Summary
Seizure induction in laboratory animals is followed by many changes in structure and function, and one of these is an increase in neurogenesis—the birth of new neurons. This phenomenon may be relevant to temporal lobe epilepsy (TLE), because one of the regions of the brain where seizure-induced neurogenesis is most robust is the dentate gyrus—an area of the brain that has been implicated in the pathophysiology of TLE. Although initial studies predicted that neurogenesis in the dentate gyrus would be important to normal functions, such as learning and memory, the new neurons that are born after seizures may not necessarily promote normal function. There appears to be a complex functional and structural relationship between the new dentate gyrus neurons and preexisting cells, both in the animal models of TLE and in tissue resected from patients with intractable TLE. These studies provide new insights into the mechanisms of TLE, and suggest novel strategies for intervention that could be used to prevent or treat TLE.
Keywords: Dentate gyrus, Granule cell, Hilus, Status epilepticus, Ectopic, Epileptogenesis
Although there has been experimental evidence that neurogenesis occurs in the adult mammalian brain for many decades (Altman 1962; Altman and Das, 1965), the concept has become widely accepted only after more recent studies were conducted using the thymidine analog bromodeoxyuridine BrdU; (Christie and Cameron, 2006; Gage 2002). BrdU is incorporated into DNA during synthesis in the S-phase of the cell cycle, allowing the identification of cells within the cell cycle or their postmitotic progeny, depending on the elapsed time between BrdU administration and cell fixation for immunohistochemical detection. These studies, which were mostly conducted in laboratory rats or mice, appear to also apply to man, because in humans that had been administered BrdU, there was evidence of ongoing neurogenesis, even at age 72 (Eriksson et al., 1998). As a result, a great deal of interest developed in the possible use of neurogenesis from a clinical perspective. For example, it was suggested that neurogenesis might be able to “repair” neuronal loss that occurs slowly during the natural aging process. In addition, there has been considerable interest in treatments that might increase the rate of neurogenesis to compensate for the loss of neurons after traumatic brain injury (Kozorovitskiy and Gould, 2003; Lie et al., 2004). In the context of epilepsy, new neurons could conceivably reverse neuronal loss due to seizure-related neuronal death. However, as will become clear below, the concept that newly born neurons in the adult brain may reverse the pathology in temporal lobe epilepsy (TLE) appears to be much more complicated than initially anticipated (Parent and Lowenstein, 2002; Scharfman 2004; Shapiro and Ribak, 2005).
In this review, we will initially provide a brief discussion of some of the fundamental aspects of neurogenesis in the adult brain, and then discuss seizure-induced neurogenesis. Next, the ability of these studies to help explain the etiology of TLE will be considered. Data from animal models of epilepsy and tissue specimens of patients with pharmacologically intractable TLE will be compared.
DENTATE GYRUS NEUROGENESIS IN LABORATORY ANIMALS: THE CURRENT PERSPECTIVE
Initially, it was agreed that neurogenesis in the adult brain largely occurs in three areas: the subventricular zone, the olfactory bulb, and the dentate gyrus. Many now would agree that adult neurogenesis can occur at other sites, such as the striatum (Parent et al., 2002; Dayer et al., 2005). In addition, although the present review focuses on neurons, it is important to consider proliferation of other types of cells, such as glia. Gliogenesis is particularly important to any discussion of epilepsy, where changes in astrocytes and microglia have long been considered a piece of the etiological puzzle.
The focus of this review in the dentate gyrus is neurogenesis, because the dentate gyrus is the primary site in the temporal lobe where the majority of neurogenesis is thought to occur in the normal adult brain, and the temporal lobe is key in TLE. In the rat, neurogenesis occurs in a zone that lies within the first 50–100 μm of the granule cell layer, the subgranular zone (SGZ), and new neurons are thought to derive from radial glia which in turn divide into so-called D cells that ultimately become dentate gyrus granule cells (Seri et al., 2004). In addition to the granule cell fate, other fates are possible, because progenitors can differentiate into glia and GABAergic neurons (Dayer et al., 2005). However, these fates appear to be relatively rare compared to the proportion of cells that become granule cells. It should be noted that the extent to which the rodent data can be generalized to man is unclear, and direct correspondence should not be assumed.
The newly born granule cells have been studied extensively. They rapidly send axon projections to their normal target zone, the mossy fiber pathway (Hastings and Gould, 1999; Markakis and Gage, 1999). Their dendritic trees resemble other granule cells, although some aspects of their dendrites and spines have been suggested to be immature (Toni et al., 2004; Pierce et al., 2005), particularly if they develop in an aged animal (Rao et al., 2005). In addition, they appear to develop electrophysiological properties like other granule cells (van Praag et al., 2002). The demonstration of functional integration of newly born dentate granule cells into hippocampal circuitry (Scharfman et al., 2000; van Praag et al., 2002; Jessberger and Kempermann, 2003) and the fact that they appear to mediate long-term potentiation (LTP) in the dentate gyrus (Schmidt-Hieber et al., 2004), has led to the hypothesis that adult neurogenesis may be important in learning and memory (Gould, 1999; Snyder et al., 2001; Shors, 2004).
MODULATION OF DENTATE GYRUS NEUROGENESIS BY NEURONAL ACTIVITY AND SEIZURES
One characteristic of adult neurogenesis in rodents that has important translational implications is that the rate of proliferation is modifiable, both by environmental cues and pathological conditions. Neuronal activity exerts a strong influence on proliferation rate. As first shown by Bengzon and colleagues (Bengzon et al., 1997), neuronal depolarization or repetitive discharge—induced either by electrical or pharmacological stimulation—increases the rate of neurogenesis in the dentate gyrus. There are only a few exceptions, such as the increase in neurogenesis that follows NMDA receptor blockade (Nacher et al., 2001) using MK-801, a noncompetitive antagonist of NMDA receptors, or CGP 43487, a competitive antagonist. Depletion of norepinephrine, which would decrease the likelihood of norepinephrine-mediated, dentate granule cell potentiation (Harley, 1991), also increases neurogenesis (Kulkarni et al., 2002).
Given that the majority of studies show that increased neuronal activity increases neurogenesis in the dentate gyrus, it is not surprising that seizure activity also increases neurogenesis. Bengzon et al. (1997) demonstrated this initially using a single afterdischarge, and subsequent studies showed that virtually all methods of seizure induction led to increased neurogenesis.
For example, status epilepticus initiated by administration of the chemoconvulsant pilocarpine intraperitoneally (Parent et al., 1997) or unilateral kainic acid intracerebroventricularly (Gray and Sundstrom, 1998) led to a bilateral increase in neurogenesis. Amygdala kindling (Parent et al., 1998; Scott et al., 1998) is another example of seizure induction in rodents that increases neurogenesis. Electroconvulsive shock also increases neurogenesis in rodents (Madsen et al., 2000; Scott et al., 2000). Subsequent studies have provided further support of these initial investigations (Covolan et al., 2000; Nakagawa et al., 2000; Ferland et al., 2002; Jiang et al., 2003).
The mechanism of the increased cell proliferation after seizures is largely unknown, although 5HT-1A (Radley and Jacobs, 2003; Zucchini et al., 2005) and galanin type 2 receptors appear important (Mazarati, 2004). Furthermore, neuropeptide Y (NPY) via its Y1 receptor (Howell et al., 2005), and fibroblast growth factor 2 (FGF-2) (Yoshimura, 2001) may contribute. Sonic hedgehog (Shh) signaling has also been implicated in seizure-induced progenitor proliferation (Banerjee, 2005).
A role for seizure-induced injury in stimulating proliferation is suggested by studies in culture, which show that kainic acid-induced injury precedes the increase in proliferation (Sadgrove et al., 2005). However, events associated with seizure-induced damage, such as up-regulation of neurotrophins, could be more important than damage per se (Hagihara, 2005; Scharfman et al., 2005). Besides neurotrophins, cysteine protease cystatin C, which is expressed on astrocytes and microglia after status epilepticus, may play a role in seizure-induced increase in neurogenesis (Pirttila, 2005). An argument against a fundamental role of seizure-induced cell death is the fact that a proliferative progenitor response to seizures can occur independent of cell death (Smith, 2005).
Seizures may exert their effect by facilitating certain steps during progenitor differentiation. In other words, do seizures stimulate the transition from Type 1 (GFAP-immunoreactive, radial glia-like cells) to Type 2a or Type 3 (doublecortin-immunoreactive)? Interestingly, after NMDA receptor antagonism, which increases neurogenesis, there is an increase in radial glia-like (Type 1) cells (Nacher et al., 2001). To address this question, Huttman et al. (2003) examined Type 1 cells using transgenic FVB/n mice expressing GFP in cells with the GFAP promoter. They found a greater number of Type 1 cells in the SGZ at a time after kainic acid-induced status epilepticus when neurogenesis is likely to be maximal (72 h). This result suggested a greater proportion of proliferating Type 1 cells (Huttmann et al., 2003). An increase in the number of proliferating astrocytes with radial glia-like features has also been reported seven days after kainic acid-induced status, based on expression for ribonucleotide reductase, an endogenously expressed cytoplasmic marker of cell proliferation (Zhu et al., 2005). Interestingly, this study also found that the number of clusters of proliferating cells in the SGZ increased after seizures, but GFAP expression in each cluster was unchanged, suggesting that more Type 1 cells were recruited into the cell cycle after seizures.
Jessberger et al. (Jessberger et al., 2005) examined Type 3 cells (doublecortin immunoreactive) after kainic acid-induced status and found that status stimulated division. They did not find evidence of increased numbers of Type 1 cells, but they evaluated animals nine days after status, a time when some could have differentiated. Therefore, there is evidence that seizures can influence the proliferation of both primitive GFAP-expressing precursors and more committed neuronal-like precursors.
Seizures also modify the survival of new neurons (Ekdahl et al., 2001). The severity of seizures appears to play an important role, with more severe seizures decreasing survival of new neurons (Mohapel et al., 2004; Scott and Burnham, 2004). However, the spontaneous seizures that follow status epilepticus do not appear to influence survival (Scharfman et al., 2000; Ekdahl et al., 2003; McCloskey et al., 2006). It is important to consider that the net increase in granule cells is not only a function of the number of surviving new neurons, but it also depends on the numbers of mature neurons that may die due to seizure-induced apoptosis. Although granule cells are relatively resistant to seizure-induced death, it has been shown that granule cells die by apoptosis after seizures in laboratory animals (Sloviter et al., 1996; Bengzon et al., 2002), and granule cell loss is evident in severely sclerotic tissue from individuals with intractable TLE (for review, see Scharfman and Pedley, 2006). Seizures also may have other effects on new neurons that complement their ability to increase the rate of proliferation. This possibility is raised by a recent study showing that the granule cells born in mice after severe seizure are able to more rapidly develop dendrites and other structural features of mature granule cells (Overstreet-Wadiche et al., 2006). They also appear to integrate more readily into the circuitry of the dentate gyrus (Overstreet-Wadiche et al., 2006). These data suggest that, in addition to the previous demonstration that seizures increase the rate of dentate gyrus neurogenesis, seizures also facilitate the maturation and assimilation of new cells into the hippocampus. Based on these results, one might conclude that seizures are one of the most robust means to increase functional, mature dentate granule cells in the adult brain.
SEIZURE-INDUCED NEUROGENESIS AND ITS RELEVANCE TO ANIMAL MODELS OF EPILEPSY
Although an intriguing phenomenon, and striking in its robust nature, the significance of seizure-induced neurogenesis to TLE has remained elusive. Is it relevant to epileptogenesis in TLE? Is it relevant to other aspects of TLE? Or might it simply be one of many phenomenons that have been reported in animal models of epilepsy for which there is no critical role?
In vivo studies
Arguments against a role of seizure-induced neurogenesis in epileptogenesis are based on studies which demonstrate that the newly born neurons do not necessarily survive for long periods of time (Bengzon et al., 2002; Mohapel et al., 2004). If the neurons do not live for long periods of time, it would seem unlikely that they could be influential. However, a transient population of new cells could be important (Fig. 1). Furthermore, some newly born cells can survive for long periods of time. This may depend on the severity of the initial seizures used to stimulate proliferation. The reason for this suggestion is based on studies of seizure severity (Mohapel et al., 2004), and also the fact that long survival was described in a study that truncated status severely (Scharfman et al., 2000).
FIG. 1.
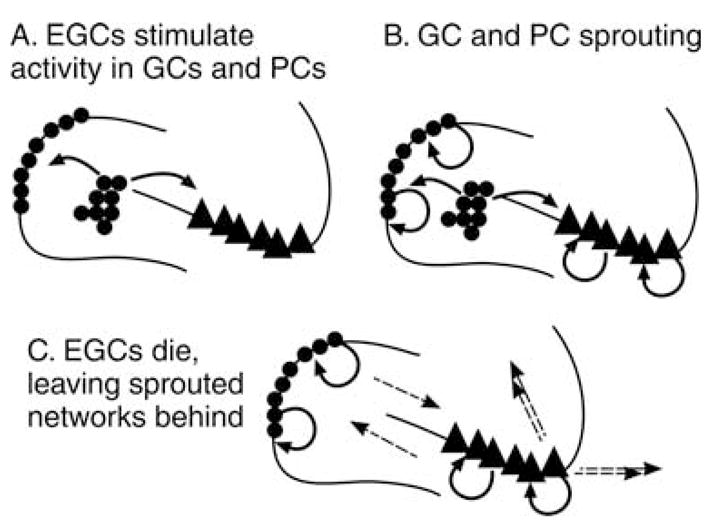
An illustration of the ways that neurogenesis, and ectopic granule cells (EGCs) could contribute to persistent changes in the circuitry of the hippocampus, even if the ectopic cells did not persist. (A) The initial step would be the formation of ectopic granule cells. It is hypothesized that their development would stimulate new circuits to form among neighboring neurons, for example, the granule cells and the CA3 pyramidal cells. In addition, non-principal cells might be involved in these circuits (not shown). (B) Some of the new circuits that form could be recurrent excitatory circuits among granule cells (mossy fiber sprouting) or strengthening of the recurrent collaterals among pyramidal cells. These may not all include ectopic granule cells. (C) If ectopic granule cells die, the recurrent excitatory circuits that are independent of them may be unaffected by their loss, and interact by preexisting interconnections (dotted lines) which could lead to an influence on downstream targets (double dotted lines).
Other studies have also suggested that seizure-induced neurogenesis may not have a profound influence on chronic epilepsy. Using pilocarpine to initiate status and subsequent recurrent seizures, Parent et al. (1999) administered radiation during the days immediately after status, when neurogenesis normally increases greatly. They found that animals still developed spontaneous seizures, suggesting that a reduction in neurogenesis could not prevent all seizures that developed in this animal model. However, it might have reduced seizures, a question that was unanswered because it was not the focus of the study. Indeed, the answer would have required rigorous seizure quantification.
A second study provided strong evidence that seizure-induced neurogenesis can influence chronic seizures in animal models. This study used i.c.v. infusion of the mitotic inhibitor arabinoside-C to reduce neurogenesis, and quantified both neurogenesis and seizures. They showed that reduction of new neurons after lithium-pilocarpine-induced status epilepticus was associated with a reduction in chronic seizure frequency (Jung et al., 2004). A caveat was that arabinoside-C influenced glia in area CA1, so factors besides reduced neurogenesis may be why seizure frequency declined. A selective means to ablate newly born neurons after seizures would be extremely valuable to evaluate their functional role.
In vitro studies
Experiments in vitro support the hypothesis that seizure-induced neurogenesis may contribute to increased excitability in animals that have had status followed by chronic seizures. These data were initially surprising because they did not support the prevailing view at the time, that new neurons in the adult brain would benefit CNS function.
The data were based on the new neurons that develop in the hilus, the region outside the granule cell layer that lies between the dentate gyrus and area CA3 (for review, see Scharfman, 1999). These cells are commonly termed “ectopic” granule cells because of their location outside the layer (Parent et al., 1997; Dashtipour et al., 2001; Scharfman et al., 2000). After pilocarpine-induced status epilepticus, it was found that a substantial population of new granule cells develop in this location (Parent et al., 1997; Scharfman et al., 2000).
The very fact that a large population of abnormally situated granule cells develops after status epilepticus in laboratory animals indicates that seizures, or seizure-induced neurogenesis, may lead to disorganization of the dentate gyrus, a potential problem for normal information processing in the hippocampal formation. This can be appreciated simply by the axonal projection of the new hilar granule cells, which projects both to area CA3, like a normal granule cell, and to the inner molecular layer (Scharfman et al., 2000). It is also supported by ultrastructural studies showing increased excitatory afferent input to ectopic granule cells in the hilus (Dashtipour et al., 2001; Pierce et al., 2005). Furthermore, physiological recordings in hippocampal slices showed that the ectopic granule cells discharged spontaneous bursts of action potentials, which is unusual for granule cells (Scharfman et al., 2000). The spontaneous discharges were synchronized with area CA3 pyramidal cell population discharges, suggesting that the activity was abnormal (Scharfman et al., 2000). Epileptiform burst discharges are not normally recorded in granule cells that are located in the granule cell layer, even after exposure to convulsants (Scharfman, 1994). The network burst discharges could play a role in the recurrent seizures that occur in these animals, because each burst could activate target neurons in CA1, the contralateral hippocampus, and ultimately the cortex. These discharges could also reverberate in the dentate-CA3 network because the ectopic cells have axon projections that contribute to the network of mossy fiber axons which sprout into the inner molecular layer (Scharfman et al., 2000). However, potential for the new neurons to innervate GABAergic neurons, or for the new neurons to express GABA like other “epileptic” granule cells (Gutierrez, 2005), remain unclear, and could dampen excitability.
In light of the possibility that the new granule neurons could influence the dentate gyrus and area CA3, it is important to consider the evidence that the abnormal network of ectopic granule cells could contribute to seizure activity in the pilocarpine animal model. To date, this question has not been fully addressed. However, it is known that after a spontaneous seizure in this animal model of epilepsy, c-fos is expressed in the ectopic granule cells, suggesting that at the very least, they are activated during a spontaneous seizure (Scharfman et al., 2001). In addition, quantification of the number of ectopic granule cells is correlated with seizure frequency (McCloskey et al., 2006). When this population is reduced, seizure frequency is also reduced (Jung et al., 2004). The similarity in the time to maturity of new ectopic neurons and the time to spontaneous seizures is suggestive, but not definitive proof that the maturation of new cells contributes to spontaneous seizures. Therefore, an increase in ectopic granule cells after status could contribute to epileptogenesis (Fig. 2). Epileptogenesis after status epilepticus may not only be due to factors associated with seizure-induced damage, but also seizure-induced cell birth.
FIG. 2.
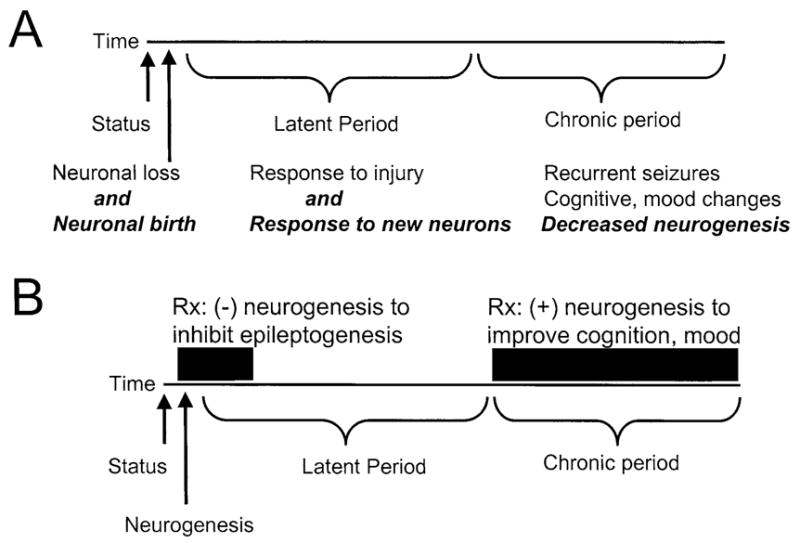
A schematic illustrating the ways that seizure-induced neurogenesis may influence epileptogenesis in TLE, and potential for therapeutic intervention. (A) A theoretical timeline is shown to reflect the hypothesis that TLE is due to an initial precipitating event, a latent period, and a subsequent chronic state of recurrent seizures. The timeline begins when an initial precipitating insult occurs, such as status epilepticus. It is suggested that status-induced neuronal damage, as well as the increase in neurogenesis following status (italicized text), contributes to epileptogenesis. Reduced neurogenesis in the chronic period may contribute to cognitive changes, which are common in TLE, and animal models of TLE. (B) The same timeline is used to suggest therapeutic intervention, which would potentially impede epileptogenesis if neurogenesis were inhibited initially. Increasing neurogenesis during the chronic period might be used to alleviate cognitive dysfunction and depression.
SEIZURE-INDUCED NEUROGENESIS AND ITS RELEVANCE TO TLE
What is the evidence that seizure-induced neurogenesis of ectopic granule cells is relevant to patients with TLE? In addition, how do the new granule cells that develop in the correct location, that is, the granule cell layer, possibly contribute to TLE pathophysiology?
Neurogenesis in the granule cell layer
Regarding the new granule cells that develop in the granule cell layer after seizures, that is, normally positioned granule cells, initial studies suggested a discordance between the data in laboratory animals and TLE. Thus, in studies of tissue resected from patients with intractable TLE, Blümcke and colleagues found little evidence of newly born granule cells in the granule cell layer (Blümcke et al., 2001). They reported that in pediatric cases less than 2 years old, evidence was present, but supportive data were sparse in older patients. A more recent study of tissue resected from patients with pharmacologically resistant TLE provided substantial evidence for progenitor cells, but similar to the findings of Blumcke and colleagues; there was no strong evidence that the progenitors ultimately became neurons. This conclusion was based on the lack of coexpression of markers of progenitors with antibodies to NeuN, a neuronal nuclear antigen found in all adult neurons (Crespel et al., 2005). Instead, the progenitors expressed markers of a glial phenotype, such as nestin, and vimentin (Blümcke et al., 2001; Crespel et al., 2005). Other studies of tissue specimens from patients with intractable TLE showed that there was decreased expression of cell markers that reflect immature neurons (Mathern et al., 2002). These data are consistent with studies discussed above, that few immature neurons exist in the individuals with TLE that come to surgery. They are also consistent with the animal studies showing that the most severe cases are associated with new neurons that do not survive for long periods of time (Mohapel et al., 2004). However, at some point in the course of TLE, neurogenesis is likely to be increased, based on the evidence of new granule cells in a recent study (Parent et al., 2006).
The different reports from studies of TLE may be due to the difference in the severity of the epilepsy as discussed above, or other factors. The age of the patient at the onset of epilepsy or epileptogenesis may play a role, because studies in rodents show that seizures during development lead to a different outcome relative to seizures during adulthood (Sankar et al., 2000; McCabe et al., 2001; Cha et al., 2004; Porter et al., 2004). There are also technical issues that can lead to different conclusions about neurogenesis in TLE: markers of an immature neuron vary in the duration of their expression, potentially causing distinct interpretations about the extent of proliferation.
Granule cell dispersion and seizure induced neurogenesis
Houser (1990) described a dispersed appearance of the granule cell layer characterized by an irregular widening of the granule cell layer. Fifty percent of patients with hippocampal sclerosis have a dispersed granule cell layer (Lurton et al., 1998). Clinically, there is a strong correlation between granule cell dispersion and a history of seizures in the first four years of life (Houser, 1990; Lurton et al., 1998). Complicated or prolonged febrile convulsions appear to be particularly associated with granule cell dispersion, while simple febrile convulsions are not (Lurton et al., 1998). Animal studies also support the observation that granule cell dispersion is associated with an initial, severe period of status epilepticus (Suzuki et al., 1995; Bouilleret et al., 1999). While dispersion of the granule cell layer has been hypothesized to be due to an initial excessive production of new neurons with later pruning, new evidence suggests it is not the case. Indeed, reelin deficiency and displacement of mature neurons, rather than neurogenesis, underlies granule cell dispersion in the epileptic hippocampus, as recently demonstrated by Heinrich et al. (2006).
Ectopic granule cells
There is some support that the ectopic location of granule cells increases in patients with TLE. For example, Houser et al. discussed that some granule cells were present in the hilus in tissue showing granule cell dispersion (Houser, 1990). Other studies of tissue resected from patients with intractable TLE demonstrated granule-like cells in the hilus (Sloviter et al., 1991; Thom, 2002). The evidence that they were granule cells was based on their similar morphology to granule cells, and their expression of calbindin. Importantly, more recent studies using a granule cell-specific marker have shown that ectopic granule cells exist in patients with intractable TLE (Parent et al., 2006).
It is important to point out that some studies do not detect ectopic granule cells in tissue from patients with intractable TLE. This could be due to the fact that they are not always present. Alternatively, they may not exist in subsets of TLE with severe hippocampal damage, when hilar neurons of all kinds are lost. Patients without severe sclerosis may contain more ectopic granule cells. Indeed, it would be interesting to study those patients, so that more comprehensive understanding of ectopic granule cells in all patients with TLE could be obtained.
Even in those tissue samples that have been examined, however, it is possible that the ectopic cells are underestimated. This is because multiple markers for these cells are not always used. This is true for studies of progenitors in the hilus, as well as hilar ectopic granule cells. For example, NG2-immunoreactive progenitors that exist in the hilus in some patients with intractable TLE have recently been identified (Sosunov et al., 2003); previous studies had not examined NG2 immunoreactivity.
Another reason why ectopic granule cells may be underestimated is that patients with a long period of chronic seizures prior to surgery, or a long history of antiepileptic (AED) therapy, may have reduced numbers of ectopic granule cells relative to those that are more rapidly recommended for surgical resection. This may occur because the more severe seizures damage the cells (Mohapel et al., 2004). Little is known about the effects of AEDs on dentate gyrus neurogenesis, although it has been shown that chronic treatment with valproate increased neurogenesis in the normal rodent dentate gyrus (Hao et al., 2004). Thus, tissue from patients with TLE for many decades may not show evidence of hilar progenitors or ectopic hilar granule cells, because the cells had developed at initial stages of the disease, but did not survive.
If this is the case, how could these cells be important to epileptogenesis, or epilepsy? It could be that they initially were born early in the process of epileptogenesis, and stimulated abnormal circuits to develop (Fig. 1). After the abnormal circuits developed, increased excitability could persist even if the ectopic granule cells died, assuming that the new circuits became independent of ectopic granule cells.
Reduced neurogenesis after chronic seizures as a mediator of cognitive dysfunction
Given the emerging role of adult neurogenesis in hippocampal-dependent learning and behaviour (Shors, 2004; Aimone et al., 2006; Dranovsky and Hen, 2006), changes in neurogenesis in chronic epilepsy may alter cognitive function and mood because it changes the production and/or integration of newly born neurons within the dentate gyrus. Central to this hypothesis has been the work of Hattiangady and colleagues (2004), who demonstrated that dentate gyrus neurogenesis initially rose after status epilepticus, and then declined in subsequent months, during the time of recurrent seizures. Interestingly, neurogenesis in the dispersed dentate gyrus has also been reported to be significantly reduced (Kralic et al., 2005). The cause of reduced neurogenesis is unknown, but may reflect disruption of the SGZ, and appears to also occur in patients with chronic epilepsy (Mathern et al., 2002; Crespel et al., 2005). It is tempting to suggest that a chronic reduction in neurogenesis may lead to cognitive dysfunction and depression in patients with TLE.
SUMMARY AND PERSPECTIVE
A substantial body of evidence now exists which demonstrates that seizures increase the rate of dentate gyrus neurogenesis in adult rodents. The consequences for our understanding of epileptogeneis and chronic epilepsy remain to be fully elucidated, but some surprising suggestions have been made already. For example, hilar ectopic granule cells develop after severe seizures, and appear to contribute to epileptogenesis rather than resolve it by replacing damaged hilar neurons. Furthermore, although there appears to be an initial surge in neurogenesis after an initial period of seizures in normal animals, chronic recurrent seizures may not be as influential, and indeed are associated with the opposite—reduced neurogenesis. These findings from animal models have been echoed by studies of tissue from patients with intractable TLE, because there is little evidence for ongoing, increased proliferation, yet there are abnormally situated neurons that could reflect abnormal development of new neurons in the history of the patient.
How to use our growing understanding of dentate gyrus neurogenesis in epilepsy for therapeutic benefit is an important question. From studies to date, one would predict that interventions early in the process of epileptogenesis that would reduce new ectopic granule cells would impede the development of recurrent seizures. In contrast, after epileptogenesis has occurred, that is, patients develop chronic seizures, the opposite type of intervention might be beneficial: enhancing neurogenesis might ameliorate cognitive dysfunction and depressive symptoms. Although these clinical possibilities are attractive, it is important to recognize that the effect of such interventions are difficult to predict in the epileptic brain, where multiple seizure-induced changes develop in addition to altered neurogenesis (Scharfman and Pedley, 2006; Scharfman and Schwarcz, in press). Therefore, a greater understanding of seizure-induced neurogenesis will be required before its therapeutic potential can be fulfilled.
Acknowledgments
We thank Dr. Guy McKhann II for discussion. Supported by NIH NS 37562, NS 41490, the Human Science Frontiers Program, and the Medical Research Council.
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nature Neuroscience. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Altman J. Are neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. Journal of Comparative Neurology. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Banerjee SB. Recruitment of the Sonic hedgehog signalling cascade in electroconvulsive seizure-mediated regulation of adult rat hippocampal neurogenesis. European Journal of Neuroscience. 2005;22:1570–1580. doi: 10.1111/j.1460-9568.2005.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Elmer E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengzon J, Mohapel P, Ekdahl CT, Lindvall O. Neuronal apoptosis after brief and prolonged seizures. Progress in Brain Research. 2002;135:111–119. doi: 10.1016/S0079-6123(02)35011-8. [DOI] [PubMed] [Google Scholar]
- Blümcke I, Schewe JC, Normann S, Brustle O, Schramm J, Elger CE, Wiestler OD. Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus. 2001;11:311–321. doi: 10.1002/hipo.1045. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Ridoux V, Depaulis A, Marescaux C, Nehlig A, Le Gal La Salle G. Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience. 1999;89:717–729. doi: 10.1016/s0306-4522(98)00401-1. [DOI] [PubMed] [Google Scholar]
- Cha BH, Akman C, Silveira DC, Liu X, Holmes GL. Spontaneous recurrent seizure following status epilepticus enhances dentate gyrus neurogenesis. Brain Research. 2004;26:394–397. doi: 10.1016/j.braindev.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- Covolan L, Ribeiro LT, Longo BM, Mello LE. Cell damage and neurogenesis in the dentate granule cell layer of adult rats after pilocarpine- or kainate-induced status epilepticus. Hippocampus. 2000;10:169–180. doi: 10.1002/(SICI)1098-1063(2000)10:2<169::AID-HIPO6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Crespel A, Rigau V, Coubes P, Rousset MC, deBock F, Okano H, Baldy-Moulinier M, Bockaert J, Lerner-Natoli M. Increased number of neural progenitors in human temporal lobe epilepsy. Neurobiological Disorder. 2005;19:436–450. doi: 10.1016/j.nbd.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Dashtipour K, Tran PH, Okazaki MM, Nadler JV, Ribak CE. Ultrastructural features and synaptic connections of hilar ectopic granule cells in the rat dentate gyrus are different from those of granule cells in the granule cell layer. Brain Research. 2001;890:261–271. doi: 10.1016/s0006-8993(00)03119-x. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. Journal of Cellular Biology. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biological Psychology. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Mohapel P, Elmer E, Lindvall O. Caspase inhibitors increase short-term survival of progenitor-cell progeny in the adult rat dentate gyrus following status epilepticus. European Journal of Neuroscience. 2001;14:937–945. doi: 10.1046/j.0953-816x.2001.01713.x. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Zhu C, Bonde S, Bahr BA, Blomgren K, Lindvall O. Death mechanisms in status epilepticus-generated neurons and effects of additional seizures on their survival. Neurobiological Disorder. 2003;14:513–523. doi: 10.1016/j.nbd.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ferland RJ, Gross RA, Applegate CD. Increased mitotic activity in the dentate gyrus of the hippocampus of adult C57BL/6J mice exposed to the flurothyl kindling model of epileptogenesis. Neuroscience. 2002;115:669–683. doi: 10.1016/s0306-4522(02)00514-6. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. Journal of Neuroscience. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neuroscience. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gray WP, Sundstrom L. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Research. 1998;790:52–59. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- Gutierrez R. The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends in Neuroscience. 2005;28:297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Hagihara H. Tonic-clonic seizures induce division of neuronal progenitor cells with concomitant changes in expression of neurotrophic factors in the brain of pilocarpine-treated mice. Molecular Brain Research. 2005;139:258–266. doi: 10.1016/j.molbrainres.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, Gould TD, Manji HK, Chen G. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. Journal of Neuroscience. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. Noradrenergic and locus coeruleus modulation of the perforant path-evoked potential in rat dentate gyrus supports a role for the locus coeruleus in attentional and memorial processes. Progress in Brain Research. 1991;88:307–321. doi: 10.1016/s0079-6123(08)63818-2. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. Journal of Comparative Neurology. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiological Disorder. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Heinrich C, Nitta N, Flubacher A, Muller M, Fahrner A, Kirsch M, Freiman T, Suzuki F, Depaulis A, Frotscher M, Haas CA. Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. Journal of Neuroscience. 2006;26:4701–4713. doi: 10.1523/JNEUROSCI.5516-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Research. 1990;535:195–204. doi: 10.1016/0006-8993(90)91601-c. [DOI] [PubMed] [Google Scholar]
- Howell OW, Doyle K, Goodman JH, Scharfman HE, Herzog H, Pringle A, Beck-Sickinger A, Gray WP. Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus. Journal of Neurochemistry. 2005;93:560–570. doi: 10.1111/j.1471-4159.2005.03057.x. [DOI] [PubMed] [Google Scholar]
- Huttmann K, Sadgrove M, Wallraff A, Hinterkeuser S, Kirchhoff F, Steinhauser C, Gray WP. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. European Journal of Neuroscience. 2003;18:2769–2778. doi: 10.1111/j.1460-9568.2003.03002.x. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. European Journal of Neuroscience. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Romer B, Babu H, Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Experiments in Neurology. 2005;196:342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Jiang W, Wan Q, Zjang ZJ, Wang WD, Huang YG, Rao ZR, Zhang X. Dentate granule cell neurogenesis after seizures induced by pentylenetrazol in rats. Brain Research. 2003;977:141–148. doi: 10.1016/s0006-8993(03)02438-7. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Kim M, Jeong SW, Song YM, Lee ST, Kim JY, Lee SK, Roh JK. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. European Journal of Neuroscience. 2004;19:3219–3226. doi: 10.1111/j.0953-816X.2004.03412.x. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gould E. Adult neurogenesis: a mechanism for brain repair? Journal of Clinical and Experimental Neuropsychology. 2003;25:721–732. doi: 10.1076/jcen.25.5.721.14580. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Ledergerber DA, Fritschy JM. Disruption of the neurogenic potential of the dentate gyrus in a mouse model of temporal lobe epilepsy with focal seizures. European Journal of Neuroscience. 2005;22:1916–1927. doi: 10.1111/j.1460-9568.2005.04386.x. [DOI] [PubMed] [Google Scholar]
- Kulkarni VA, Jha S, Vaidya VA. Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. European Journal of Neuroscience. 2002;16:2008–2012. doi: 10.1046/j.1460-9568.2002.02268.x. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annual Review of Pharmacology and Toxicology. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Lurton D, El Bahh B, Sundstrom L, Rougier A. Granule cell dispersion is correlated with early epileptic events in human temporal lobe epilepsy. Journal of Neurological Science. 1998;154:133–136. doi: 10.1016/s0022-510x(97)00220-7. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biological Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections fo the field CA3 and are surrounded by synaptic vesicles. Journal of Comparative Neurology. 1999;406:449–460. [PubMed] [Google Scholar]
- Mathern GW, Leiphart JL, DeVera A, Adelson PD, Seki T, Neder L, Leite JP. Seizures decrease postnatal neurogenesis and granule cell development in the human fascia dentata. Epilepsia. 2002;43:68–73. doi: 10.1046/j.1528-1157.43.s.5.28.x. [DOI] [PubMed] [Google Scholar]
- Mazarati A. Galanin type 2 receptors regulate neuronal survival, susceptibility to seizures and seizure-induced neurogenesis in the dentate gyrus. European Journal of Neuroscience. 2004;19:3235–3244. doi: 10.1111/j.0953-816X.2004.03449.x. [DOI] [PubMed] [Google Scholar]
- McCabe BK, Silveira DC, Cilio MR, Cha BH, Liu X, Sogawa Y, Holmes GL. Reduced neurogenesis after neonatal seizures. Journal of Neuroscience. 2001;21:2094–2103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DP, Hintz T, Pierce JP, Scharfman HE. Stereological methods reveal the robust size and stability of an ectopic population of hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. European Journal of Neuroscience. 2006 doi: 10.1111/j.1460-9568.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapel P, Ekdahl CT, Lindvall O. Status epilepticus severity influences the long-term outcome of neurogenesis in the adult dentate gyrus. Neurobiological Disorder. 2004;15:196–205. doi: 10.1016/j.nbd.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Nacher J, Rosell DR, Alonso-Llosa G, McEwen BS. NMDA receptor antagonist treatment induces a long-lasting increase in the number of proliferating cells, PSA-NCAM-immunoreactive granule neurons and radial glia in the adult rat dentate gyrus. European Journal of Neuroscience. 2001;13:512–520. doi: 10.1046/j.0953-816x.2000.01424.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa E, Aimi Y, Yasuhara O, Tooyama I, Shimada M, McGeer P, Kimura H. Enhancement of progenitor cell division in the dentate gyrus triggered by initial limbic seizures in rat models of epilepsy. Epilepsia. 2000;41:10–18. doi: 10.1111/j.1528-1157.2000.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. Seizures accelerate functional integration of adult-generated granule cells. Journal of Neuroscience. 2006;26:4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Lowenstein DH. Seizure-induced neurogenesis: are more new neurons good for an adult brain? Progress in Brain Research. 2002;135:121–131. doi: 10.1016/S0079-6123(02)35012-X. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. Journal of Neuroscience. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Janumpalli S, McNamara JO, Lowenstein DH. Increased dentate granule cell neurogenesis following amygdala kindling in the adult rat. Neuroscience Letters. 1998;247:9–12. doi: 10.1016/s0304-3940(98)00269-9. [DOI] [PubMed] [Google Scholar]
- Parent JM, Tada E, Fike JR, Lowenstein DH. Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. Journal of Neuroscience. 1999;19:4508–4519. doi: 10.1523/JNEUROSCI.19-11-04508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Annals of Neurology. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Annals of Neurology. 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Punsoni M, McCloskey DP, Scharfman HE. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Experiments in Neurology. 2005;196:316–331. doi: 10.1016/j.expneurol.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirttila TJ. Cystatin C modulates neurodegeneration and neurogenesis following status epilepticus in mouse. Neurobiological Disorder. 2005;20:241–253. doi: 10.1016/j.nbd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Porter BE, Maronski M, Brooks-Kayal AR. Fate of newborn dentate granule cells after early life status epilepticus. Epilepsia. 2004;45:13–19. doi: 10.1111/j.0013-9580.2004.23903.x. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Jacobs BL. Pilocarpine-induced status epilepticus increases cell proliferation in the dentate gyrus of adult rats via a 5-HT1A receptor-dependent mechanism. Brain Research. 2003;966:1–12. doi: 10.1016/s0006-8993(02)03989-6. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. European Journal of Neuroscience. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Sadgrove M, Chad JE, Gray WP. Kainic acid induces rapid cell death followed by transiently reduced cell proliferation in the immature granule cell layer of rat organotypic hippocampal slice cultures. Brain Research. 2005;1035:111–119. doi: 10.1016/j.brainres.2004.11.059. [DOI] [PubMed] [Google Scholar]
- Sankar R, Shin D, Liu H, Katsumori H, Wasterlain CG. Granule cell neurogenesis after status epilepticus in the immature rat brain. Epilepsia. 2000;41:S53–S56. doi: 10.1111/j.1528-1157.2000.tb01557.x. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. EPSPs of dentate gyrus granule cells during epileptiform bursts of dentate hilar “mossy” cells and area CA3 pyramidal cells in disinhibited rat hippocampal slices. Journal of Neuroscience. 1994;14:6041–6057. doi: 10.1523/JNEUROSCI.14-10-06041.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. The role of nonprincipal cells in dentate gyrus excitability and its relevance to animal models of epilepsy and temporal lobe epilepsy. In: Delgado-Esqueta AV, Wilson W, Olsen RW, Porter RJ, editors. Basic mechanisms of the epilepsies: molecular and cellular approaches. 3. New York: Lippincott-Raven; 1999. pp. 805–820. [PubMed] [Google Scholar]
- Scharfman HE. Functional implications of seizure-induced neurogenesis. Advances in Experimental Medicine and Biology. 2004;548:192–212. doi: 10.1007/978-1-4757-6376-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Pedley TA. Temporal lobe epilepsy. In: Gilman A, editor. The Neurobiology of Disease. Academic Press; New York: 2006. [Google Scholar]
- Scharfman HE, Schwarcz R. Neuromodulators in seizures, epileptogenesis, and epilepsy. In: Engel P, Pedley TA, editors. Epilepsy: a comprehensive textbook. 2. Lippincott-Raven; New York: in Press. [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. Journal of Neuroscience. 2000;201:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Goodman JH. Spontaneous recurrent seizures after pilocarpine-induced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neuroscience. 2001;2111:71–81. doi: 10.1016/s0306-4522(01)00599-1. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, MacLeod A, Phani S, Antonelli C, Croll SD. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Experiments in Neurology. 2005;192:384–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Scott BW, Burnham WM. Kindled seizures enhance young neuron survival in the adult rat dentate gyrus. Acta Neuropathology (Berlin) 2004;111:364–371. doi: 10.1007/s00401-006-0039-y. [DOI] [PubMed] [Google Scholar]
- Scott BW, Wang S, Burnham WM, DeBoni U, Wojtowicz JM. Kindling-induced neurogenesis in the dentate gyrus of the rat. Neuroscience Letters. 1998;248:73–76. doi: 10.1016/s0304-3940(98)00355-3. [DOI] [PubMed] [Google Scholar]
- Scott BW, Wojtowicz JM, Burnham WM. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Experiments in Neurology. 2000;165:231–236. doi: 10.1006/exnr.2000.7458. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, BSM, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. Journal of Comparative Neurology. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ribak CE. Integration of newly born dentate granule cells into adult brains: hypotheses based on normal and epileptic rodents. Brain Research Rev. 2005;48:43–56. doi: 10.1016/j.brainresrev.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Memory traces of trace memories: neurogenesis, synaptogenesis and awareness. Trends in Neuroscience. 2004;27:250–256. doi: 10.1016/j.tins.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Sollas AL, Barbaro NM, Laxer KD. Calcium-binding protein (calbindin-D28K) and parvalbumin immunocytochemistry in the normal and epileptic human hippocampus. Journal of Comparative Neurology. 1991;308:381–396. doi: 10.1002/cne.903080306. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dean E, Sollas AL, Goodman JH. Apoptosis and necrosis induced in different hippocampal neuron populations by repetitive perforant path stimulation in the rat. Journal of Comparative Neurology. 1996;366:516–533. doi: 10.1002/(SICI)1096-9861(19960311)366:3<516::AID-CNE10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Smith PD. Seizures, not hippocampal neuronal death, provoke neurogenesis in a mouse rapid electrical amygdala kindling model of seizures. Neuroscience. 2005;136:405–415. doi: 10.1016/j.neuroscience.2005.07.055. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowica JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. Journal of Neurophysiology. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Sosunov AA, Wu X, Crino PD, Goodman RR, McKhann GM. Discrete populations of nestin expressing cells in human epileptic hippocampus: implications for cell replacement strategies. Epilepsia. 2003;44:174. [Google Scholar]
- Suzuki F, Junier MP, Guilhem D, Sorensen JC, Onteniente B. Morphogenetic effect of kainate on adult hippocampal neurons associated with a prolonged expression of brain-derived neurotrophic factor. Neuroscience. 1995;64:665–674. doi: 10.1016/0306-4522(94)00463-f. [DOI] [PubMed] [Google Scholar]
- Thom M. Cytoarchitectural abnormalities in hippocampal sclerosis. Journal of Neuropathology and Experimental Neurology. 2002;61:510–519. doi: 10.1093/jnen/61.6.510. [DOI] [PubMed] [Google Scholar]
- Toni N, Bushong EA, Teng EM, Jones Y, vanPraag H, Martone ME, Ellisman MH, Gage FH. Synaptogenesis of new neurons in the adult hippocampus. Washington, DC: Society for Neuroscience; 2004. [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Dahlstrom A, Hansson HA. Characterization of cell proliferation in the adult dentate under normal conditions and after kainate induced seizures using ribonucleotide reductase and BrdU. Brain Research. 2005;1036:7–17. doi: 10.1016/j.brainres.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Zucchini S, Barbieri M, Simonato M. Alterations in seizure susceptibility and in seizure-induced plasticity after pharmacologic and genetic manipulation of the fibroblast growth factor-2 system. Epilepsia. 2005;46:52–58. doi: 10.1111/j.1528-1167.2005.01009.x. [DOI] [PubMed] [Google Scholar]


