Abstract
Vascular endothelial growth factor (VEGF) is a vascular growth factor which induces the development of new blood vessels (angiogenesis), vascular permeability, and inflammation. In brain, receptors for VEGF have been localized to vascular endothelium, neurons, and glia. VEGF is upregulated after hypoxic injury to the brain, such as occurs with cerebral ischemia or high-altitude edema, and has been implicated in the blood-brain barrier breakdown which occurs during these conditions. Given its recently-described role as an inflammatory mediator, VEGF could also contribute to the inflammatory responses observed in cerebral ischemia. After seizures, blood-brain barrier breakdown and inflammation is also observed in brain, albeit on a lower scale than that observed after stroke. Recent evidence has suggested a role for inflammation in seizure disorders. We have described striking increases in VEGF protein in both neurons and glia after pilocarpine-induced status epilepticus in the brain. Increases in VEGF could contribute to the blood-brain barrier breakdown and inflammation observed after seizures. However, VEGF has also been shown to be neuroprotective across several experimental paradigms, and hence could potentially protect vulnerable cells from damage associated with seizures. Therefore, the role of VEGF after seizures could be either protective or destructive. Although only further research will determine the exact nature of VEGF's role after seizures, preliminary data indicate that VEGF plays a protective role after seizures.
Introduction
During and after cerebral ischemia in animal models, there is a well-documented breakdown of the blood-brain barrier that peaks 1-3 days after the ischemic insult.1,2,3,4,5,6,7,8 Edema can be severe after ischemia, and much of the edema is thought to be vasogenic in nature, that is, caused by leakage of plasma fluids into the brain parenchyma. After seizures, blood-brain barrier breakdown has also been described, although the scope and magnitude of the breakdown is substantially milder than that seen after cerebral ischemia.9,10,11 Further, ischemic and post-ischemic vasculature upregulates adhesion molecules which cause leukocytes to adhere to the luminal wall of vascular endothelium.12,13,14,15,16,17,18 Chemokine upregulation then leads to the extravasation of leukocytes, which infiltrate the brain parenchyma in an inflammatory reaction. These vascular-mediated post-ischemic responses are likely to contribute to the damage observed after stroke.15,19,20,21,22,23 Inflammatory cells can also be found in the brain after seizures.24 There is, in fact, accumulating evidence that inflammatory cytokines are involved in the expression of seizures (see also Vezzani et al, this volume).25,26,27,28 Because of the qualitative, albeit not quantitative, similarities in inflammation and blood-brain barrier breakdown after both stroke and seizures, it is reasonable to investigate the proposed mediators of post-ischemic vascular abnormalities after seizures. Among the protein factors recently implicated in post-ischemic blood-brain barrier breakdown are vascular growth factors.
Vascular Endothelial Growth Factor (VEGF)
In the past decade, there has been a surge of interest in vascular growth factors. During development, these factors have potent effects on endothelial cells, and are thought to regulate proliferation, migration, endothelial tube formation, vascular differentiation, permeability, and regression (for reviews see 29 and 30). Although much still remains to be understood regarding the effects of these factors on adult vasculature, current data suggest that they play similar roles in the changes that occur in both normal and pathological states (for review see 30). Cerebral ischemia is one such pathological state in which vascular changes are striking. During and after cerebral ischemia, alterations in the cerebral vasculature include blood-brain barrier breakdown, endothelial cell apoptosis, upregulation of adhesion molecules, and angiogenesis (the development of new blood vessels from existing blood vessels).1,2,3,4,5,6,7,31,32,33,34,35,36,37,38,39 Vascular abnormalities, such as blood-brain barrier breakdown, have been observed after seizures also. Because these vascular alterations might contribute to the brain pathology observed after ischemia or seizures, it is important to understand how changes in the levels of various vascular growth factors might contribute to these pathologies.
There are a large number of protein factors that act on vasculature including, but not limited to, fibroblast growth factor, platelet-derived growth factor, transforming growth factor, hepatocyte growth factor (scatter factor), vascular endothelial growth factor (VEGF), and the angiopoietins. Proteins in the VEGF family of factors have potent vascular effects, and will be the topic of this chapter. These factors modulate the structure and function of vasculature in both developing and adult organisms.
VEGF was originally described as vascular permeability factor (VPF) because of its potent permeabilizing effects on endothelium.40 Since the discovery of VEGF, four additional VEGF-like family members have been described. These additional VEGF-like proteins are placental growth factor (PlGF), VEGFB, VEGFC, and VEGFD (the original VEGF has been termed “VEGFA”) (for review see 41; refer to Fig. 1). The receptors currently described for the VEGF family are VEGFR1 (Flt-1), VEGFR2 (Flk-1 or KDR), VEGFR3 (Flt-4), and the neuropilins (see Fig. 1).41,42,43,44 The primary receptors for VEGFA, VEGFR1 and VEGFR2, are localized predominantly to the vascular endothelium, including cerebral endothelium. However, several recent papers have reported neuronal localization of VEGFR2 in cultured hippocampal or dorsal root ganglion cells.45,46 In addition, neurons located in peri-infarct regions after focal cerebral ischemia or in VEGF-treated brain express VEGFR2.47,48 It is possible that VEGFR2, while not normally detectable in resting neurons, is upregulated during neuronal perturbation. One could argue that cultured neurons are in some way “perturbed,” having been removed from their normal neural microenvironment. VEGFR2 has also been described on glial cells, particularly after cerebral ischemia.49 VEGFR1 has been localized almost exclusively to vascular endothelium, but has been described on circulating inflammatory cells and VEGF-treated astroglia.48,50 In addition to VEGFR1 and VEGFR2, members of the neuropilin receptor family bind to VEGFA. Although neuropilin can be found on vascular endothelium, it is most densely expressed in nonendothelial cells, especially in the nervous system.51,52
Figure 1.
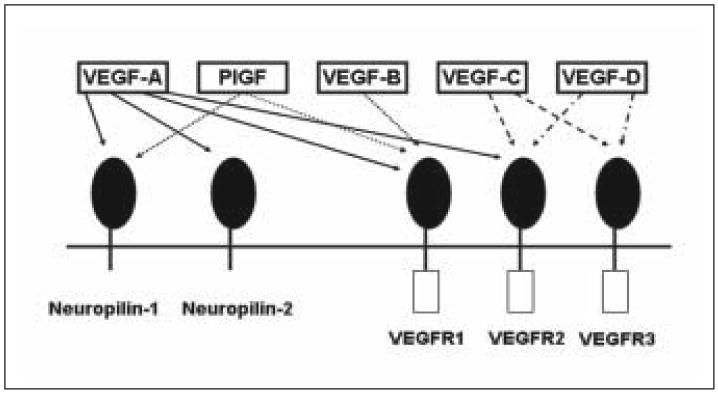
Schematic illustrating the VEGF family of proteins and their receptors.
There are 5 known isoforms of VEGFA in humans which are termed VEGF 121, 145, 165, 189 and 206, corresponding to the number of amino acids found in each isoform (each isoform has 1 amino acid fewer in rodents).53 While all isoforms of VEGFA bind with high affinity to both VEGFR1 and VEGFR2, binding of VEGFA to the neuropilins is isoform-specific (e.g., the 165 amino acid isoform of VEGFA binds to neuropilin-1, while the 121 amino acid isoform does not).44 In addition, the various VEGF family members have different receptor specificities (e.g., VEGFA binds to VEGFR1 and 2, but PlGF only binds to VEGFR1; (Fig. 1); for review see 30). There is still much to be learned about the roles of the diverse members of the VEGF family of proteins, as well as of the various VEGFA isoforms. Because more is currently known about VEGFA than its protein relatives, the remainder of this chapter will be devoted to discussions of VEGFA (hereafter referred to simply as “VEGF”).
In recent years, most of the in vivo research on VEGF has focused on its role as a potent angiogenic factor, responsible for the development of new vascular sprouts (for reviews see 54 and 55). Gene deletion studies have shown that VEGF is critical for the development of new blood vessels during development. Mutant mice lacking even a single VEGF allele die during embryonic development with a striking lack of secondary vasculature (i.e., deficient angiogenesis).56,57 Both VEGFR1and VEGFR2 null mutants are early embryonic lethal and show a lack of vasculature (i.e., deficient vasculogenesis).58,59
In adult animals, application of recombinant VEGF protein induces the formation of new blood vessels from preexisting blood vessels (angiogenesis) in a variety of tissues, including brain.48,60,61,62,63,64,65 However, the blood vessels formed by application of VEGF to adult tissues are grossly abnormal, characterized by profound permeability and a disorganized, dilated, tortuous morphology.48,63,64,65,66,67
Because of the leaky nature of the new blood vessels formed by exogenous administration of VEGF, increasing attention has been given to VEGF's originally-described function as a vascular permeabilizing agent. Application of VEGF to adult tissues or cells results in edema and vascular leak. VEGF results in vascular leak in every tissue to which it has been applied including, but not limited to, brain, lung, testis, bladder, skin, duodenum, mesentery, and intestine.65,66,68,69,70,71 VEGF's effects on vascular leak in the brain occur rapidly, within 30 minutes of exposure to VEGF.68 In ischemic brain, the timing of VEGF mRNA and protein upregulation corresponds closely to the peak of vasogenic edema.35,47,49,72,73,74,75,76,77,78,79 VEGF expression is increased in both glia and neurons of the ischemic brain, as determined by both immunostaining and in situ hybridization.47,49,75,76 Presumably, the VEGF is secreted by the neurons and glia, and binds to VEGF receptors on local vasculature to mediate the increases in vascular permeability. Upregulation of VEGF mRNA occurs both in vivo and in vitro under hypoxic conditions.36,80,81,82,83 Hypoxia-induced upregulation of VEGF mRNA is associated with increases in the transcription factor hypoxia inducible factor (HIF)1-α, which is also upregulated after cerebral ischemia.36,80,81,82,84 Interestingly, upregulation of VEGF mRNA after cerebral ischemia sometimes occurs in cells not directly affected by the ischemic insult, such as the cingulate cortex and hippocampus, suggesting that triggers other than hypoxia could lead to VEGF upregulation.47 Possible secondary mechanisms of VEGF upregulation could include damage to neuronal afferents or efferents, pressure effects of edema, or ischemia-related physiological phenomena such as cortical spreading depression (for review see 85). Cortical spreading depression has been shown to induce increases in other cytokines.86 Because some phenomena observed during cerebral ischemia can also be observed in association with seizures, we hypothesized that VEGF might increase in neural cells after seizures. Specifically, post-ischemic cortical spreading depression follows increases in synchronous neuronal firing such as those observed during seizures. Additionally, prolonged seizures can lead to hypoxic states due to breathing compromise during tonus, and hypoxia is the most consistently confirmed trigger of VEGF upregulation.
VEGF Regulation After Seizures
To study the possibility that seizures could lead to increases in VEGF, rats were treated with 380mg/kg pilocarpine to induce status epilepticus (SE) as previously described.87 Animals were sacrificed one day, one week, or one to two months after status epilepticus, and their brains were processed for VEGF immunostaining.88 One day after SE, VEGF protein is dramatically increased both in neurons and glia in the hippocampus and limbic cortex (Fig. 2C and D and Fig. 3). Specifically, what appears to be cytosolic immunostaining for VEGF is observed in the neurons of CA1 and/or CA3 of the hippocampus as well as some of the pyramidal neurons in entorhinal and perirhinal cortex. The increases in neuronal VEGF are similar in intensity to those observed in CA1 pyramidal neurons 24h after middle cerebral artery occlusion (Croll et al, unpublished observations). These increases in neuronal VEGF reflected increased expression of VEGF mRNA in CA1 of the hippocampus, demonstrating that neurons can make VEGF (Fig. 2A and B).47 Interestingly, the hippocampus does not suffer direct ischemia during middle cerebral artery occlusion, and therefore the increases in VEGF mRNA and protein are likely to be caused by widespread consequences of focal ischemia. One interesting candidate mechanism is spreading depression, a phenomenon characterized by abnormal and synchronous cell firing not unlike that observed with seizures. Glial staining after seizures appears to be at least partially cytosolic, but even more convincing is punctuate cell surface staining on glial profiles (Fig. 3). Presumably, this staining pattern represents staining of VEGF protein bound to its receptors. This same punctuate cell surface staining can also be observed on vascular endothelial cells in the region of VEGF upregulation.
Figure 2.
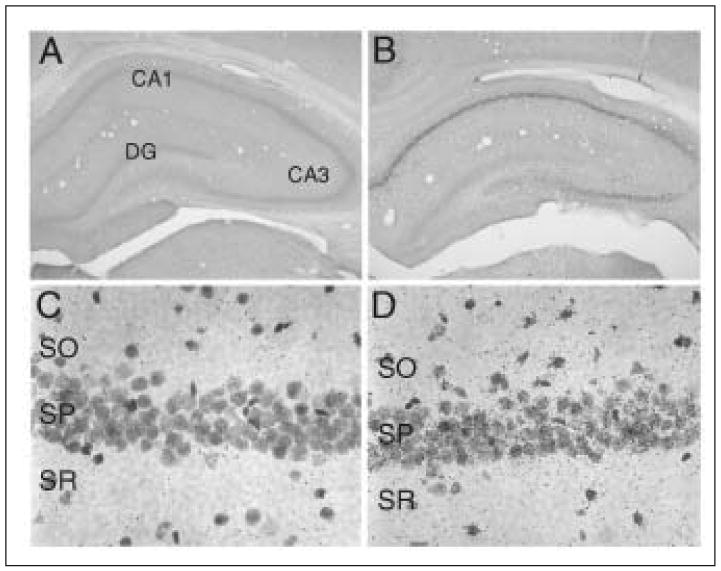
Upregulation of VEGF protein in CA1 of the hippocampus after seizures and VEGF mRNA in CA1 after cerebral ischemia. A) Control brain section stained for VEGF-ir 24h after the animal received a vehicle injection. DG = dentate gyrus. B) Post-status epilepticus (SE) (1 hour of status followed by diazepam injection) brain section stained for VEGF-ir from an animal that was perfused 24h after pilocarpine injection. C) In situ hybridization for VEGF mRNA in CA1 neurons 24h after a sham surgery. SO= stratum radiatum, SP= stratum pyramidale, SR = stratum radiatum. D) In situ hybridization for VEGF mRNA in CA1 neurons 24h after MCAO. Note that MCAO does not cause the hippocampus to be ischemic, but rather leads to indirect effects.
Figure 3.
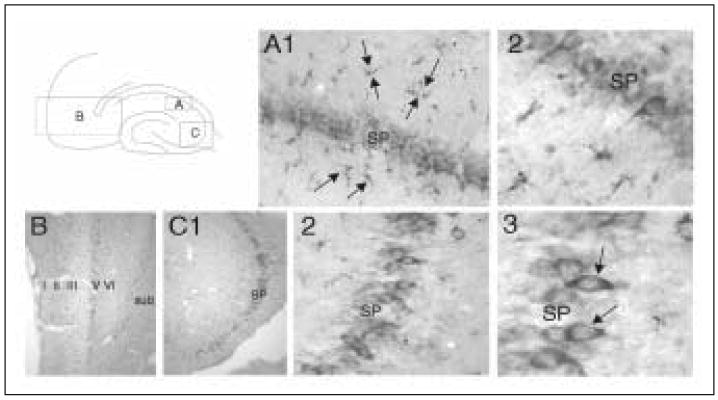
Both neurons and glia upregulate VEGF 24h after SE. All sections are stained for VEGF-ir. A schematic in the upper left shows the locations of parts A-C. A) A low (1) and high (2) magnification view of a post-SE brain section in dorsal CA1. Arrows point to glial VEGF-ir. SP = stratum pyramidale. B) Low magnification view of the entorhinal cortex of the same brain with the major layers of the medial entorhinal cortex marked. Sub = subiculum. C) Low (1), high (2), and higher (3) magnification of ventral CA3 in the same brain. Arrows point to neuronal VEGF-ir.
The trigger for increased neural VEGF after seizure activity is unclear. As previously discussed, hypoxia is the best known trigger of VEGF expression across cell types, and hypoxia can occur during seizures. However, heretofore undescribed triggers of VEGF upregulation are likely, particularly given that the upregulation of neuronal VEGF has been observed after cerebral ischemia in areas not directly affected by the hypoxic insult (Fig. 2C and D).47 For example, the upregulation of VEGF could result from increased neuronal activity, although there is little prior evidence for activity-dependent increases in VEGF. The paucity of evidence arises not from negative findings, but rather because the question has not been studied yet. Increases in neuronal activity induced by exposure of rats to complex environments increased microvascular density in the brains of these animals.89 Because VEGF has been shown to be critical for angiogenesis across a wide variety of tissues, one could speculate that active neural cells in stimulated brain could upregulate VEGF in an attempt to induce increased vascular flow, hence metabolically supporting their increased activity. The fact that increased neuronal activity does lead to increased blood flow and an increased need for vascular investment is well-documented. This premise serves as the basis for much of the current work in human functional brain imaging. Both fMRI and PET scans commonly use increased cerebral blood flow as surrogate markers of increased neuronal activity.
VEGF as a Neurotrophic Factor
Although all original research, and most current research, on VEGF has focused on its effects on vasculature, there is a growing body of literature studying VEGF as a potential neurotrophic factor (for review, see 90). Many neurons constitutively contain neuropilins, and have been shown under some circumstances, such as after ischemia, during development, and in culture, to express VEGFR2.46,47,49,91 Glial cells have also been reported to express VEGF receptors. Although the exact roles of these receptors in neural cell function remain to be determined, accumulating evidence exists for such roles. VEGF has been shown to protect neurons across a wide variety of circumstances. For instance, VEGF has been reported to have a neuroprotective effect in the context of cerebral ischemia.45,92,93 In addition, VEGF supports developing sensory cells in retina and dorsal root ganglia.46,94,95 Further, VEGF has been shown to speed the recovery of damaged peripheral neurons, although increased vascular density in the injured area could have partially or fully accounted for this effect.96,97 Elegant evidence for a role of VEGF in protecting adult neurons, either directly or indirectly, came in a recent study showing that deletion of the hypoxia response element from the VEGF promoter resulted in motor neuron disease in adult mice.98
Perhaps most relevant to a neuroprotective role in seizureinduced damage is the recent report that VEGF protects cultured hippocampal neurons against glutamate excitotoxicity.99 Using antisense oligonucleotides, Matsuzaki et al99 showed that this protective effect of VEGF is mediated through VEGFR2. In addition, blockade of VEGFR2 synthesis blocked induction of the Akt survival pathway in these neurons. Because the Akt survival pathway has been repeatedly demonstrated to be activated after VEGF treatment, it is possible that VEGF directly protects cells from excitotoxic damage by increasing signaling pathways important to survival.100,101 Because these cultures only contained neurons, a direct protective role of VEGF could be inferred. Further evidence for a direct protective role of VEGF through VEGFR2 and Akt signaling has been presented in cultured hypoxic neurons.45,93,102 In these hypoxic neurons, caspase-3 levels were increased when VEGF was blocked, providing further evidence for a direct effect of VEGF on pathways mediating cell survival.93
One final possibility is that VEGF produces its protective effects, at least in part, through neuropilin-1, which is located on neurons. VEGF and semaphorin 3A compete for binding at neuropilin-1. The semaphorins have traditionally been thought to induce cell death during development. VEGF could block this death pathway by binding to neuropilin-1, hence preventing semaphorin binding.103 Recent evidence suggests, however, that the relationship between VEGF and semaphorin could be complex.103 In addition, a recent direct attempt to induce neuroprotection in hypoxic cells with neuropilin-1 activation failed to show protection.45 Therefore, although the potential role of neuropilin in VEGF-mediated neuroprotection cannot be overlooked, neuroprotection mediated via VEGFR2 activation is currently the most parsimonious explanation.
Potential Effects of VEGF on Seizures and their Sequelae
VEGF could be a double-edged sword for the epileptic brain, because it results in has multiple effects. Its proposed vascular effects might be expected to aggravate seizures and post-seizure brain damage, while its direct effects on neurons could be neuroprotective. VEGF also activates glia, which could potentially impact seizures or their sequelae.48,65
As previously discussed, VEGF has dramatic effects on brain vasculature, and results in blood-brain barrier breakdown. At low concentrations, VEGF has minimal angiogenic effects, especially within a short time frame. That is, VEGF's effects on vascular leak and permeability are more striking than its effects on angiogenesis in low amounts or after brief exposures.65 In addition to its very potent effects as a vascular permeabilizing agent, VEGF also potently induces inflammation. At the same low doses and early time points which induce blood-brain barrier leak, local application of VEGF causes a striking inflammation characterized by a primarily monocytic infiltrate.65,66 This effect could be caused by a direct chemoattractant effect on monocytes, which express VEGFR1, but it could also result from the complex pattern of upregulation of multiple inflammatory mediators such as ICAM-1 and Mip-1α, both of which have been observed after VEGF administration to brain.50,65 Interestingly, VEGF is upregulated by both IL-1 and TNF-α.104,105,106 These inflammatory cytokines are upregulated after seizures.25,26,27,28 Evidence accumulated to date suggests that these inflammatory cytokines increase the potential for seizures.27,28,107 Because both of these cytokines upregulate VEGF, it is possible that VEGF is one common pathway through which these proteins exert their effects. Given the data suggesting detrimental effects of inflammation on seizures and their sequelae, one might hypothesize that VEGF aggravates seizures or seizurerelated brain compromise. In cerebral ischemia, VEGF has been shown to worsen post-ischemic edema and VEGF antagonism protects against damage.108,109 However, there is also evidence that anti-inflammatory agents could lead to more post-seizure cell damage.110 Therefore, an increased understanding of the role of inflammation in seizures will be necessary before we can fully appreciate the implications of VEGF's pro-inflammatory and vascular (leak) effects on epileptic brain.
In contrast to the potentially detrimental vascular effects of VEGF, we have summarized the developing evidence for a neuroprotective role of VEGF. There is accumulating evidence that VEGF directly protects neurons from damage, including excitotoxic damage.99 Direct protective effects of VEGF on any cell containing VEGF receptors, including potential induction of VEGFR2 on neurons and glia, is quite possible given VEGF's activation of the Akt intracellular survival pathway.100,101
We have conducted preliminary studies to assess the effects of VEGF on cell damage after seizures. By infusing VEGF (30-60ng/day; continuously for 5 days into adult rat brains via osmotic minipump) before inducing status epilepticus with pilocarpine (350mg/kg), we have begun to address the role of VEGF upregulation after seizures. These initial experiments have revealed a statistically significant neuroprotective effect of VEGF on cell damage in CA1 and CA3 of the hippocampus, assessed 24 hours following status epilepticus (Fig. 4). Therefore, it seems possible that VEGF, while inducing inflammation, has the ability to protect cells from any damage that could occur as a result of either the inflammation or the excitotoxic insult.
Figure 4.
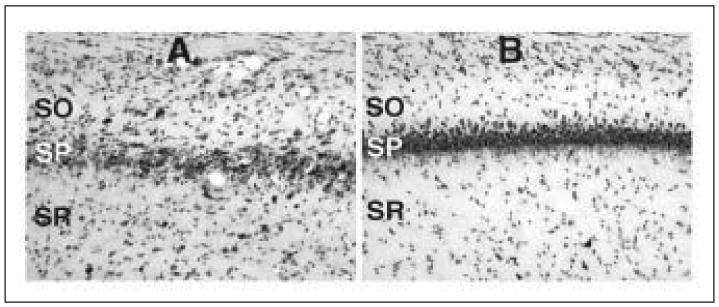
Cresyl violet stained hippocampal sections taken from animals 24 hours after status epilepticus induced by pilocarpine. A) Hippocampus from the median animal in the PBS-infused group showing damage in stratum pyramidale (SP) of area CA1. SO= stratum oriens, SR = stratum radiatum. B) Hippocampus from the median animal in the VEGF-infused group. Note the lack of cell damage in area CA1 of the animal treated with VEGF.
Much additional research needs to be conducted before we can fully understand the implications of VEGF upregulation after seizures. Based on currently available data, VEGF could be a double-edged sword, directly protecting neurons from post-seizure cell death, but simultaneously inducing blood-brain barrier breakdown and inflammation. VEGF could, however, also be the rare pro-inflammatory cytokine that possesses the ability to directly protect neurons from the potentially detrimental effects of the very inflammation that it induces.
Acknowledgments
We thank our many colleagues, especially Drs. Stan Wiegand and John Rudge, for collaborative experiments and stimulating discussions which have helped lead to the premises presented in the current review. In addition, we acknowledge the technical contributions of our students and technicians, Sachin Shah, Ahmed Elkady, and Jamee Nicoletti from Queens College and Anne Sollas and Adam MacLeod from Helen Hayes Hospital, who made possible the generation of the data presented in this chapter.
References
- 1.Gotoh O, Asano T, Koide T, et al. Ischemic brain edema following occlusions of the middle cerebral artery in the rat. I: The time courses of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125I-albumin. Stroke. 1985;16:101–109. doi: 10.1161/01.str.16.1.101. [DOI] [PubMed] [Google Scholar]
- 2.Hatashita S, Hoff JT. Role of blood-brain barrier permeability in focal ischemic brain edema. Adv Neurol. 1990a;52:327–333. [PubMed] [Google Scholar]
- 3.Hatashita S, Hoff JT. Brain edema and cerebrovascular permeability during cerebral ischemia in rats. Stroke. 1990b;21:582–588. doi: 10.1161/01.str.21.4.582. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa Y, Fujimoto N, Matsumoto K, et al. Morphological changes in acute cerebral ischemia after occlusion and reperfusion in the rat. Adv Neurol. 1990;52:21–27. [PubMed] [Google Scholar]
- 5.Kitagawa K, Matsumoto M, Tagaya M, et al. Temporal profile of serum albumin extravasation following cerebral ischemia in a newly established reproducible gerbil model for vasogenic brain edema: A combined immunohistochemical and dye tracer analysis. Acta Neuropathol (Berl) 1991;82:164–171. doi: 10.1007/BF00294441. [DOI] [PubMed] [Google Scholar]
- 6.Menzies SA, Betz AL, Hoff JT. Contributions of ions and albumin to the formation and resolution of ischemic brain edema. J Neurosurg. 1993;78:257–266. doi: 10.3171/jns.1993.78.2.0257. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto K, Lo EH, Pierce AR, et al. Role of vasogenic edema and tissue cavitation in ischemic evolution on diffusion-weighted imaging: Comparison with multiparameter MR and immunohistochemistry. Am J Neuroradiol. 1995;16:1107–1115. [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg GA. Ischemic brain edema. Prog Cardiovasc Dis. 1999;42:209–216. doi: 10.1016/s0033-0620(99)70003-4. [DOI] [PubMed] [Google Scholar]
- 9.Ates N, Esen N, Ilbay G. Absence epilepsy and regional blood-brain barrier permeability: The effects of pentylenetetrazole-induced convulsions. Pharmacol Res. 1999;39:305–310. doi: 10.1006/phrs.1998.0441. [DOI] [PubMed] [Google Scholar]
- 10.Cornford EM. Epilepsy and the blood brain barrier: Endothelial cell responses to seizures. Adv Neurol. 1999;79:845–862. [PubMed] [Google Scholar]
- 11.Roch C, Leroy C, Nehlig A, et al. Magnetic resonance imaging in the study of the lithium-pilocarpine model of temporal lobe epilepsy in adult rats. Epilepsia. 2002;43:325–335. doi: 10.1046/j.1528-1157.2002.11301.x. [DOI] [PubMed] [Google Scholar]
- 12.Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann NY Acad Sci. 1997;825:179–193. doi: 10.1111/j.1749-6632.1997.tb48428.x. [DOI] [PubMed] [Google Scholar]
- 13.del Zoppo GJ, Wagner S, Tagaya M. Trends and future developments in the pharmacological treatment of acute ischaemic stroke. Drugs. 1997;54:9–38. doi: 10.2165/00003495-199754010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Becker KJ. Inflammation and acute stroke. Curr Opin Neurol. 1998;11:45–49. doi: 10.1097/00019052-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 15.DeGraba TJ. The role of inflammation after acute stroke: Utility of pursuing anti-adhesion molecule therapy. Neurology. 1998;51:S62–68. doi: 10.1212/wnl.51.3_suppl_3.s62. [DOI] [PubMed] [Google Scholar]
- 16.Jean WC, Spellman SR, Nussbaum ES, et al. Reperfusion injury after focal cerebral ischemia: The role of inflammation and the therapeutic horizon. Neurosurgery. 1998;43:1382–1396. doi: 10.1097/00006123-199812000-00076. [DOI] [PubMed] [Google Scholar]
- 17.del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thromb Res. 2000;98:73–81. doi: 10.1016/s0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- 18.Stanimirovic D, Satoh K. Inflammatory mediators of cerebral endothelium: A role in ischemic brain inflammation. Brain Pathol. 2000;10:3–126. doi: 10.1111/j.1750-3639.2000.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang RL, Chopp M, Jiang N, et al. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995;26:1438–1442. doi: 10.1161/01.str.26.8.1438. [DOI] [PubMed] [Google Scholar]
- 20.Chopp M, Li Y, Jiang N, et al. Antibodies against adhesion molecules reduce apoptosis after transient middle cerebral artery occlusion in rat brain. J Cereb Blood Flow Metab. 1996;16:578–584. doi: 10.1097/00004647-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Chopp M, Zhang ZG. Anti-adhesion molecule and nitric oxide protection strategies in ischemic stroke. Curr Opin Neurol. 1996;9:68–72. doi: 10.1097/00019052-199602000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Goussev AV, Zhang Z, Anderson DC, et al. P-selectin antibody reduces hemorrhage and infarct volume resulting from MCA occlusion in the rat. J Neurol Sci. 1998;161:16–22. doi: 10.1016/s0022-510x(98)00262-7. [DOI] [PubMed] [Google Scholar]
- 23.Jiang N, Chopp M, Chahwala S. Neutrophil inhibitory factor treatment of focal cerebral ischemia in the rat. Brain Res. 1998;788:25–34. doi: 10.1016/s0006-8993(97)01503-5. [DOI] [PubMed] [Google Scholar]
- 24.Peltola J, Laaksonen J, Haapala AM, et al. Indicators of inflammation after recent tonic-clonic epileptic seizures correlate with plasma interleukin-6 levels. Seizure. 2002;11:44–46. doi: 10.1053/seiz.2001.0575. [DOI] [PubMed] [Google Scholar]
- 25.Vezzani A, Conti M, De Luigi A, et al. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: Functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Simoni MG, Perego C, Ravizza T, et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12:2623–2633. doi: 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- 27.Vezzani A, Moneta D, Conti M, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci USA. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vezzani A, Moneta D, Richichi C, et al. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43(S5):30–35. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- 29.Tomanek RJ, Schatteman GC. Angiogenesis. New insights and therapeutic potential. Anat Rec. 2000;261:126–135. doi: 10.1002/1097-0185(20000615)261:3<126::AID-AR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 31.Bednar MM, Raymond S, McAuliffe T, et al. The role of neutrophils and platelets in a rabbit model of thromboembolic stroke. Stroke. 1991;22:44–50. doi: 10.1161/01.str.22.1.44. [DOI] [PubMed] [Google Scholar]
- 32.Clark RK, Lee EV, Fish CJ, et al. Development of tissue damage, inflammation and resolution following stroke: An immunohistochemical and quantitative planimetric study. Brain Res Bull. 1993;31:565–572. doi: 10.1016/0361-9230(93)90124-t. [DOI] [PubMed] [Google Scholar]
- 33.Morioka T, Kalehua AN, Streit WJ. Characterization of microglial reaction after middle cerebral artery occlusion in rat brain. J Comp Neurol. 1993;327:123–132. doi: 10.1002/cne.903270110. [DOI] [PubMed] [Google Scholar]
- 34.Okada Y, Copeland BR, Mori E, et al. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25:202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- 35.Beck H, Acker T, Wiessner C, et al. Expression of angiopoietin-1, angiopoietin-2 and tie receptors after middle cerebral artery occlusion in the rat. Amer J Pathol. 2000;157:1473–1483. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marti HJ, Bernaudin M, Bellail A, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwab JM, Nguyen TD, Postler E, et al. Selective accumulation of cyclooxygenase-1-expressing microglial cells/macrophages in lesions of human focal cerebral ischemia. Acta Neuropathol (Berl) 2000;99:609–614. doi: 10.1007/s004010051170. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Yue TL, Barone FC, et al. Demonstration of increased endothelial-leukocyte adhesion molecule-1 mRNA expression in rat ischemic cortex. Stroke. 1995;26:1665–1668. doi: 10.1161/01.str.26.9.1665. [DOI] [PubMed] [Google Scholar]
- 39.Zubkov AY, Ogihara K, Bernanke DH, et al. Apoptosis of endothelial cells in vessels affected by cerebral vasospasm. Surg Neurol. 2000;53:260–266. doi: 10.1016/s0090-3019(99)00187-1. [DOI] [PubMed] [Google Scholar]
- 40.Senger DR, Perruzzi CA, Feder J, et al. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- 41.Frelin C, Ladoux A, D'Angelo G. Vascular endothelial growth factors and angiogenesis. Ann Endocrinol (Paris) 2000;61:70–74. [PubMed] [Google Scholar]
- 42.Quinn TP, Peters KG, De Vries C, et al. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci USA. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soker S, Takashima S, Miao H, et al. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 44.Gluzman-Poltorak Z, Cohen T, Herzog Y, et al. Neuropilin-2 and neuropilin-1 are receptors for the 165-amino acid form of vascular endothelial growth factor (VEGF) and of placenta growth factor-2, but only neuropilin-2 functions as a receptor for the 145-amino acid form of VEGF. Biol Chem. 2000;275:18040–5. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 45.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: Direct neuroprotective effect in in vivo ischemia. Proc Natl Acad Sci. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12:4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 47.Croll SD, Wiegand SJ. Vascular growth factors and cerebral ischemia. Mol Neurobiol. 2001;23:121–35. doi: 10.1385/MN:23:2-3:121. [DOI] [PubMed] [Google Scholar]
- 48.Krum JM, Mani N, Rosenstein JM. Angiogenic and astroglial responses to vascular endothelial growth factor administration in adult rat brain. Neuroscience. 2002;110:589–604. doi: 10.1016/s0306-4522(01)00615-7. [DOI] [PubMed] [Google Scholar]
- 49.Lennmyr F, Ata KA, Funa K, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) following permanent and transient occlusion of the middle cerebral artery in the rat. J Neuropathol Exp Neurol. 1998;57:874–882. doi: 10.1097/00005072-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Sawano A, Iwai S, Sakurai Y, et al. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97:785–791. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]
- 51.Kawakami A, Kitsukawa T, Takagi S, et al. Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J Neurobiol. 1996;29:1–17. doi: 10.1002/(SICI)1097-4695(199601)29:1<1::AID-NEU1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 52.Chédotal A, Del Rio JA, Ruiz M, et al. Semaphorins III and IV repel hippocampal axons via two distinct receptors. Develop. 1998;125:4313–4323. doi: 10.1242/dev.125.21.4313. [DOI] [PubMed] [Google Scholar]
- 53.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 54.Carmeliet P, Collen D. Molecular basis of angiogenesis. Roles of VEGF and VE-cadherin. Ann NY Acad Sci. 2000;902:249–262. doi: 10.1111/j.1749-6632.2000.tb06320.x. [DOI] [PubMed] [Google Scholar]
- 55.Dvorak HF. VPF/VEGF and the angiogenic response. Semin Perinatol. 2000;24:75–78. doi: 10.1016/s0146-0005(00)80061-0. [DOI] [PubMed] [Google Scholar]
- 56.Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 57.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 58.Fong GH, Rossant J, Gertsenstein M, et al. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 59.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 60.Bauters C, Asahara T, Zheng LP, et al. Physiological assessment of augmented vascularity induced by VEGF in ischemic rabbit hindlimb. Am J Physiol. 1994;267:H1263–1271. doi: 10.1152/ajpheart.1994.267.4.H1263. [DOI] [PubMed] [Google Scholar]
- 61.Takeshita S, Zheng LP, Brogi E, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearlman JD, Hibberd MG, Chuang ML, et al. Magnetic resonance mapping demonstrates benefits of VEGF-induced myocardial angiogenesis. Nat Med. 1995;1:1085–1089. doi: 10.1038/nm1095-1085. [DOI] [PubMed] [Google Scholar]
- 63.Rosenstein JM, Mani N, Silverman WF, et al. Patterns of brain angiogenesis after vascular endothelial growth factor administration in vivo and in vivo. Proc Natl Acad Sci USA. 1998;95:7086–7091. doi: 10.1073/pnas.95.12.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Springer ML, Chen AS, Kraft PE, et al. VEGF gene delivery to muscle: Potential role for vasculogenesis in adults. Mol Cell. 1998;2:549–558. doi: 10.1016/s1097-2765(00)80154-9. [DOI] [PubMed] [Google Scholar]
- 65.Kasselman LJ, Ransohoff RM, Cai N, et al. Vascular endothelial growth factor (VEGF)-mediated inflammation precedes angiogenesis in adult rat brain. Soc Nsci Abstr. 2002 doi: 10.1016/j.expneurol.2004.02.010. in press. [DOI] [PubMed] [Google Scholar]
- 66.Proescholdt MA, Heiss JD, Walbridge S, et al. Vascular endothelial growth factor (VEGF) modulates vascular permeability and inflammation in rat brain. J Neuropathol Exp Neurol. 1999;58:613–627. doi: 10.1097/00005072-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Carmeliet P. VEGF gene therapy: Stimulating angiogenesis or angioma-genesis? Nat Med. 2000;6:1102–1103. doi: 10.1038/80430. [DOI] [PubMed] [Google Scholar]
- 68.Dobrogowska A, Lossinsky AS, Tarnawski M, et al. Increased blood-brain barrier permeability and endothelial abnormalities induced by vascular endothelial growth factor. J Neurocytol. 1998;27:63–173. doi: 10.1023/a:1006907608230. [DOI] [PubMed] [Google Scholar]
- 69.Zhao L, Zhang MM, Ng K. Effects of vascular permeability factor on the permeability of cultured endothelial cells from brain capillaries. J Cardiovasc Pharmacol. 1998;32:1–4. doi: 10.1097/00005344-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Kaner RJ, Ladetto JV, Singh R, et al. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol. 2000;22:657–664. doi: 10.1165/ajrcmb.22.6.3779. [DOI] [PubMed] [Google Scholar]
- 71.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 72.Kovacs Z, Ikezaki K, Samoto K, et al. VEGF and flt: Expression time kinetics in rat brain infarct. Stroke. 1996;27:1865–1873. doi: 10.1161/01.str.27.10.1865. [DOI] [PubMed] [Google Scholar]
- 73.Hayashi T, Abe K, Suzuki H, et al. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- 74.Cobbs CS, Chen J, Greenberg DA, et al. Vascular endothelial growth factor expression in transient focal cerebral ischemia in the rat. Neurosci Lett. 1998;249:79–82. doi: 10.1016/s0304-3940(98)00377-2. [DOI] [PubMed] [Google Scholar]
- 75.Issa R, Krupinski J, Bujny T, et al. Vascular endothelial growth factor and its receptor, KDR, in human brain tissue after ischemic stroke. Lab Invest. 1999;79:417–425. [PubMed] [Google Scholar]
- 76.Lee MY, Ju WK, Cha JH, et al. Expression of vascular endothelial growth factor mRNA following transient forebrain ischemia in rats. Neurosci Lett. 1999;265:107–110. doi: 10.1016/s0304-3940(99)00219-0. [DOI] [PubMed] [Google Scholar]
- 77.Pichiule P, Chavez JC, Xu K, et al. Vascular endothelial growth factor upregulation in transient global ischemia induced by cardiac arrest and resuscitation in rat brain. Brain Res Mol Brain Res. 1999;74:83–90. doi: 10.1016/s0169-328x(99)00261-2. [DOI] [PubMed] [Google Scholar]
- 78.Plate KH, Beck H, Danner S, et al. Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J Neuropathol Exp Neurol. 1999;58:654–666. doi: 10.1097/00005072-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 79.Lin TN, Wang CK, Cheung WM, et al. Induction of angiopoietin and tie receptor mRNA expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2000;20:387–395. doi: 10.1097/00004647-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 80.Chiarugi V, Magnelli L, Chiarugi A, et al. Hypoxia induced pivotal tumor angiogenesis control factors including p53, vascular endothelial growth factor and the NFKB-dependent inducible nitric oxide synthase and cyclo-oxygenase-2. J Cancer Res Clin Oncol. 1999;125:525–528. doi: 10.1007/s004320050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El Awad B, Kreft B, Wolber EM, et al. Hypoxia and interleukin-1 stimulate vascular endothelial growth factor production in human proximal tubular cells. Kidney Intl. 2000;58:43–50. doi: 10.1046/j.1523-1755.2000.00139.x. [DOI] [PubMed] [Google Scholar]
- 82.Tsuzuki Y, Fukumura D, Oosthuyse B, et al. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1 - hypoxia response element- VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res. 2000;60:6248–6252. [PubMed] [Google Scholar]
- 83.Yuan HT, Yang SP, Woolf AS. Hypoxia up-regulates angiopoietin-2, a Tie-2 ligand, in mouse mesangial cells. Kidney Int. 2000;58:1912–1919. doi: 10.1111/j.1523-1755.2000.00363.x. [DOI] [PubMed] [Google Scholar]
- 84.Bergeron M, Yu AY, Solway KE, et al. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci. 1999;11:4159–4170. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- 85.Hossmann KA. The hypoxic brain. Insights from ischemia research. Adv Exp Med Biol. 1999;474:155–169. [PubMed] [Google Scholar]
- 86.Jander S, Schroeter M, Peters O, et al. Cortical spreading depression induces proinflammatory cytokine gene expression in the rat brain. J Cereb Blood Flow Metab. 2001;21:218–225. doi: 10.1097/00004647-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 87.Scharfman HE, Goodman JH, Sollas AL, et al. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp Neurol. 2002;174:201–214. doi: 10.1006/exnr.2002.7869. [DOI] [PubMed] [Google Scholar]
- 88.Croll SD, Goodman JH, Sollas A, et al. Vascular endothelial growth factor (VEGF) is upregulated after pilocarpine-induced seizures in rats. Soc Nsci Abs. 2000 in press. [Google Scholar]
- 89.Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. III. Neuronal and glial nuclei, boutons, dendrites and capillaries. Brain Res. 1987;424:320–332. doi: 10.1016/0006-8993(87)91477-6. [DOI] [PubMed] [Google Scholar]
- 90.Carmeliet P, Storkebaum E. Vascular and neuronal effects of VEGF in the nervous system: Implications for neurological disorders. Semin Cell Dev Biol. 2002;13:39–53. doi: 10.1006/scdb.2001.0290. [DOI] [PubMed] [Google Scholar]
- 91.Ogunshola OO, Antic A, Donoghue MJ, et al. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277:11410–11415. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- 92.Hayashi T, Abe K, Itoyama Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J Cereb Blood Flow Metab. 1998;18:887–895. doi: 10.1097/00004647-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 93.Jin K, Mao XO, Batteur SP, et al. Caspase-3 and the regulation of hypoxic neuronal death by vascular endothelial growth factor. Neuroscience. 2001;108:351–358. doi: 10.1016/s0306-4522(01)00154-3. [DOI] [PubMed] [Google Scholar]
- 94.Yourey PA, Gohari S, Su JL, et al. Vascular endothelial cell growth factors promote the in vivo development of rat photoreceptor cells. J Neurosci. 2000;20:6781–6788. doi: 10.1523/JNEUROSCI.20-18-06781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robinson GS, Ju M, Shih SC, et al. Nonvascular role for VEGF: VEGFR-1, 2 activity is critical for neural retinal development. FASEB J. 2001;15:1215–1217. doi: 10.1096/fj.00-0598fje. [DOI] [PubMed] [Google Scholar]
- 96.Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat. 2000;197:591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schratzberger P, Schratzberger G, Silver M, et al. Favorable effect of VEGF gene transfer on ischemic peripheral neuropathy. Nat Med. 2000;6:405–413. doi: 10.1038/74664. [DOI] [PubMed] [Google Scholar]
- 98.Oosthuyse B, Moons L, Storkebaum E, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 99.Matsuzaki H, Tamatani M, Yamaguchi A, et al. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: Signal transduction cascades. FASEB J. 2001;15:1218–1220. [PubMed] [Google Scholar]
- 100.Mazure NM, Chen EY, Laderoute KR, et al. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signlaing pathway in Ha-ras-transformed cells through a hypoxia-inducible factor-1 transcriptional element. Blood. 1997;90:3322–3331. [PubMed] [Google Scholar]
- 101.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 102.Wick A, Wick W, Waltenberger J, et al. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci. 2002;22:6401–6407. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bagnard D, Vaillant C, Khuth ST, et al. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci. 2001;21:3332–3341. doi: 10.1523/JNEUROSCI.21-10-03332.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li J, Perrella MA, Tsai JC, et al. Induction of vascular endothelial growth factor gene expression by interleukin-1 beta in rat aortic smooth muscle cells. J Biol Chem. 1995;270:308–312. doi: 10.1074/jbc.270.1.308. [DOI] [PubMed] [Google Scholar]
- 105.Ryuto M, Ono M, Izumi H, et al. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem. 1996;271:28220–28228. doi: 10.1074/jbc.271.45.28220. [DOI] [PubMed] [Google Scholar]
- 106.Jung YD, Liu W, Reinmuth N, et al. Vascular endothelial growth factor is upregulated by interleukin-1 beta in human vascular smooth muscle cells via the P38 mitogen-activated protein kinase pathway. Angiogenesis. 2001;4:155–162. doi: 10.1023/a:1012291524723. [DOI] [PubMed] [Google Scholar]
- 107.Shandra AA, Godlevsky LS, Vastyanov RS, et al. The role of TNF-alpha in amygdala kindled rats. Neurosci Res. 2002;42:147–153. doi: 10.1016/s0168-0102(01)00309-1. [DOI] [PubMed] [Google Scholar]
- 108.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Bruggen N, Thibodeaux H, Palmer JT, et al. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104:1613–1620. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baik EJ, Kim EJ, Lee SH, et al. Cyclooxygenase-2 selective inhibitors aggravate kainic acid induced seizure and neuronal cell death in the hippocampus. Brain Res. 1999;843:118–129. doi: 10.1016/s0006-8993(99)01797-7. [DOI] [PubMed] [Google Scholar]


