Abstract
Background
A hallmark of visuospatial neglect syndrome is that patients with lesions to right parietal cortex misbisect horizontal lines far rightward of veridical center. Neurologically normal subjects misbisect lines with a systematic leftward bias (pseudoneglect). Both phenomena, as well as neuroimaging studies, disclose a predominant right hemisphere control of spatial attention. Numerous studies of patients with schizophrenia have implicated global deficits of either right or left hemisphere function, as well as compromised integrity of the corpus callosum.
Methods
To better understand the functional implications of schizophrenia we utilized a forced-choice tachistoscopic line bisection task to probe the status of right hemisphere control of spatial attention, and compared left- versus right-hand unimanual responses to index the degree of callosal transfer of visuospatial information in both patient and control groups.
Results
In contrast to the significant leftward bisection errors of control subjects, patients exhibit no significant leftward error. Whereas control subjects evince a significant correlation between left- and right-hand bisection errors, patients lack a significant intermanual correlation.
Conclusions
The lack of significant leftward bisection error of patients implies a deficit of right hemisphere function. The lack of a significant correlation between left- and right-hand bisection errors in patients implies a loss of callosal integrity.
Keywords: Line Bisection, Pseudoneglect, Visuospatial Attention, Corpus Callosum, Schizophrenia, Magnocellular
1. INTRODUCTION
1.1. Visuospatial neglect and pseudoneglect
Visuospatial attention refers to that type of environmental monitoring which is deployed across the visual field. Disruptions of visuospatial attention are a hallmark of the clinical neurological syndrome known as hemispatial neglect, which is associated with vascular lesions of the right parietal and/or temporal-parietal-occipital (TPO) cortex (Cappa et al., 1991; Kerkhoff, 2001; Mesulam, 1982; 2000; Na et al., 2000; Vallar & Perani, 1987). Line bisection is a frequently used task for the clinical assessment of hemispatial neglect. In line bisection tasks subjects manually mark, or otherwise indicate, the perceived midpoint of lines. Patients with hemispatial neglect bisect horizontal lines far rightward of veridical center (Heilman et al., 1993, Mesulam, 2000; Robertson & Halligan, 1999). Converging evidence from both the laboratory and the clinic indicates that neural circuits in the right cerebral hemisphere play a dominant role in the allocation of visuospatial attention (Corbetta et al., 2000; Coull et al.,2001; Fink et al., 2000; Foxe et al., 2003; Karnath, 2001; Karnath et al., 2001). That the right hemisphere is dominant for this function is revealed in dramatic fashion by the severe deficits of attention directed into contralesional (left) space in hemineglect syndrome (Cappa et al., 1991; Kerkhoff, 2001; Mesulam, 1982; 2000; Na et al., 2000; Vallar & Perani, 1987). The paucity of leftward-directed attention leads to gross rightward errors on line bisection tasks.
There is general agreement that whereas both hemispheres participate in the deployment of spatial attention to right hemispace, the right hemisphere is uniquely responsible for allocating attention into left hemispace (Heilman & Van Den Abell, 1980; Weintraub & Mesulam, 1987). Thus, lesions to the right hemisphere frequently render neglect patients pathologically inattentive to left hemispace, whereas lesions to homologous regions of the left hemisphere produce only modest deficits of spatial attention.
The hemispheric specialization hypothesis of neglect is further supported by the phenomenon known as “pseudoneglect” (Bowers & Heilman, 1980; Jewell & McCourt, 2000), which refers to the systematic leftward misbisection of horizontal lines made by neurologically intact observers. The theoretical connection is that pseudoneglect is proposed to result from the same profound asymmetry in the magnitudes of the attentional vectors projected into left versus right hemispace that underlie hemineglect syndrome (McCourt & Jewell, 1999; McCourt et al., 2000, 2001).
1.2. Line bisection in patients with schizophrenia
Five studies to date have reported line bisection performance in patients diagnosed with schizophrenia. The earliest study (Mather et al., 1990) reported a significant leftward bisection error for control subjects, and no significant difference between the patient and control groups. A study by Barnett (2006) also found leftward errors for control subjects, but the errors made by patients did not differ significantly from zero. Two studies from the same laboratory (Cavezian et al., 2007; Michel et al., 2007) reported bisection errors in control subjects that were not significantly different from zero, and significant leftward errors for patients. Finally, Zivotofsky et al. (2007) reported bisection errors in patients diagnosed with schizophrenia which were not significantly different from zero. Obviously, there is considerable disagreement with respect to both data and interpretation across these studies. It should be noted that all of the previous studies utilized method-of-adjustment manual line bisection procedures which require subjects to make gross motor gestures using the hand and arm, and allow for unlimited oculomotor scanning of the line stimuli. Oculomotor or limb scanning is a significant contributor to bisection error, as disclosed by a recent meta-analysis (Jewell & McCourt, 2000), and unilateral motor activity has also been shown to significantly affect bisection error (McCourt et al., 2001a). When gross motor activation is minimized, and scanning eye movements are disallowed by tachistoscopic presentation, the effect size (Cohen’s d-statistic; Cooper & Hedges, 1994) of pseudoneglect is tripled relative to that found using manual line bisection procedures (d = −1.32 versus −0.40, respectively; Jewell & McCourt, 2000). In addition, most of the prior studies tested only a modest sample of patients: N=24 (Mather et al., 1990); N=10 (Barnett, 2006); N=8 (Michel et al., 2007); and N=10 (Cavezian et al., 2007). In the largest study to date, Zivotofsky et al. (2007) report data from 45 schizophrenic patients, but the lack of a control group makes interpretation of their results problematic. Finally, only Mather et al. (1990) and Barnett (2006) examined the effects of hand used to bisect; the other studies examined only unimanual responses by the dominant hand. For these reasons it is conceivable that important (and statistically significant) differences in the bisection performance of patients and controls were overlooked. The current study was therefore conducted on a large sample of patients (N=34) using a tachistoscopic forced-choice visual line bisection technique. This procedure is three times more sensitive to visuospatial asymmetries than is method-of-adjustment since it minimizes the confounding influences of oculomotor and manual scanning, and isolates the contribution of perceptual/attentional mechanisms. We find that control and patient groups differ significantly on two dimensions. The first is with respect to both global asymmetries of spatial attention, where patients fail to exhibit the typical leftward bisection error which signifies right-hemisphere dominance in control subjects. The second is with respect to intermanual coordination, where the lack of a significant correlation between left- and right-hand bisection errors in patients implies a deficit of transcallosal hemispheric integration.
2. METHOD
2.1. Participants
The control group consisted of twenty neurologically normal right-handed subjects (10 male; mean age = 36.4 years). The patient group consisted of 34 medicated right-handed patients with a diagnosis of schizophrenia/schizoaffective disorder (28 male; mean age = 37.3 years). All patients were inpatients and were recruited from the Clinical Research and Evaluation Facility (CREF) at the Nathan Kline Institute for Psychiatric Research (NKI), an inpatient and outpatient New York state psychiatric facility. Diagnosis was determined by means of chart review, consultation with physicians, and the Structured Clinical Interview for DSM-IV, SCID (First et al., 1997). Both patients and controls were excluded from participating if they exhibited any neurological or ophthalmologic disorders, or if they met criteria for substance dependence within the previous six months, or substance abuse within the month preceding testing. Laterality was assessed using a standard instrument (Oldfield, 1971; expanded scale) on which a composite score of −220 denotes exclusive left-handedness, and +220 denotes exclusive right-handedness. The mean laterality score of control subjects was 165.8; mean laterality score for patients was 185.5. There was no significant difference between groups with respect to age, t(52) = 0.28, p = 0.779; the patient group scored modestly higher on the laterality index, t(52) = 2.17, p = 0.035.
The patient group exhibited primarily negative symptomology. See Table 1 for demographic and clinical data for the patient group.
Table 1.
Demographic and Clinical Characteristics of Schizophrenia Patient Group (n = 34).
| Characteristic | Mean ± SD |
|---|---|
| Diagnosis: Schizophrenia/Schizoaffective Disorder | 26/8 |
| Neuroleptics: Typical/Atypical/Both | 21/3/10 |
| Chlorpromazine daily equivalent, mg | 1252.6 ± 557.8 |
| Education, years | 11.3 ± 2.2 |
| Illness Duration, years* | 17.6 ± 9.6 |
| IQ (Quick Test) score* | 95.1 ± 9.7 |
| BPRS Positive Symptom* | 9.9 ± 4.5 |
| SANS (total score without global baseline)* | 26.6 ± 11.5 |
Data were missing for a single patient in each case.
Abbreviations: BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms. Diagnoses were obtained using the Structured Clinical Interview for DMSIV and all available clinical information. Doses of anti-psychotic medications were translated into chlorpromazine equivalents using the best available literature at the time of data analysis.
The work described here has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Prior to their participation in the study, all participants provided written informed consent. All procedures were approved by the Institutional Review Boards of North Dakota State University and the Nathan Kline Institute for Psychiatric Research.
2.2. Instrumentation and calibration
Subject responses were sensed and collected, and stimuli were presented on a CRT, by microcomputer. Display resolution was 1024 × 768 pixels (34.6° × 26.4°), and the monitor frame refresh rate was 70 Hz. The generation and sequencing of stimuli, and the collection of subject responses, were accomplished using an application written in C++ which utilized the DirectX graphics library.
2.3. Stimuli
Stimuli were horizontally oriented lines of 100% Michelson contrast presented on a homogeneous background whose mean luminance was 35 cd/m2. At a viewing distance of 65 cm the CRT screen subtended 34.6° (w) × 26.4° (h) visual angle; the horizontal line stimuli subtended 13° × 0.44°. All lines were pre-transected prior to presentation.
Figure 1 illustrates examples of line stimuli. Both members of the upper pair of lines (A, B) are transected to the left of veridical line midpoint, by −0.51° and −0.05°, respectively. Both members of the lower pair of lines (D, E) are transected to the right of veridical line midpoint, by +0.51° and +0.22°, respectively. Line C is transected at the veridical midpoint. The members of line pairs (A, B) and (D, E) differ in contrast polarity. Lines of each contrast polarity appeared with equal frequency throughout the experiment.
Figure 1.
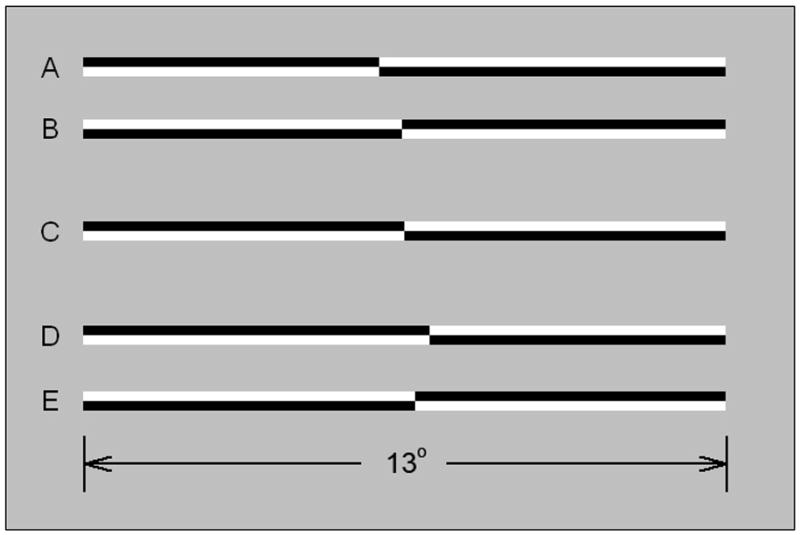
Examples of line stimuli used in the experiments. The members of the upper pair (A, B) are transected to the left of veridical line midpoint (by −0.51° and −0.05°, respectively). The members of the lower pair (D, E) are transected to the right of veridical center (by +0.51° and +0.22°, respectively). Line C is veridically transected. The members of line pairs (A, B) and (D, E) differ in contrast polarity. Lines of opposite polarity appeared with equal frequency and were counterbalanced within and across blocks of trials.
2.4. Procedure
Subjects were seated upright in straight-backed chairs with their midsagittal planes aligned with the display monitor. On each trial subjects made single-interval forced-choice decisions regarding transector location relative to perceived line midpoint by depressing either the left or right mouse button, as appropriate. Button orientation corresponded to the axis of perceptual discrimination, such that the “left” response button was situated to the left of the “right” response button. Subjects responded using both right and left hands in separate blocks of trials. The order of hand use was counterbalanced across subjects and blocks.
Tachistoscopic presentation (McCourt & Olafson, 1997) was used to control for the possible confounding effects of scanning eye movements. Pre-transected lines were presented for 143 ms; inter-trial intervals were variable since subsequent trials began approximately 500 ms following previous responses.
Perceived line midpoint was assessed using an adaptive psychophysical staircase procedure (Cornsweet, 1962). Each block of trials incorporated ten randomly interleaved staircases. Each staircase was initiated with a transector location randomly selected to lie in the range of ±0.15° from veridical line midpoint. A one-down one-up rule was employed such that each staircase converged on the transector location producing a 50:50% left-right response rate; the transector location corresponding to a 50:50% left-right response rate is defined as the point of subjective equality (p.s.e.). From trial to trial, based on subject responses, transector location was adjusted in step sizes of 0.034° (i.e., a single pixel). Each of the ten interleaved staircases ran until six reversals were obtained. For purposes of analysis the first two reversal values in each staircase were discarded (as practice trials), and p.s.e. was calculated as the mean of the four remaining reversal values. Subsequent inferential statistical tests were conducted on these mean p.s.e.’s. Each block of staircases was completed in approximately 200 trials. Subjects completed either one or two blocks of staircases using each hand (average number of blocks/subject = 1.8).
3. RESULTS
Figure 2 plots mean p.s.e (±2 s.e.m.), for both control and patient groups, as a function of hand used to respond. Averaged across hands, bisection error measured −0.16° for controls and −0.02° for patients (negative values indicate leftward errors). A 2 (hand) × 2 (group) mixed ANOVA revealed no significant main effect of hand used to respond, F(1, 52) = 0.066, p = 0.798, and no significant hand × group interaction, F(1, 52) = 0.154, p = 0.696. There was, however, a significant main effect of group, F(1, 52) = 5.178, p = 0.027, such that control subjects demonstrated a greater mean leftward bisection error than did patients. Single-sample t-tests revealed that mean bisection error in control subjects deviated significantly leftward of veridical line midpoint (zero error): Left hand, t(19) = −3.13, p = 0.005; Right hand, t(19) = −4.57, p < 0.001; Average, t(19) = −4.30, p < 0.001. Bisections made by patients did not differ significantly from veridical line midpoint in any condition: Left hand, t(33) = −0.66, p = 0.948; Right hand, t(33) = −1.19, p = 0.242; Average, t(33) = −0.52, p = 0.606. In addition, there were no significant (p < 0.05) sex-related differences in bisection error for either controls or patients.
Figure 2.
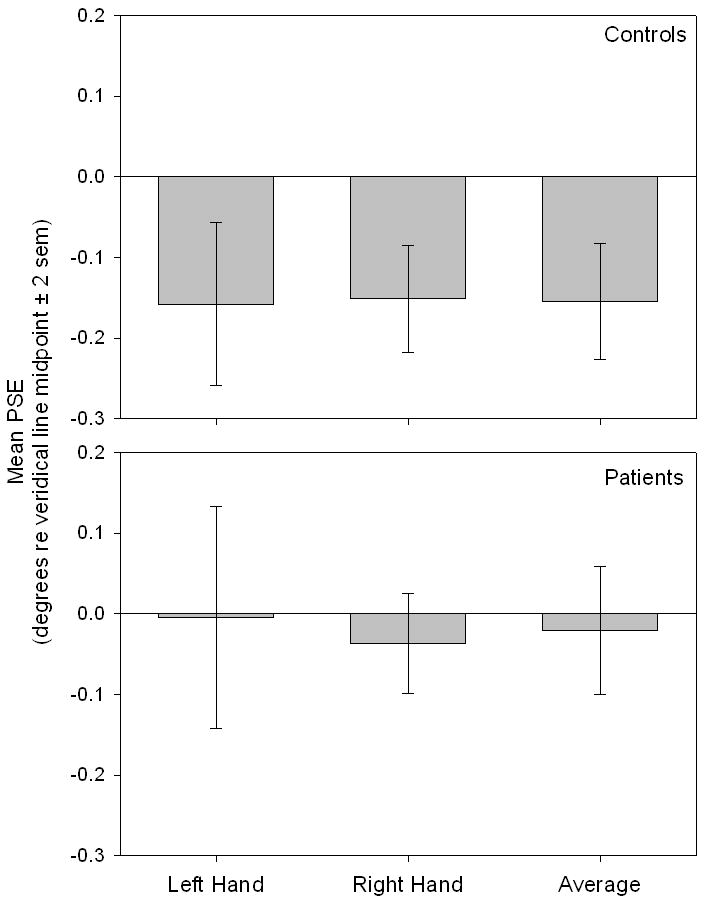
Mean p.s.e (±2 s.e.m.) is plotted for control and patient groups as a function of hand used to respond. Control subjects demonstrated a significantly greater mean leftward bisection error than did patients. Average bisection error was −0.16° for controls, and was significantly leftward of zero. Average error was −0.02° for patients and was not significantly leftward of zero.
Figure 3 shows correlograms which plot right- versus left-hand bisection errors (p.s.e.) for the control group (panel a) and for the patient group (panel b). There is a significant correlation between bisection errors made using the right- and left-hands for controls, r(19) = 0.46, p = 0.041, whereas this correlation is nonsignificant for patients, r(33) = 0.15, p = 0.409. Because the number of control subjects tested in the current study is modest, panel (c) plots right- versus left-hand bisection judgments obtained from a large group (N=184) of control subjects from a previously published study (McCourt et al., 2001a) which utilized a nearly identical methodology. The degree of consistency between bisections executed with the two hands in this larger control group is highly significant, r(183) = 0.76, p < .001, in agreement with results from the current control group, but in stark contrast to the uncorrelated hand-related bisections exhibited by patients. Each control subject executed four separate blocks of bisection trials (two with each hand). The inter-block correlation for mean bisection errors was r(38) = 0.79, p < 0.001. Only twenty-two of the patient group completed all four blocks of bisection trials, but for those who did the inter-block correlation for mean bisection errors was r(42) = 0.48, p = 0.001. Thus, while the magnitude of inter-block correlation for patients is somewhat diminished relative to that seen in control subjects, general inconsistency of behavior in the bisection task itself is unlikely to account for the observed lack of a significant intermanual correlation.
Figure 3.
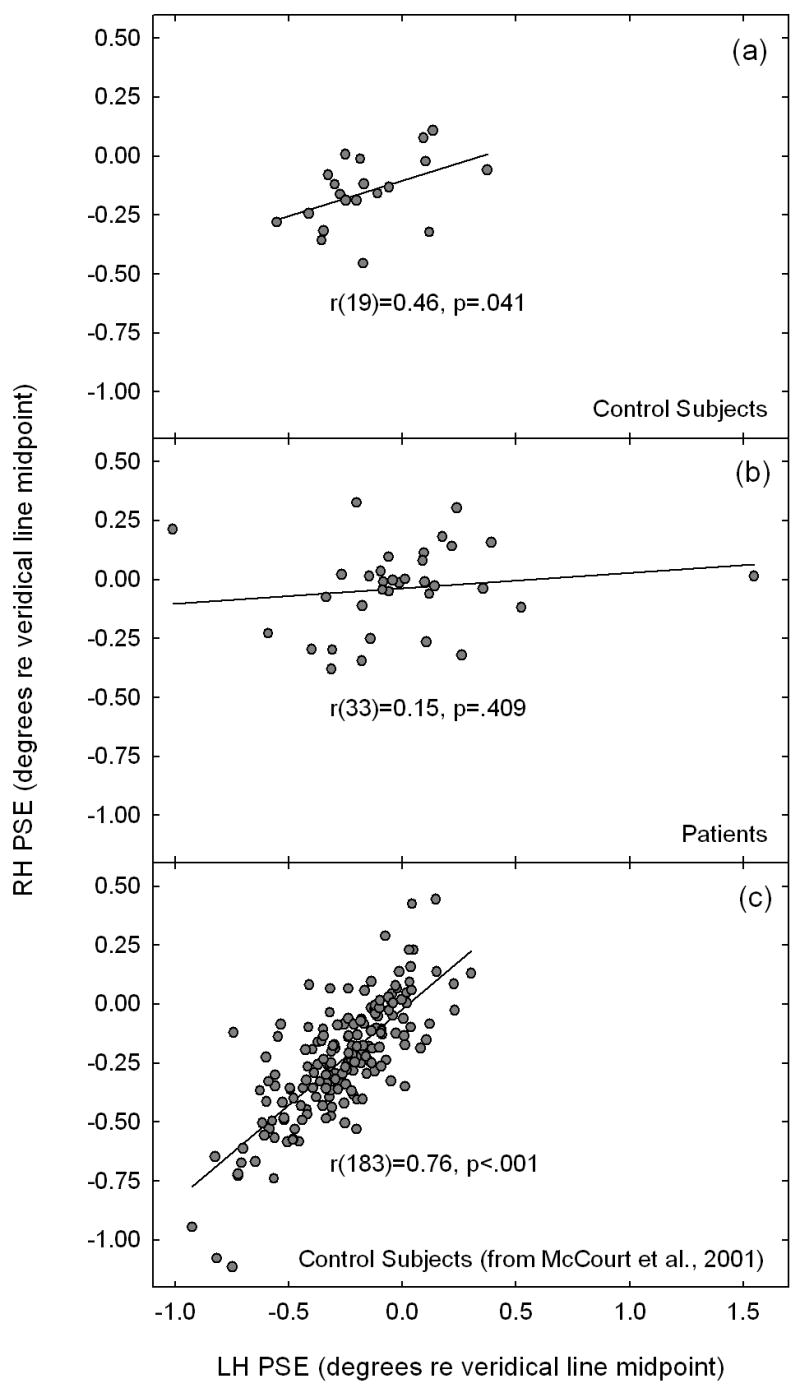
Correlograms plot right- versus left-hand bisection errors (p.s.e.) for the control group (panel a) and for the patient group (panel b). There is a significant correlation between bisection errors made using the right- and left-hands for controls, whereas this correlation is nonsignificant for patients. Panel (c) replots right- versus left-hand bisection judgments obtained from a large group (N=184) of control subjects from a previously published study (McCourt, Freeman, Tahmakera-Stevens & Chaussee, 2001a) which utilized a nearly identical methodology. Intermanual correlations are significant for control subjects, but are uncorrelated for the patient group.
4. DISCUSSION
The mean leftward bisection error of the control group (−0.155°; 1.2% line length) is comparable to that reported in numerous previous studies using the forced-choice tachistoscopic line bisection procedure (Foxe et al., 2003; McCourt & Jewell, 1999; McCourt & Garlinghouse, 2000; 2001; McCourt, 2001; McCourt et al., 2000, 2001a, 2001b, 2005), and replicates the nearly universal finding that neurologically normal observers exhibit a modest, yet significant and systematic, leftward error on line bisection tasks, i.e., pseudoneglect; see Jewell & McCourt (2000), for a meta-analytic treatment and review of this literature. The trend for greater leftward errors of control subjects when making bisections using the left hand, while not achieving statistical significance in the present study, is also congruent with previous literature (McCourt et al., 2001a).
The first noteworthy finding is that patients do not, as a group, exhibit the normal pattern of significant leftward bisection error (pseudoneglect) which characterizes the right-hemisphere dominance for the allocation of visuospatial attention observed in neurologically normal subjects. One interpretation of this finding is that some aspect(s) of right hemisphere structure/function are compromised in schizophrenia. While initial studies of schizophrenia patients identified selective deficits in functions associated with the left hemisphere (Gruzelier & Hammond, 1980; Gur & Chin, 1999; Kugler & Henley, 1979; Magaro & Chamrad, 1983; Posner et al., 1987), evidence is accumulating to suggest right hemispheric deficits as well (Barnett & Kirk, 2005; Barnett et al., 2005; Minor & Park, 1999; Mizuno et al., 1997; Murphy & Cutting, 1990). In addition to identifying global abnormalities of hemispheric function a number of recent studies has probed the integrity of specific functional neuranotomical pathways, disclosing that schizophrenia involves selective deficits within the magnocellular (dorsal) processing stream (Butler et al., 2001; Foxe et al., 2001, 2005; Schechter et al., 2003; 2005). This is potentially significant since parietal cortex is a major recipient of magnocellular input (Merigan & Maunsell, 1993; Ungerleider & Mishkin, 1982), and houses neural networks demonstrated to play a crucial role in the allocation of visuospatial attention (Corbetta et al., 2000; Coull et al., 2001; Fink et al., 2000; Foxe et. al, 1998; 2003; Karnath, 2001; Karnath et al., 2001), including those activated during line bisection tasks. Thus, deficits of magnocellular function are a likely candidate to underlie the abnormalities of spatial attention we describe. In summary, the reduction in right-hemisphere control of visuospatial attention in the patient group might accrue from either a global hemispheric dysfunction, or alternatively, from a specific magnocellular deficit. In either case, additional line bisection studies of patients utilizing the tachistoscopic forced-choice methodology, in conjunction with high-density EEG recording (see, for example, Foxe, et. al, 2003) would be revealing in this regard.
A second noteworthy and novel finding is that, whereas control subjects evince a highly significant correlation between bisection errors committed using the two hands (see also McCourt et al., 2001a), the patient sample exhibits a remarkable lack of intermanual correlation. This finding is intriguing since, in addition to the cited evidence that schizophrenia involves alterations of normal cerebral laterality, there is a growing consensus that patients with schizophrenia manifest structural and/or functional deficits of the corpus callosum (Barnett, 2006; Green, 1978; Mohr et al., 2000; Woodruff et al., 1995). Non-schizophrenic subjects with frank lesions of the corpus callosum exhibit a pattern of intermanual line bisection performance in which the direction of errors differs in sign when bisections are executed using the right versus the left hands (Hausmann et al., 2003; Heilman et al., 1984; Wolk & Coslett, 2004). Further, callosal immaturity in young children has been suggested to explain similar dissociations of bisection error as a function of hand used to respond (Bradshaw et al., 1988; Failla et al., 2003; Hausmann et al., 2003). The absence of a significant correlation between right and left hand bisections in our patient group strongly suggests an impairment of normal interhemispheric information transfer.
A detailed consideration of intermanual bisection performance is informative in this regard. Controls subjects exhibit a highly significant correlation between left-hand and right-hand responses (Figs. 3a, c). This correlation implies that efficient callosal transfer of information pertaining to spatial attention flows from the dominant to the non-dominant hemisphere, effectively yoking the responses of both hands to the output of the dominant hemisphere. Such intermanual coordination is independent of which hemisphere happens to be dominant for spatial attention; however, the data of Fig. 2 (as well as the preponderance of evidence from line bisection research, see: Jewell & McCourt, 2000; Foxe et al., 2003) discloses that for neurologically normal subjects the right hemisphere is overwhelmingly dominant in this regard. Patients with schizophrenia, by contrast, exhibit no significant correlation between line bisection errors committed using the left- and right-hands (Fig. 3b). Instead, when using either hand to perform the line bisection task, patients commit bisection errors which reflect the controlling hemisphere’s degree of attentional control, consistent with the activation-orientation theory of attention (Kinsbourne, 1970; 1993).
Figure 4.
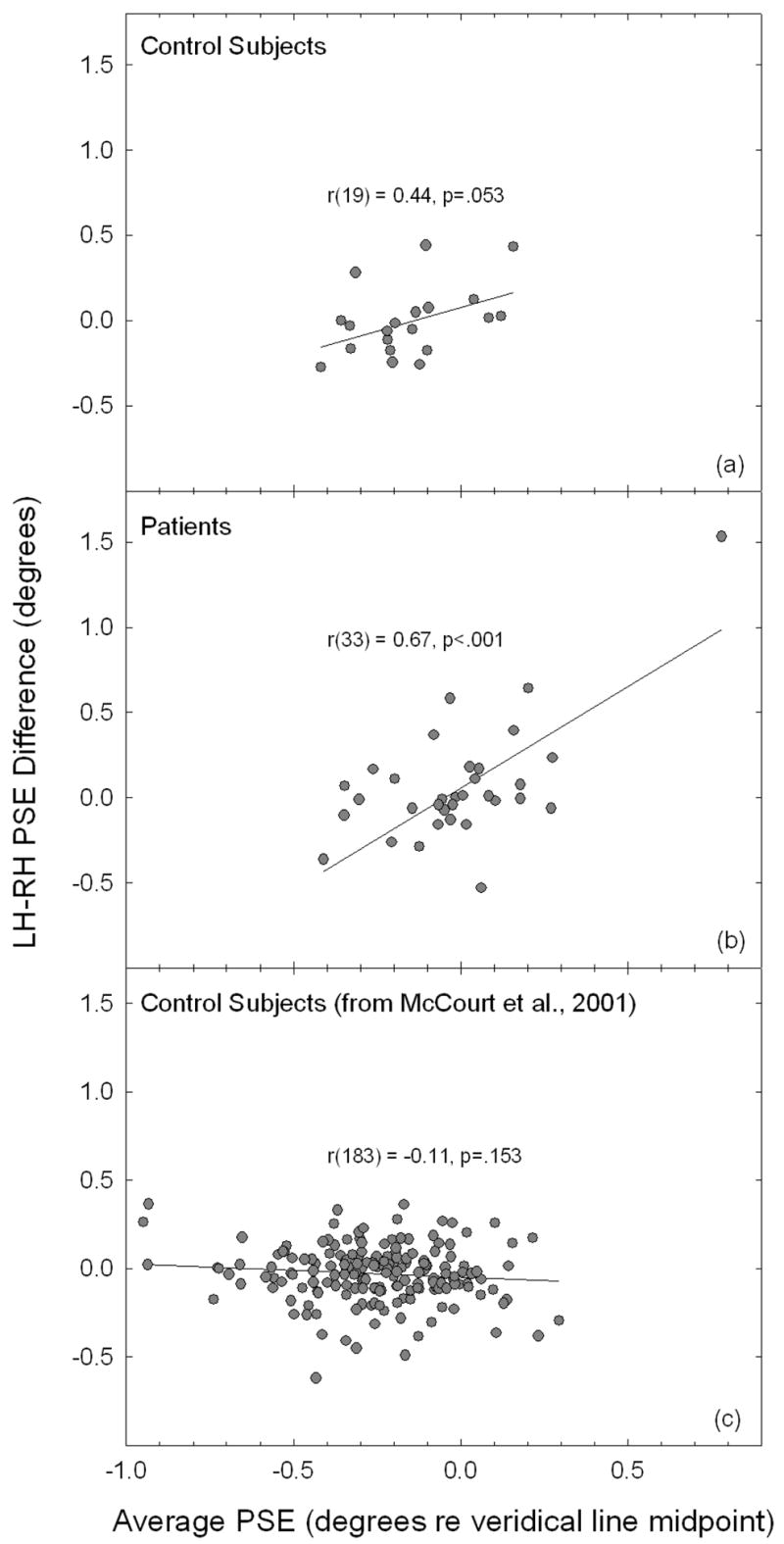
Correlograms plot the difference between right- versus left-hand bisection error (p.s.e.) against the average error for the control (panel a) and patient groups (panel b). The correlation between these measures is not significant for controls, whereas it is highly significant for patients. Panel (c) plots the difference between right- versus left-hand bisection error against the average bisection error obtained from a large group of control subjects from a previously published study (McCourt et al., 2001a), using an essentially identical methodology. The correlation between these measures does not approach significance.
Acknowledgments
The authors thank Gail Silipo for patient recruitment and Roey Pasternak for assistance in running the experiment.
Role of funding source: This publication was made possible by following grants: NIH P20 RR020151 (MEM); NSF EPS-0132289 (MEM); NIH R01 MH65350 (JJF); and NIH R01 MH49334 (DCJ). The National Center for Research Resources (NCRR) and the National Institute for Mental Health (NIMH) are components of the National Institutes of Health (NIH). EPSCoR (EPS) is a division of the National Science Foundation (NSF). The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NSF, NIH, NCRR, or NIMH. These agencies had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest: All authors declare that they have no conflicts of interest. Individual contributions: Authors MEM, JJF and DCJ designed the study. Author MS implemented the study, collected and scored data. Author MEM ran statistical analyses and wrote the first draft of the manuscript. All authors contributed to and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnett KJ, Kirk IJ. Lack of asymmetrical transfer for linguistic stimuli in schizophrenia: An ERP study. Clinical Neurophysiology. 2005;116:1019–1027. doi: 10.1016/j.clinph.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Barnett KJ. Schizophrenia and rightward bias in line bisection. Laterality. 2006;11:36–42. doi: 10.1080/13576500500233628. [DOI] [PubMed] [Google Scholar]
- Barnett KJ, Kirk IJ, Corballis MC. Right hemisphere dysfunction in schizophrenia. Laterality. 2005;10:29–35. doi: 10.1080/13576500442000175. [DOI] [PubMed] [Google Scholar]
- Bowers D, Heilman KM. Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychologia. 1980;18:491–498. doi: 10.1016/0028-3932(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Spataro JA, Harris M, Nettleton NC, Bradshaw J. Crossing the midline by four- to eight-year-old children. Neuropsychologia. 1988;26:221–235. doi: 10.1016/0028-3932(88)90076-0. [DOI] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Guariglia C, Messa C, Pizzamiglio L, Zoccolotti P. Computed tomography correlates of chronic unilateral neglect. Neuropsychology. 1991;5:195–204. [Google Scholar]
- Cavezian C, Danckert J, Lerond J, Dalery J, d’Amato T, Saoud M. Visual-perceptual abilities in healthy controls, depressed patients, and schizophrenic patients. Brain and Cognition. 2007;64:257–264. doi: 10.1016/j.bandc.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Cooper H, Hedges LV. The Handbook of Research Synthesis. Russel Sage Foundation; N.Y: 1994. [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Cornsweet T. The staircase method in psychophysics. American Journal of Psychology. 1962;75:485–491. [PubMed] [Google Scholar]
- Coull JT, Nobre AC, Frith CD. The noradrenergic a2 agonist clonidine modulates behavioural and neuranotomical correlates of human attentional orienting and alerting. Cerebral Cortex. 2001;11:73–84. doi: 10.1093/cercor/11.1.73. [DOI] [PubMed] [Google Scholar]
- Failla CV, Sheppard DM, Bradshaw JL. Age and responding-hand related changes in performance of neurologically normal subjects on the line-bisection and chimeric-faces tasks. Brain and Cognition. 2003;52:353–363. doi: 10.1016/s0278-2626(03)00181-7. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Shah NJ, Weiss PH, Halligan PW, Grosse-Ruyken M, Ziemons K, Zilles K, Freund HJ. Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology. 2000;28:1324–1331. doi: 10.1212/wnl.54.6.1324. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders-patient edition. New York, NY: New York State Psychiatric Institute; 1997. [Google Scholar]
- Foxe JJ, Doniger GM, Javitt DC. Visual processing deficits in schizophrenia: Impaired P1 generation revealed by high-density electrical mapping. NeuroReport. 2001;12:3815–3820. doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, McCourt ME, Javitt DC. Parietal control of visuospatial attention: Line bisection judgments evaluated with high-density electrical mapping and source analysis. NeuroImage. 2003;19:710–726. doi: 10.1016/s1053-8119(03)00057-0. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Murray MM, Javitt DC. Filling-in in schizophrenia: A high-density electrical mapping and source-analysis investigation of illusory contour processing. Cerebral Cortex. 2005;15:1914–1927. doi: 10.1093/cercor/bhi069. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Cued shifts of intermodal attention: Parieto-occipital ~10 Hz activity reflects anticipatory state of visual attention mechanisms. NeuroReport. 1998;9:3929–3933. doi: 10.1097/00001756-199812010-00030. [DOI] [PubMed] [Google Scholar]
- Green P. Defective interhemispheric transfer in schizophrenia. Journal of Abnormal Psychology. 1978;87:472–480. doi: 10.1037//0021-843x.87.5.472. [DOI] [PubMed] [Google Scholar]
- Gruzelier JH, Hammond NV. Lateralized deficits and drug influences on the dichotic listening of schizophrenia patients. Biological Psychiatry. 1980;15:759–779. [PubMed] [Google Scholar]
- Gur RE, Chin S. Laterality in functional brain imaging studies of schizophrenia. Schizophrenia Bulletin. 1999;25:141–156. doi: 10.1093/oxfordjournals.schbul.a033361. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Corballis MC, Fabri M. Line bisection in the split brain. Neuropsychology. 2003;17:602–609. doi: 10.1037/0894-4105.17.4.602. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Waldie KE, Corballis MC. Developmental changes in line bisection: A result of callosal maturation? Neuropsychology. 2003;17:155–160. [PubMed] [Google Scholar]
- Heilman KM, Bowers D, Watson R. Pseudoneglect in a patient with partial callosal disconnection. Brain. 1984;107:519–532. doi: 10.1093/brain/107.2.519. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Van Den Abell T. Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect) Neurology. 1980;30:327–330. doi: 10.1212/wnl.30.3.327. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. 3. Oxford University Press; N.Y: 1993. pp. 279–336. [Google Scholar]
- Jewell G, McCourt ME. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38:93–110. doi: 10.1016/s0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Karnath HO. New insights into the functions of the superior temporal cortex. Nature Review Neuroscience. 2001;2:568–576. doi: 10.1038/35086057. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411:950–953. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- Kerkhoff G. Spatial hemineglect in humans. Prog Neurobiol. 2001;63:1–27. doi: 10.1016/s0301-0082(00)00028-9. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. The cerebral basis of lateral asymmetries in attention. Acta Psychologica. 1970;33:193–201. doi: 10.1016/0001-6918(70)90132-0. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. In: Orientational bias model of unilateral neglect: Evidence from attentional gradients within hemispace, in: Unilateral Neglect: Clinical and Experimental Studies. Robertson IH, Marshall JC, editors. Lawrence Erlbaum Associates; Hove, U.K: 1993. pp. 63–86. [Google Scholar]
- Kugler BT, Henley SH. Laterality effects in the tactile modality in schizophrenia. In: Gruzelier J, Flor-Henry P, editors. Hemisphere Asymmetries of Function in Psychopathology. Elsevier; New York: 1979. [Google Scholar]
- Magaro PA, Chamrad DI. Information processing and lateralization in schizophrenia. Biological Psychiatry. 1983;18:29–44. [PubMed] [Google Scholar]
- Mather JA, Neufeld RWJ, Merskey H, Nicholas C, Russell NC. Schizophrenic performance on line bisection: No simple lateralization defects. Journal of Psychiatric Research. 1990;24:185–190. doi: 10.1016/0022-3956(90)90058-x. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Freeman P, Tahmahkera-Stevens C, Chaussee M. The influence of unimanual response on pseudoneglect magnitude. Brain and Cognition. 2001a;45:52–63. doi: 10.1006/brcg.2000.1255. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M. Stimulus modulation of pseudoneglect: Influence of line geometry. Neuropsychologia. 2000;38:520–524. doi: 10.1016/s0028-3932(99)00085-8. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M. Asymmetries of visuospatial attention are modulated by viewing distance and visual field elevation: Pseudoneglect in peripersonal and extrapersonal space. Cortex. 2001;36:715–732. doi: 10.1016/s0010-9452(08)70548-3. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Jewell G. Visuospatial attention in line bisection: Stimulus modulation of pseudoneglect. Neuropsychologia. 1999;37:843–855. doi: 10.1016/s0028-3932(98)00140-7. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Olafson C. Cognitive and perceptual influences on visual line bisection: Psychometric and chronometric analyses of pseudoneglect. Neuropsychologia. 1997;35:369–380. doi: 10.1016/s0028-3932(96)00143-1. [DOI] [PubMed] [Google Scholar]
- McCourt ME. Performance consistency of normal observers in forced-choice tachistoscopic visual line bisection. Neuropsychologia. 2001;39:1065–1076. doi: 10.1016/s0028-3932(01)00044-6. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Butler J. The Influence of viewing eye on pseudoneglect magnitude. Journal of the International Neuropsychological Society. 2001b;7:391–395. doi: 10.1017/s1355617701003137. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Reuter-Lorenz PA. A common origin for the effects of unilateral cueing and line geometry in the modulation of pseudoneglect. Cortex. 2005;41:499–511. doi: 10.1016/s0010-9452(08)70190-4. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Slater J. Centripetal versus centrifugal bias in visual line bisection: Focusing attention on two hypotheses. Frontiers in Bioscience. 2000;5:d58–71. doi: 10.2741/a496. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JR. How parallel are the primate visual pathways? Annual Review Neuroscience. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1982;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Attentional networks, confusional states, and neglect syndromes. In: Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology. 2. Oxford University Press; Oxford: 2000. pp. 174–256. [Google Scholar]
- Michel C, Cavezian C, d’Amato T, Dalery J, Rode G, Saoud M, Rosetti Y. Pseudoneglect in schizophrenia: A line bisection study with cueing. Cognitive Neuropsychiatry. 2007;12:222–234. doi: 10.1080/13546800601033266. [DOI] [PubMed] [Google Scholar]
- Minor K, Park S. Spatial working memory: Absence of gender differences in schizophrenia patients and healthy controls. Biological Psychiatry. 1999;46:1003–1005. doi: 10.1016/s0006-3223(99)00149-3. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Kato M, Sartori G, Okawara H, Kashima H. Performance characteristics of chronic schizophrenia on attention tests sensitive to unilateral brain damage. Journal of Nervous and Mental Disease. 1997;185:427–433. doi: 10.1097/00005053-199707000-00002. [DOI] [PubMed] [Google Scholar]
- Mohr B, Pulvermuller F, Cohen R, Rochstroh B. Interhemispheric cooperation during word processing: Evidence for callosal transfer dysfunction in schizophrenia patients. Schizophrenia Research. 2000;46:231–239. doi: 10.1016/s0920-9964(00)00020-7. [DOI] [PubMed] [Google Scholar]
- Murphy D, Cutting J. Prosodic comprehension and expression in schizophrenia. Journal of Neurology, Neurosurgery & Psychiatry. 1990;53:727–730. doi: 10.1136/jnnp.53.9.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na DL, Adair JC, Hye Choi S, Won Seo D, Kang Y, Heilman KM. Ipsilesional versus contralesional neglect depends on attentional demands. Cortex. 2000;36:455–467. doi: 10.1016/s0010-9452(08)70532-x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Posner MI, Early TS, Reiman EM, Pardo PJ, Dhawan M. Asymmetries in hemispheric control of attention in schizophrenia. Office of Naval Research Technical Report 87–8. 1987:1–28. doi: 10.1001/archpsyc.1988.01800330038004. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Halligan PW. Spatial Neglect: A Clinical Handbook for Diagnosis and Treatment. Psychology Press; 1999. [Google Scholar]
- Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophrenia Research. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, Jalbrzikowski M, Pasternak R, Silipo G, Javitt DC. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clinical Neurophysiology. 2005;116:2204–2215. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. The Analysis of Visual Behavior. MIT Press; Cambridge, MA: 1982. pp. 549–586. [Google Scholar]
- Vallar G, Perani D. The anatomy of spatial neglect in humans. In: Jeannerod M, editor. Neurophysiological and Neuropsychological Aspects of Spatial Neglect. North Holland; Amsterdam: 1987. pp. 235–258. [Google Scholar]
- Weintraub S, Mesulam MM. Right cerebral dominance in spatial attention. Further evidence based on ipsilateral neglect. Archives of Neurology. 1987;44:621–625. doi: 10.1001/archneur.1987.00520180043014. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Coslett HB. Hemispheric mediation of spatial attention: Pseudoneglect after callosal stroke. Annals of Neurology. 2004;56:434–436. doi: 10.1002/ana.20213. [DOI] [PubMed] [Google Scholar]
- Woodruff PWR, McManus IC, David AS. Meta-analysis of corpus callosum size in schizophrenia. Journal of Neurology, Neurosurgery & Psychiatry. 1995;58:457–461. doi: 10.1136/jnnp.58.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivotofsky AZ, Edelman S, Green T, Fostick L, Strous RD. Hemisphere asymmetry in schizophrenia as revealed through line bisection, line trisection, and letter cancellation. Brain Research. 2007;1142:70–79. doi: 10.1016/j.brainres.2007.01.046. [DOI] [PubMed] [Google Scholar]


