was induced in ciliated cells pre-treated with control (Scr), AurA-targeted (siA), or HEF1-targeted (siH) siRNA by supplementing growth media with serum. At 2, 12, and 24 hours after addition of serum, AurA was immunoprecipitated and used for an in vitro kinase assay as in (Pugacheva and Golemis, 2005). Shown, 32P-labelled phosphorylated histone H3 (top) and total histone H3 in the reaction (stained with Coomassie Blue, bottom). C. Length of cilia in untreated hTERT-RPE1 cells (--), or the hTERT-RPE1 cells treated with control (Scr) or HEF1 targeting siRNA, at the indicated time points. D. Ciliated hTERT-RPE1 cells were treated with AurA inhibitor (PHA-680632) or DMSO, then disassembly of cilia tracked for 24 hours post serum addition. The in vitro IC50 of PHA-680632 is 27 nM for AurA; this compound also less potently inhibits AurC, AurB, and FGFR1 (IC50 120, 185, and 390 nM, respectively, (Soncini et al., 2006)). Results were confirmed using anti-glutamylated tubulin, as shown in Supplemental Figure S4D. E. Analysis performed in parallel with experiments described in D demonstrates PHA-680632 blocks appearance of T288-phospho-AurA (visualized with antibody from BioLegend), and HEF1 phosphorylation (115 kDa form), in reference to DMSO (-) at the 2 and 24 hour time points. Black arrows marks phosphorylated AurA, and hyperphosphorylated (p115) HEF1; gray arrow indicates p105 HEF1. See also Figure S1I. F. Cells were treated with indicated concentrations of the AurA inhibitor PHA-680632, and then AurA immunoprecipitated, and used for in vitro kinase reactions (left) or whole cell lysates used for Western analysis with antibody to total or phosphorylated AurA (right). G. Immunofluorescence analysis of appearance of phospho-AurA at times indicated after serum stimulation in DMSO- or PHA-680632-treated cells. DNA (blue), acetylated α-tubulin (red), and T288-phospho-AurA (green). In 18 hr DMSO/ph-AurA, an asterisk (*) marks a rare observation of phospho-AurA at the base of a shortened cilium. H. FACS analysis of cells treated with DMSO vehicle or PHA-680632 at the times indicated after serum stimulation.
Second, we used the small molecule AurA kinase inhibitor PHA-680632 (Nerviano Medical Sciences (Soncini et al., 2006)) to inactivate AurA kinase (Figures 2D,E). Disassembly of cilia was strongly reduced in cells pre-treated for 3 hours with 500 nM PHA-680632 (Figure 2D). Although some ciliary disassembly was observed at 1 and 2 hours after serum stimulation, the percentage was lower than in DMSO-treated cells, and disassembly was not maintained, with cilia consistently re-established at the 8- and 12-hour time points. The second wave of ciliary disassembly, at the time of mitosis, was completely eliminated in PHA-680632-treated cells (Figure 2D). In cells with inhibited AurA, hyper-phosphorylated HEF1 did not accumulate significantly at either wave of ciliary disassembly, indicating AurA dependence of this phosphorylation (Figure 2E). Western blot (Figures 2E, 2F (right panels)), in vitro kinase assays (Figure 2F, left panels) and immunofluorescence (Figure 2G) confirmed the effectiveness of the compound in blocking AurA activation.
Together, these data imply that activation of AurA by HEF1 contributes to resorption of cilia at 2 and 18 hours following serum stimulation (Figure 2A-E) and that active AurA is necessary to stably complete the disassembly process, but that HEF1 may not be the sole factor driving AurA activation and ciliary resorption (Figure 2A). Further, FACS analysis of cells with siRNA-depleted HEF1 or AurA (Supplemental Figure S4C), or drug-inhibited AurA (Figure 2H) AurA indicated that the blocked resorption of cilia at the 2h time point does not reflect an indirect consequence of altered cell cycle compartmentalization due to AurA inhibition. Cells indeed show predictable siRNA- and drug-induced accumulation in G2 at 18-24h after serum stimulation, which may account for the reduced resorption at these time points. However all cells at 2h post-serum treatment have similar cell cycle profiles, remaining predominantly in G0/G1. Hence, the role of HEF1 and AurA at this early non-mitotic time point represents an unexpected direct action of these proteins.
AurA activation is sufficient to induce rapid disassembly of cilia
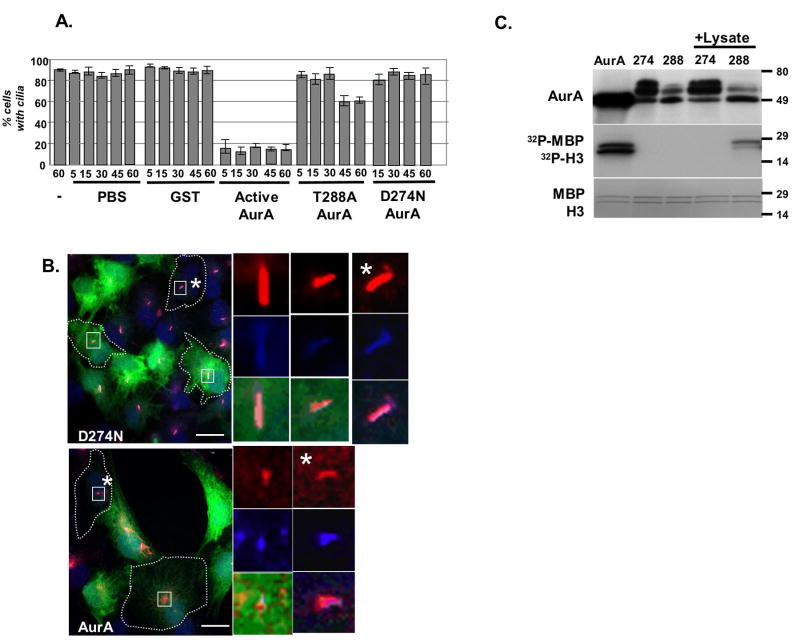
Next, as a direct approach to establish sufficiency of active AurA to induce disassembly, we microinjected pre-activated wild type AurA (aAurA), T288A AurA (a hypomorphic mutant, (Satinover et al., 2004)), D274N AurA (an inactive mutant), GST, or buffer alone, together with fluorescent marker dye, into hTERT-RPE1 cells with pre-formed cilia. Microinjection of aAurA rapidly induced the disappearance of cilia from cells maintained in low serum medium: essentially as soon as cells could be fixed after microinjection, more than 80% of injected cells lacked cilia (Figures 3A, B). In contrast, injection of GST or buffer did not induce loss of cilia. Of the two mutants, D274N did not induce loss of cilia, while T288A caused eventual partial loss of cilia (Figure 3A) and ciliary shortening (results not shown). The ability of aAurA, T288A, and D274N paralleled the behavior of these proteins in in vitro kinase assays performed in parallel to microinjections (Figure 3C). Whereas aAurA was highly active and D274N was completely inactive, T288A became weakly active following brief incubation with cell lysates. Hence, the delayed resorption of cilia and ciliary shortening induced by T288A likely reflects the gradual emergence of an active pool of AurA following microinjection.
Figure 3. Microinjection of active AurA causes rapid loss of cilia.
A. Microinjection of wild type AurA, T288A or D274N mutant AurA, or GST, or PBS buffer, into hTERT-RPE1 cells with pre-formed cilia. (-), uninjected controls. Time reflects minutes from injection to initiating fixation of slides. Experiments repeated 3 times, with >100 injected cells scored in each experiment. B. Cilia 45 minutes post-injection of AurA or D274N. Red, acetylated α-tubulin; blue, glutamylated α-tubulin (a second independent marker of cilia); blue, DNA; green, Dextran488 indicates injected cells. High magnification images to right are from boxed cells; * marks magnification of uninjected cells. C. AurA and mutants (D274N, T288A) were incubated with histone H3 (17 kD) and MBP (22 kD) substrates in an in vitro kinase assay, confirming the activity of kinase. +Lysate indicates that mutants were incubated for 3 hours at 4°C with hTERT-RPE1 cell lysate, then pulled down and used for the kinase assay.
HDAC6 is required for ciliary disassembly
Little is known about the cellular machinery necessary for disassembling cilia. In seeking targets of AurA phosphorylation that might be relevant to this process, we considered the possibility that the acetylated α-tubulin commonly used to visualize cilia might play an active role in stabilizing the ciliary axoneme, based on reports that α-tubulin deacetylation promoted the in vivo destabilization of microtubules (Matsuyama et al., 2002). In particular, histone deacetylase 6 (HDAC6) has been identified as an important cytoplasmic tubulin deacetylase that influences mitosis and chemotaxis through regulating tubulin stability (Hubbert et al., 2002).
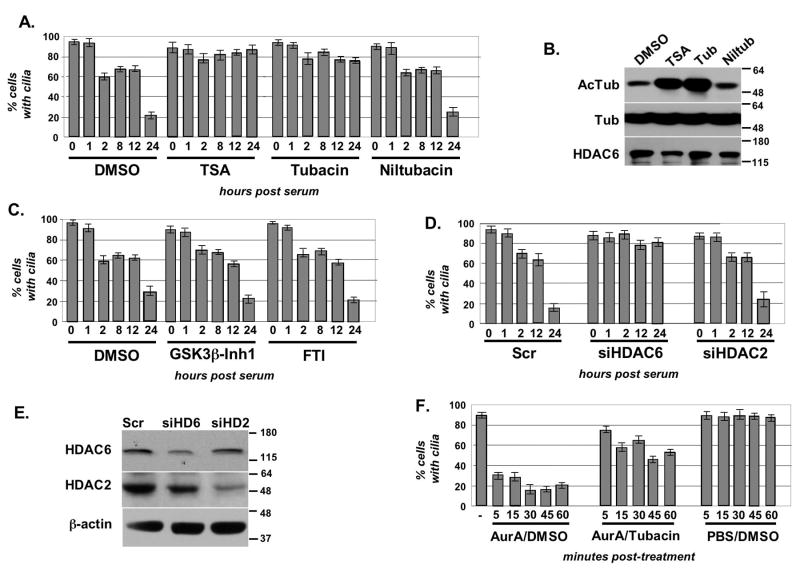
To assess whether altered regulation of tubulin acetylation might mediate HEF1/AurA signaling, we treated ciliated hTERT-RPE1 cells with small molecule deacetylase inhibitors, and established the ciliary disassembly profile (Figure 4A). Both the broad-spectrum HDAC inhibitor trichostatin A (TSA), and tubacin, an inhibitor specifically targeting HDAC6 (Hideshima et al., 2005), completely blocked serum-induced ciliary disassembly, whereas niltubacin, an inactive analog of tubacin, and vehicle alone had no effect. Levels of acetylated tubulin were measured in treated cells, confirming that these were increased in cells treated with TSA and tubacin, but not in cells treated with niltubacin or control vehicle (Figure 4B). As a control, because both AurA and HDAC inhibitors blocked ciliary disassembly, we considered the possibility that regulated ciliary disassembly might be generally sensitive to signaling inhibitors because of non-specific toxicities. However, serum induced disassembly with a normal profile in cells treated with inhibitors of GSK-3β and farnesyltransferase (FTI), indicating that blocked ciliary disassembly was specific response to impaired AurA and HDAC6 signaling (Figure 4C).
Figure 4. HDAC6 activity is necessary for resorption of cilia.
A. Treatment of hTERT-RPE1 cells with histone deacetylase inhibitors prevents ciliary resorption. Cells were incubated with indicated compounds or vehicle (DMSO) at concentrations described in Methods for 2 hours prior to induction of ciliary disassembly. The assay was performed 3 times, with an average of 100 cells counted/time point. B. TSA and tubacin increase intracellular levels of acetylated tubulin. Shown, Western blot with indicated antibodies showing levels of acetylated tubulin in cells treated with TSA, tubacin, niltubacin, or vehicle (DMSO) C. GSK3β inhibitor and farnesyltransferase inhibitor (FTI) do not inhibit ciliary disassembly. D. Depletion of HDAC6 restricts serum-induced disassembly of cilia in hTERT-RPE1 cells transfected for 48 hrs with siRNAs to HDAC6, HDAC2, or a scrambled control. E. Western analysis of hTERT-RPE1 cells treated with siRNA to HDAC6, HDAC2, or scrambled control. F. Active AurA or PBS were microinjected into hTERT-RPE1 cells pretreated for 2 hours with tubacin or DMSO.
To further confirm a specific requirement for HDAC6, we next established that cilia do not disassemble in serum-treated cells with siRNA-depleted HDAC6 (Figure 4D, 4E). Finally, we have microinjected aAurA into ciliated cells pre-treated for 3 hours with tubacin (Figure 4F). Tubacin pre-treatment substantially limited the ability of microinjected AurA to disassemble cilia. Initial disassembly was slower, and in some cases transient, with a significant percentage of injected cells re-forming cilia by 1 hour after injection. As for AurA, neither tubacin treatment nor siRNA to HDAC6 influenced cell cycle profile at 2h after serum stimulation, although both treatments led to accumulation in G2 at the later time point (Figures S4F,S4G). As a final control, we again used antibody to glutamylated tubulin as an independent means of scoring ciliary disassembly (Figure S4E). The results of these experiments are equivalent to those obtained using antibody to acetylated α-tubulin (Figures S5A-C). Based on these data, we concluded that HDAC6 is an important downstream AurA effector for ciliary disassembly.
AurA phosphorylates HDAC6 to activate tubulin deacetylase activity
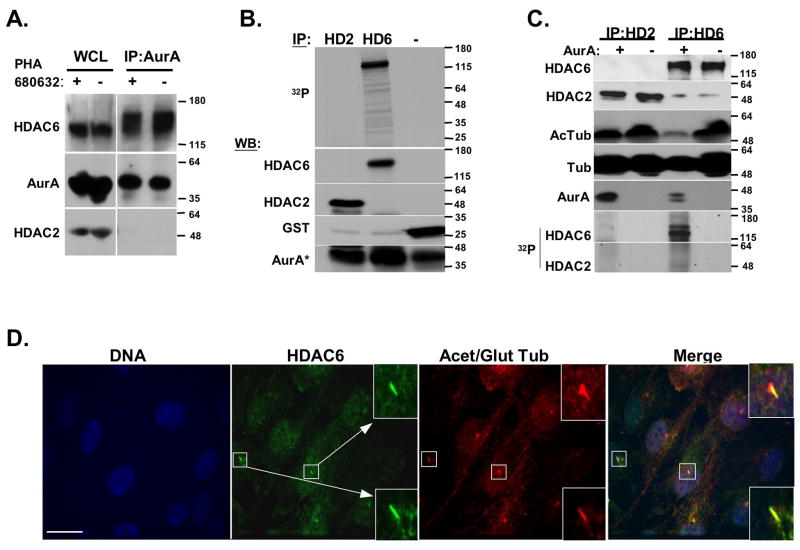
Taken together, our data suggested that the mechanism of ciliary disassembly by AurA requires intact HDAC6 deacetylation activity, to destabilize microtubules. AurA-dependent regulation of tubulin deacetylation may be direct or indirect. Importantly, although microinjection of AurA induced loss of ciliary α-acetylated tubulin as cilia disassemble, the non-ciliary α-acetylation of cytoplasmic microtubule networks were unaffected, suggesting a specific action of AurA and HDAC6 at the cilia (Figure S5C). Further supporting this idea, HDAC6 localized to cilia in serum-starved cells and during the ciliary disassembly process (Figure 5D and unpublished results), providing a ready target for AurA phosphorylation. Demonstrating a direct AurA-HDAC6 connection, antibody to AurA coimmunoprecipitated HDAC6 from hTERT-RPE1 cells (Figure 5A). AurA-HDAC6 coimmunoprecipitation was not eliminated by pre-treatment of cells with PHA-680632, indicating that the association was not regulated by AurA activation status (Figure 5A).
Figure 5. Direct phosphorylation by AurA activates HDAC6 tubulin deacetylase activity.
A. hTERT-RPE1 whole cell lysate (WCL) of cells treated with AurA inhibitor PHA 680632 (+) or with vehicle (-) was analyzed by Western blot directly, or following immunoprecipitation (IP) with antibody to AurA, using antibodies as indicated. The immunoprecipitation of the slow-migrating form of HDAC6 is not impacted by treatment of cells with PHA-680632, indicating that it most likely represents HDAC6 modified by an additional (unknown) cellular kinase/s. B. AurA phosphorylates HDAC6. In vitro translated and immunoprecipitated HDAC2 or HDAC6 (HD2, HD6), or recombinant GST (-), were mixed with recombinant AurA and used in an in vitro kinase assay. Reaction was split and used for autoradiography (32P) or Western Blot (WB) C. In vitro translated HDAC2 or HDAC6 (HD2, HD6) were immunoprecipitated (IPed). IPs were mixed with AurA (+) or buffer (-), then used for either an in vitro tubulin deacetylation assay, or in an in vitro kinase assay using γ-32P-ATP (see Methods). Reaction mix was visualized by Western blot and by autoradiography, as indicated. D. HDAC6 localizes to disassembling cilia 2 hours after serum treatment. Scale bar, 15 μM.
To directly determine whether HDAC6 might be an AurA substrate, recombinant activated AurA was used in an in vitro kinase assay with purified HDAC6, HDAC2, or GST, as in (Pugacheva and Golemis, 2005). AurA phosphorylated HDAC6, but not HDAC2 or the GST negative control (Figure 5B). We next immunoprecipitated in vitro translated HDAC6 and a negative control, HDAC2, and gauged the relative ability of AurA to phosphorylate these proteins, and stimulate a tubulin deacetylase activity, in a defined in vitro assay. In reactions containing comparable levels of HDAC2 and HDAC6, only HDAC6 was phosphorylated by AurA (Figure 5C). Moreover, AurA-phosphorylated HDAC6 was much more potent than unphosphorylated HDAC6 in deacetylating α-tubulin (Figure 5C). These results lead us to conclude that AurA phosphorylation of HDAC6 stimulates HDAC6 deacetylase activity.
Ciliary disassembly and intraflagellar transport (IFT)
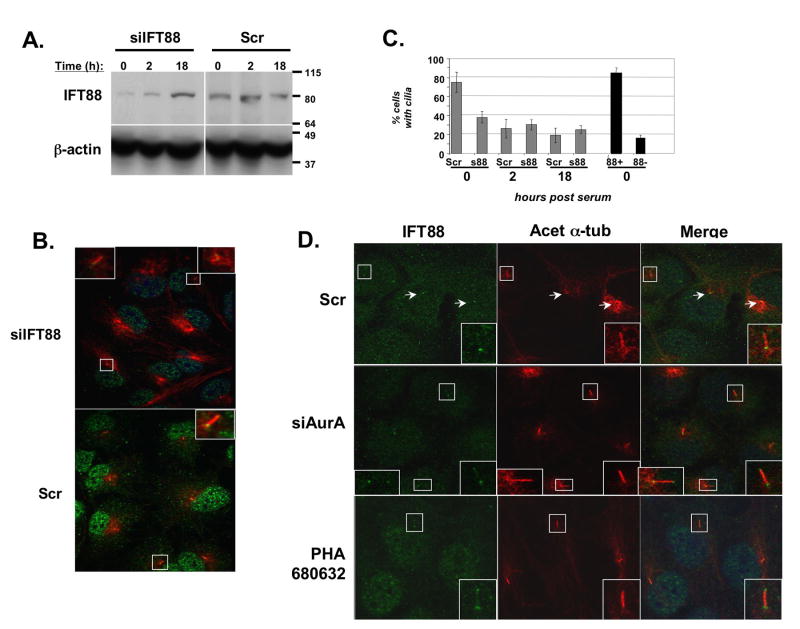
Intraflagellar transport proteins perform important roles in mediating transport of proteins to and from the apical tip of cilia, and in many cases mutations in IFT proteins have been linked to ciliary dysfunction, loss of cilia, and pathological conditions (Sloboda, 2005). In contrast to depletion of HEF1 or AurA, depletion of representative IFT proteins IFT88 (Figures 6A-C) and IFT20 (Supplemental Figure S6) limits the initial formation of cilia in hTERT-RPE1 cells similar to reports in other cell types (Follit et al., 2006; Pazour et al., 2000). Based on immunofluorescence, cilia were only observed in IFT-depleted cells that retain at least some detectable IFT protein (Figure 6C). This clear requirement of IFT proteins for ciliary assembly hinders the dissection of the contribution of these proteins in disassembly. However, intriguingly, the existing cilia in IFT88- or IFT20-depleted cells undergo minimal disassembly following serum stimulation, with the difference particularly noticeable at the early (2h) timepoint (Figures 6C, S6). Further, depletion or inhibition of AurA alters the localization of IFT88 during the ciliary disassembly process. In untreated cells, IFT88 is seen intensely at the basal body and more diffusely along the axoneme of residual cilia two hours after serum stimulation, whereas in cells lacking active AurA, IFT88 accumulates at both the basal body and apical tip at this time point (Figure 6D). It is likely that as in Chlamydomonas (Pan and Snell, 2005), IFT signaling mediates some aspects of ciliary disassembly.
Figure 6. A role for IFT proteins in AurA-induced ciliary resoprtion.
A. Western blot demonstrating siRNA depletion of IFT88 (siIFT88) in ciliated hTERT-RPE1 cells at times following serum treatment, relative to scramble-depleted control. B. Immunofluorescence matching Figure 6A at time 0, indicating relative degree of depletion of IFT88 at the basal body. C. Ciliary disassembly in IFT88- depleted (s88) versus Scr-depleted cells, at 0, 12, or 18 hours after serum treatment, based on the total cell population (gray bars). Black bars (right) indicate % ciliated cells at time 0 calculated specifically from cells confirmed by immunofluorescence to have significant IFT88 staining (88+), or to be well-depleted for IFT88 (88-). D. Cells treated with scrambled (Scr) or AurA-targeting (siAurA) siRNAs, or with PHA-680632 were fixed 2 hours after serum-initiated disassembly. Shown, immunofluorescence indicating cilia (anti-acetylated α-tubulin, red) and IFT88 (green). Insets are enlargements of boxed ciliary structures; arrows indicate direction of ciliary projection relative to basal body. Scale bars, 10 μm.
Discussion
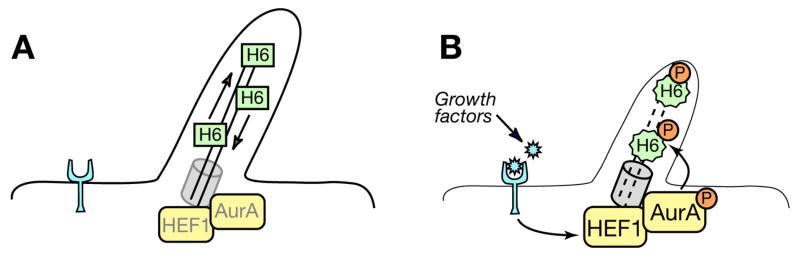
Cilia and flagella have been described as cellular “antennas”, sensing a multiplicity of extracellular stimuli to induce an intracellular response (Singla and Reiter, 2006). In addition to undergoing regulated resorption induced by extracellular cues, for over four decades cilia have been known to be dynamically resorbed and resynthesized throughout the cell cycle. Taken in sum, our data suggest a model (Figure 7) in which the serum growth factor-induced activation of a HEF1-AurA complex allows AurA to phosphorylate and activate HDAC6, which destabilizes the ciliary axoneme by deacetylating tubulin. Unexpectedly, activation of AurA is a central component of this cascade even during the G1 resorption wave, indicating a non-mitotic activity for AurA in animals.
Figure 7. Working Model.
A. Aurora A (AurA) and low levels of HEF1 are localized to the basal body of quiescent, ciliated cells. B. Our data are consistent with a model in which growth factors induce HEF1 expression, promoting HEF1-dependent activation of Aurora A. This results in phosphorylation of ciliary HDAC6 6 (H6) by Aurora A, thereby inducing ciliary resorption.
An important finding of this work is the novel connection between AurA and HDAC6. HDAC6 tightly interacts with α and β tubulins through its HDAC domain, which may restrict its enzymatic activity, based on reports that taxol treatment causes HDAC6 to accumulate on microtubules, and is accompanied by increased tubulin acetylation (Zhang et al., 2003). Localized phosphorylation by AurA may increase the turnover of HDAC6 at microtubules, thus increasing the active pool of HDAC6 at cilia. Interestingly, studies in Chlamydomonas indicate that an important element of flagellar resorption is destabilization of the microtubule-based axoneme, suggesting this signaling cascade may be evolutionarily conserved (Pan and Snell, 2005; Pan et al., 2004). Further supporting the idea of conservation, the C. elegans gene MEC-12 encodes an α-tubulin variant that is specifically required only in mechanosensing neurons, which depend on intact cilia: MEC-12 is the only α-tubulin in this species with a conserved site for acetylation (Fukushige et al., 1999). Interestingly, HDAC6 has been reported to associate with protein phosphatase 1 (PP1) (Brush et al., 2004), which binds microtubules (Liao et al., 1998), and dephosphorylates and inactivates AurA kinase. Such feedback may limit AurA activation at cilia.
A number of growth stimuli induce HEF1 expression and phosphorylation, influencing its protein interactions. These include PDGF, which is here shown to partially induce ciliary disassembly (Natarajan et al., 2006). Intriguingly, recent studies of p130Cas, a protein structurally similar to HEF1, indicate that p130Cas acts as a stretch sensor; HEF1 contains all sequence motifs necessary for similar function (Kostic and Sheetz, 2006). As one major function of cilium is to sense fluid flow, and overly persistent flow has been reported to induce ciliary disassembly (Iomini et al., 2004), stretch sensation may be an important action of HEF1. Our data suggest that HEF1 both activates AurA and stabilizes the protein from degradation; it will be interesting to determine if the HEF1 scaffolding activity also contributes to AurA interaction with its effector HDAC6. Our data also indicate that AurA activity influences IFT88 localization during disassembly, and suggest integrity of the IFT system is important for the disassembly process in animals, as in Chlamydomonas (Pan and Snell, 2005).
Our establishment of a HEF1-AurA-HDAC6 cascade at cilia also informs the understanding of the mitotic activities of these proteins. Dynamic changes in microtubule acetylation and deacetylation characterize the stages of mitosis, and HDAC inhibitors that inhibit family members with microtubule deacetylase activity induce mitotic arrest (Blagosklonny et al., 2002). The identification here of HDAC6 as an AurA target suggests that HEF1-AurA regulation of tubulin deacetylation at mitosis through HDAC6 might offer a mechanism to fine-tune the mechanical properties of the mitotic spindle. This signaling cascade may also influence reestablishment of focal adhesions at and following cytokinesis, given the growing appreciation of the role of microtubules in guiding the formation of these structures (Ezratty et al., 2005; Strickland et al., 2005). Further, one intriguing possibility is that the common use of an AurA-HEF1-HDAC6 switch at the basal body of quiescent cells and the centrosome of G2/M cells may serve as part of a checkpoint mechanism coordinating responsiveness to extracellular cues at different points in cell cycle. In this context, our observation that inhibition of AurA causes appearance of mitotically arrested cells possessing both spindles and cilia (results not shown) may reflect triggering of such a centrosomally based checkpoint.
These results also have implications for the understanding and treatment of cancer. Tumor cells commonly do not have cilia, and both HEF1 and AurA are often upregulated in cancer. The roles for these proteins at the centrosome and focal adhesions described earlier already offer two mechanisms by which these proteins may promote tumor initiation and progression. The current study indicates a third mechanism, in which elevation of HEF1 or AurA in tumors may destabilize cilia, thus conditioning cellular response to external cues and impacting multiple signaling pathways. Further, AurA is regarded as a promising chemotherapeutic target, with agents inhibiting this protein currently in clinical trials (Andrews, 2005). TSA and other broad-spectrum agents targeting HDACs are used in the clinic (Vanhaecke et al., 2004), with more focused agents such as tubacin in preclinical development (Hideshima et al., 2005). Our data suggest that AurA- or HDAC-targeted drugs may have previously unappreciated in vivo effects involving cilia, that may contribute to the observed efficacy and/or side effects of these agents.
PKD is one of the best-described cilia-related diseases (Wilson, 2001), with mutation of the cilia-localized polycystin proteins 1 and 2 (PKD1 and PKD2) responsible for the significant majority of PKD patients. p130Cas interacts directly with complexes containing PKD1 and PKD2, and also with nephrocystins, cilia-associated proteins that are mutated in a second renal cystic syndrome, nephronophthisis (Benzing et al., 2001). Although an association of HEF1 with these proteins has never been assessed, HEF1 is abundant in the kidney and conserves many protein interaction sequences with p130Cas. It is also tantalizing to consider that closer connections exist between dysplastic disorders leading to cysts and cancer than have previously been appreciated. One of the surprising results of a recent large study to analyze the cancer genome was the identification of the PKHD1 protein, a ciliary protein which is mutant in autosomal recessive PKD, as commonly mutated in colorectal cancer (Sjoblom et al., 2006). Overall, deregulated AurA/HEF1/HDAC6 signaling may have broad implications for studies of human development and disease.
Experimental Procedures
Cell culture and siRNA
hTERT-RPE1 cells were grown in DMEM with 10% fetal bovine serum (FBS). For analysis of ciliary disassembly, cells were plated at 30% confluence in plates containing glass cover slips, and starved for 48 hours (in Opti-MEM or regular DMEM, without added serum) to induce cilia formation, followed by treatments described in Results. Details of siRNAs used for depletion of HEF1, AurA, HDAC6, HDAC2, IFT88, IFT20, and control siRNAs, are available on request. For siRNA treatment, cells were initially plated in DMEM/10%FBS in plates containing cover slips, and 12 hours later siRNA transfection was performed in Opti-MEM with Oligofectamine (Invitrogen) according to manufacturer recommendations, and fixed 48 hours after transfection, following treatments indicated in Results. The remaining cells on plate were lysed, then either directly analyzed by Western blot analysis, or used for immunoprecipitation (IP)-kinase reaction to measure AurA activity.
Drug inhibition experiments
The Aurora kinase inhibitor PHA-680632, GSK3β-inhibitor 1 (Calbiochem), FTI-277 (Calbiochem), Tubacin, Niltubacin or DMSO vehicle were added to hTERT-RPE1 cells 2 hours prior to the initiation of ciliary disassembly. After initial titration experiments to establish effective range, PHA-680632 was used at 0.5μM, Tubacin and Niltubacin at 2μM, GSK3β-inhibitor 1 at 2μM, FTI-277 at 50nM concentration for the experiments described.
Protein expression, Western blotting, and immunoprecipitation
For microinjection, recombinant glutathione-S-transferase (GST), GST fused AurA mutants T288A and D274N) produced from BL21 (DE3) bacteria were purified using the MicroSpin GST Purification Module (Amersham Biotech.). Purified recombinant AurA was purchased from Upstate; this AurA was pre-activated based on incubation with ATP. Mutationally inactive AurA (T288A,) was also made using a baculoviral expression system (Invitrogen), and was purified by Ni-Sepharose 6FF (Amersham).
To prepare lysates for Western blotting and IP, mammalian cells were disrupted by M-PER lysis buffer (Pierce) supplemented with EDTA-free protease inhibitor cocktail (Roche). Lysates used for IP were incubated overnight with antibody at 4°C, subsequently incubated for 2 hours with protein A/G-sepharose (Pierce), washed, and resolved by SDS-PAGE. Western blotting was performed using standard procedures and proteins visualized using the West-Pico system (Pierce). Antibodies used included mouse monoclonal antibody (mAb) anti-HEF1 2G9 (Pugacheva and Golemis, 2005), anti-α-tubulin mAb (Sigma), anti-AurA (BD Bioscience) for Western blotting, anti-AurA rabbit polyclonal (Cell Signaling) for IP, anti-Phospho-AurA/T288 (BioLegend), anti-Phospho-AurA/T288 (Cell Signaling), anti-HDAC6 rabbit polyclonal (Upstate; 1:5000), anti-HDAC2 rabbit polyclonal (Invitrogen) and mAb anti-β-actin (AC15, Sigma), anti-IFT88 and anti-IFT20. Secondary horseradish peroxidase (HRP)-conjugated antibodies were from Amersham Biotech.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde (10 min) then methanol (5 min), permeabilized with 1%Triton-X100 in PBS, blocked in 1xPBS, 3%BSA, and incubated with antibodies using standard protocols. Primary antibodies included rabbit polyclonal anti-Aurora A and anti-phospho-AuroraA/T288, (Cell Signaling), mouse mAb anti-HEF1 (14A11), polyclonal anti-γ-tubulin (Sigma), anti-α-tubulin mAb (Sigma), anti-acetylated α-tubulin mAb (clone 6-11B-1, Sigma, and clone K(Ac)40 Biomol), anti-IFT88 and anti-IFT20 (gifts of G. Pazour), mouse anti-glutamylated tubulin (Sigma), and anti-HDAC6 (Upstate). Secondary antibodies labeled with Alexa-488, Alexa-568, and Alexa-633, and TOTO-3 dye to stain DNA, were from Molecular Probes/ Invitrogen. DNA was co-stained in some experiments by propidium iodine (Sigma) or Draq5 (Alexis). Confocal microscopy was performed using a Radiance 2000 laser scanning confocal microscope ((Carl Zeiss, Thornwood, NY) coupled to a Nikon Eclipse E800 upright microscope (Nikon). Statistical analysis of data by one-way ANOVA was performed using GraphPad Instat 3.0 (San Diego, CA).
Microinjection
Microinjections were performed on a Nikon TE300 Microscope (Nikon, Melville, NY) that was equipped with an Eppendorf Transjector 5246 semi-automatic microinjector and micromanipulator (Eppendorf, Westbury, NY). Cells were plated on gridded coverslips (Belco) and starved for 48 hours before cytoplasmic microinjection of 0.05μM pre-activated AurA (Upstate), inactive AurA (T288A) and (D274N), GST protein, or buffer. Proteins were pre-filtered through a 0.2-μm Milliopore membrane and mixed with Dextran Green488 (Molecular Probes) to mark injected cells. Injected cells were incubated at 37°C before fixation. Typically, 150 cells were microinjected in each of 3 experiments.
Kinase and tubulin deacetylation assays
In vitro kinase assays were performed using recombinant active AurA (Upstate), mutationally inactive AurA purified from baculovirus and BL21(DE3) bacteria, or endogenous AurA immunoprecipitated from mammalian cells. A standard kinase reaction with γ-32P(ATP) and histone H3 and MBP (Upstate) substrates was done as in (Pugacheva and Golemis, 2005). For deacetylase assays, HDAC6 and HDAC2 were in vitro translated using a TnT-Coupled Reticulocyte Lysate System (Promega), immunoprecipitated, and incubated with/without active AurA(Upstate) in the presence of (25μg) stabilized microtubules prepared from purified bovine brain tubulin (Cytoskeleton) to measure deacetylase activity (as in (Hubbert et al., 2002)) and with γ-32P-ATP (Perkin-Elmer) in AurA reaction buffer. 1/10 volume of samples were reserved for Western blotting.
Supplementary Material
Acknowledgments
This work and the authors were supported by NIH RO1 CA63366, a Department of Defense OCRF IDEA Award, Pennsylvania Tobacco Settlement Funds, and a grant from the Susan B. Komen Foundation (to EAG), and by an Appropriation from the Commonwealth of Pennsylvania, and by NIH core grant CA-06927 (to Fox Chase Cancer Center). TRH is supported by NRSA funding from the NIH. We thank Jonathan Chernoff for critical review of the manuscript. We thank Seth Bluestein for help in scoring cilia. We gratefully acknowledge Dr. Jurgen Moll and Nerviano Medical Sciences Srl., for the gift of PHA-680632, Drs. James Bradner and Ralph Mazitschek of the Broad Institute of Harvard and MIT for the gifts of tubacin and niltubacin, Dr. Gregory Pazour for antibodies to IFT proteins, and Dr. Richard Bayliss for the D274N AurA mutant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- Andrews PD. Aurora kinases: shining lights on the therapeutic horizon? Oncogene. 2005;24:5005–5015. doi: 10.1038/sj.onc.1208752. [DOI] [PubMed] [Google Scholar]
- Benzing T, Gerke P, Hopker K, Hildebrandt F, Kim E, Walz G. Nephrocystin interacts with Pyk2, p130(Cas), and tensin and triggers phosphorylation of Pyk2. Proc Natl Acad Sci U S A. 2001;98:9784–9789. doi: 10.1073/pnas.171269898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing T, Walz G. Cilium-generated signaling: a cellular GPS? Curr Opin Nephrol Hypertens. 2006;15:245–249. doi: 10.1097/01.mnh.0000222690.53970.ca. [DOI] [PubMed] [Google Scholar]
- Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. Embo J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV, Robey R, Sackett DL, Du L, Traganos F, Darzynkiewicz Z, Fojo T, Bates SE. Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol Cancer Ther. 2002;1:937–941. [PubMed] [Google Scholar]
- Bradley BA, Quarmby LM. A NIMA-related kinase, Cnk2p, regulates both flagellar length and cell size in Chlamydomonas. J Cell Sci. 2005;118:3317–3326. doi: 10.1242/jcs.02455. [DOI] [PubMed] [Google Scholar]
- Brush MH, Guardiola A, Connor JH, Yao TP, Shenolikar S. Deactylase inhibitors disrupt cellular complexes containing protein phosphatases and deacetylases. J Biol Chem. 2004;279:7685–7691. doi: 10.1074/jbc.M310997200. [DOI] [PubMed] [Google Scholar]
- Cano DA, Murcia NS, Pazour GJ, Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development. 2004;131:3457–3467. doi: 10.1242/dev.01189. [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–590. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushige T, Siddiqui ZK, Chou M, Culotti JG, Gogonea CB, Siddiqui SS, Hamelin M. MEC-12, an alpha-tubulin required for touch sensitivity in C. elegans. J Cell Sci. 1999;112(Pt 3):395–403. doi: 10.1242/jcs.112.3.395. [DOI] [PubMed] [Google Scholar]
- Goepfert TM, Adigun YE, Zhong L, Gay J, Medina D, Brinkley WR. Centrosome amplification and overexpression of aurora A are early events in rat mammary carcinogenesis. Cancer Res. 2002;62:4115–4122. [PubMed] [Google Scholar]
- Gritsko TM, Coppola D, Paciga JE, Yang L, Sun M, Shelley SA, Fiorica JV, Nicosia SV, Cheng JQ. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin Cancer Res. 2003;9:1420–1426. [PubMed] [Google Scholar]
- Hideshima T, Bradner JE, Wong J, Chauhan D, Richardson P, Schreiber SL, Anderson KC. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci U S A. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Gans JD, Nogueira C, Wang A, Paik JH, Feng B, Brennan C, Hahn WC, Cordon-Cardo C, Wagner SN, et al. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125:1269–1281. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Kostic A, Sheetz MP. Fibronectin Rigidity Response through Fyn and p130Cas Recruitment to the Leading Edge. Mol Biol Cell. 2006 doi: 10.1091/mbc.E05-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law SF, Estojak J, Wang B, Mysliwiec T, Kruh GD, Golemis EA. Human Enhancer of Filamentation 1 (HEF1), a novel p130Cas-like docking protein, associates with FAK, and induces pseudohyphal growth in yeast. Mol Cell Biol. 1996;16:3327–3337. doi: 10.1128/mcb.16.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law SF, Zhang Y-Z, Klein-Szanto A, Golemis EA. Cell-cycle regulated processing of HEF1 to multiple protein forms differentially targeted to multiple compartments. Mol Cell Biol. 1998;18:3540–3551. doi: 10.1128/mcb.18.6.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Li Y, Brautigan DL, Gundersen GG. Protein phosphatase 1 is targeted to microtubules by the microtubule-associated protein Tau. J Biol Chem. 1998;273:21901–21908. doi: 10.1074/jbc.273.34.21901. [DOI] [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Marshall WF, Qin H, Rodrigo Brenni M, Rosenbaum JL. Flagellar length control system: testing a simple model based on intraflagellar transport and turnover. Mol Biol Cell. 2005;16:270–278. doi: 10.1091/mbc.E04-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. Embo J. 2002;21:6820–6831. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, Wang W, Grammer JR, Gladson CL. HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene. 2006;25:1721–1732. doi: 10.1038/sj.onc.1209199. [DOI] [PubMed] [Google Scholar]
- O’Neill GM, Fashena SJ, Golemis EA. Integrin signaling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10:111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- Pan J, Snell WJ. Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking, and blocking anterograde cargo loading. Dev Cell. 2005;9:431–438. doi: 10.1016/j.devcel.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Pan J, Wang Q, Snell WJ. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev Cell. 2004;6:445–451. doi: 10.1016/s1534-5807(04)00064-4. [DOI] [PubMed] [Google Scholar]
- Pan J, Wang Q, Snell WJ. Cilium-generated signaling and cilia-related disorders. Lab Invest. 2005;85:452–463. doi: 10.1038/labinvest.3700253. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva EN, Golemis EA. The focal adhesion scaffolding protein HEF1 regulates activation of the Aurora-A and Nek2 kinases at the centrosome. Nat Cell Biol. 2005;7:937–946. doi: 10.1038/ncb1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva EN, Golemis EA. HEF1-aurora A interactions: points of dialog between the cell cycle and cell attachment signaling networks. Cell Cycle. 2006;5:384–391. doi: 10.4161/cc.5.4.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby LM. Cellular deflagellation. Int Rev Cytol. 2004;233:47–91. doi: 10.1016/S0074-7696(04)33002-0. [DOI] [PubMed] [Google Scholar]
- Quarmby LM, Parker JD. Cilia and the cell cycle? J Cell Biol. 2005;169:707–710. doi: 10.1083/jcb.200503053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Jensen CG, Jensen LC. The resorption of primary cilia during mitosis in a vertebrate (PtK1) cell line. J Ultrastruct Res. 1979;68:173–185. doi: 10.1016/s0022-5320(79)90152-7. [DOI] [PubMed] [Google Scholar]
- Satinover DL, Leach CA, Stukenberg PT, Brautigan DL. Activation of Aurora-A kinase by protein phosphatase inhibitor-2, a bifunctional signaling protein. Proc Natl Acad Sci U S A. 2004;101:8625–8630. doi: 10.1073/pnas.0402966101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber T, Mandelker D, Leary RJ, Ptak J, Silliman N, et al. The Consensus Coding Sequences of Human Breast and Colorectal Cancers. Science. 2006 doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- Sloboda RD. Intraflagellar transport and the flagellar tip complex. J Cell Biochem. 2005;94:266–272. doi: 10.1002/jcb.20323. [DOI] [PubMed] [Google Scholar]
- Soncini C, Carpinelli P, Gianellini L, Fancelli D, Vianello P, Rusconi L, Storici P, Zugnoni P, Pesenti E, Croci V, et al. PHA-680632, a novel Aurora kinase inhibitor with potent antitumoral activity. Clin Cancer Res. 2006;12:4080–4089. doi: 10.1158/1078-0432.CCR-05-1964. [DOI] [PubMed] [Google Scholar]
- Strickland LI, Wen Y, Gundersen GG, Burgess DR. Interaction between EB1 and p150glued is required for anaphase astral microtubule elongation and stimulation of cytokinesis. Curr Biol. 2005;15:2249–2255. doi: 10.1016/j.cub.2005.10.073. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- Tucker RW, Pardee AB, Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17:527–535. doi: 10.1016/0092-8674(79)90261-7. [DOI] [PubMed] [Google Scholar]
- Vanhaecke T, Papeleu P, Elaut G, Rogiers V. Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: toxicological point of view. Curr Med Chem. 2004;11:1629–1643. doi: 10.2174/0929867043365099. [DOI] [PubMed] [Google Scholar]
- Wilson PD. Polycystin: new aspects of structure, function, and regulation. J Am Soc Nephrol. 2001;12:834–845. doi: 10.1681/ASN.V124834. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. Embo J. 2003;22:1168–1179. doi: 10.1093/emboj/cdg115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.