Abstract
The mechanisms underlying responses to drugs of abuse have been widely investigated; however, less is known about pathways normally protective against the development of drug reinforcement. These pathways are also important since they may regulate individual differences in vulnerability to addiction. The neuropeptide galanin and its binding sites are expressed in brain areas important for drug reward. Previous studies have shown that centrally infused galanin attenuates morphine place preference and peripheral injection of galnon, a galanin agonist, decreases opiate withdrawal signs. The current studies in galanin knockout (GKO) mice examined the hypothesis that galanin is an endogenous negative regulator of opiate reward and identified downstream signaling pathways regulated by galanin. We show that GKO mice demonstrate increased locomotor activation following morphine administration, which is inhibited by acute administration of galnon. GKO mice also show enhanced morphine place preference, supporting the idea that galanin normally antagonizes opiate reward. In addition, morphine-induced ERK1/2 phosphorylation was increased in the VTA of both WT and GKO mice, but only the GKO mice showed increases in ERK1/2 and CREB phosphorylation in the amygdala or nucleus accumbens. Furthermore, a single systemic injection of galnon in GKO mice was sufficient to reverse some of the biochemical changes brought about by morphine administration. These data suggest that galanin normally attenuates behavioral and neurochemical effects of opiates; thus, galanin agonists may represent a new class of therapeutic targets for opiate addiction.
Keywords: galanin, addiction, morphine, ERK, place preference, CREB
Introduction
Although many genetic and molecular studies have focused on molecules that predispose individuals to drug abuse, it is equally important to determine which genes and gene products may be protective against the progression from drug use to addiction. The neuropeptide galanin is an excellent candidate for an endogenous protective factor against progression to opiate abuse. Central infusion of galanin attenuates morphine place preference in the mouse (Zachariou et al, 1999). Further, galanin knockout mice demonstrate increased signs of opiate withdrawal, whereas transgenic over expression of galanin or administration of the galanin agonist galnon attenuates opiate withdrawal (Zachariou et al, 2003). These findings suggest that galanin has the potential to decrease abuse liability of opiates. The present studies in galanin knockout (GKO) mice further explore the role of galanin in morphine reward and identify signaling pathways downstream of galanin by which galanin may act as a neuromodulator.
Galanin binds to G protein-coupled receptors (GPCRs) and can modulate intracellular signaling pathways that are involved in addiction (Hawes et al, 2006a, b; Nestler, 2001; Zachariou et al, 2003). All three galanin receptor subtypes, as well as significant galanin binding, are found in the ventral tegmental area (VTA), amygdala, nucleus accumbens (NAc), cingulate cortex, and locus coruleus (LC) (Burgevin et al, 1995; Gustafson et al, 1996; Hawes and Picciotto, 2004; Kolakowski et al, 1998; Waters and Krause, 2000), areas of the brain known to contribute to drug addiction and reward. Galanin also regulates the levels and release of a number of neurotransmitters, including dopamine, acetylcholine and norepinephrine (Pieribone et al, 1995; Tsuda et al, 1998; Wang et al, 1999). Acute morphine administration increases locomotor activity and results in a conditioned place preference (Suzuki et al, 1993; Wise, 1987) and these behaviors can be modulated by dopaminergic agents (Chang et al, 2004; Zarrindast et al, 2006a), noradrenergic depletion, or cholinergic receptor antagonists (Hikida et al, 2003; Olson et al, 2006; Rezayof et al, 2006; Zarrindast et al, 2006b). Thus, galanin may modulate the rewarding and locomotor activating effects through modulation of multiple neurotransmitter systems.
To study whether galanin may be an endogenous protective factor able to attenuate reinforcing properties of opiates, we measured morphine-induced locomotor activity and morphine place preference in wild type mice and GKO mice lacking the neuropeptide galanin. These studies further examined whether second messenger signaling pathways that support these morphine-regulated behaviors are modulated by galanin. At the molecular level, morphine administration leads to enhanced phosphorylation, and thus activation, of extracellular regulated kinase (ERK1/2) in the VTA, hippocampus, NAc, cingulate cortex, and amygdala (Berhow et al, 1996; Valjent et al, 2004). Increased ERK1/2 phosphorylation in the VTA is associated with morphine and psychostimulant reward (Ozaki et al, 2004) and systemic inhibition of ERK1/2 phosphorylation blocks expression of morphine and cocaine conditioned place preference (CPP)(Valjent et al, 2006a). To assess galanin effects on changes in signaling, we measured the effects of morphine administration on levels of total and phosphorylated ERK1/2 in the VTA, NAc, amygdala, cingulate cortex, substantia nigra, locus coruleus, caudate putamen and hippocampus of wild type and GKO mice and assessed whether the galanin receptor agonist galnon could reverse these changes.
NAc CREB has also been implicated in modulating motivational valance associated with drugs of abuse (Carlezon et al, 2005; Chartoff et al, 2003; Pandey et al, 2005). A number of pathways are known to be responsible for CREB activation (Carlezon et al, 2005; Chao and Nestler, 2004; Nestler, 2001). One second messenger pathway that leads to CREB activation is the cAMP pathway. D1 and D2 dopamine receptors respectively couple to stimulatory Gs proteins that, via protein kinase A (PKA) enhance CREB phosphorylation and inhibitory Gi/Go proteins that inhibit cAMP production, and downstream CREB activity (Girault and Greengard, 2004). Previous work in Cath.a cells has shown that galanin administration attenuates naloxone precipitated increases in pCREB following repeated morphine exposure, suggesting that galanin regulates CREB during opiate withdrawal (Hawes et al, 2006). The purpose of the current study was to determine whether galanin might also regulate CREB signaling in vivo, using a rewarding dosing regimen of morphine. Opiate regulation of Go/Gq pathways may also result in CREB activation (Chao et al, 2002; Widnell et al, 1996), potentially through activation of ERK signaling cascades. Therefore, we also investigated whether opiate administration leads to changes in levels of total CREB and CREB phosphorylation following morphine exposure in areas of the brain thought to support opiate reinforcement in wild type and GKO animals.
These studies support the hypothesis that galanin is normally protective against the development of behaviors related to opiate addiction and further identify neuronal signaling pathways by which galanin might regulate behaviors that support addiction.
Materials and Methods
Animals
GKO mice were generated on the 129OlaHsd background (Wynick et al, 1998). As described previously, exons one to five of the galanin gene were replaced with a PGK-Neocassette in reverse orientation removing the signal peptide, coding region and the majority of the galanin associated peptide. Galanin expression was undetectable in all tissues examined in homozygous GKO mice (Wynick et al, 1998). Once generated, the chimeras were bred to co-isogenic 129OlaHsd wild type mice. Mice used in the current study were generated by crossing GKO mice to co-isogenic wild type mice of the 129OlaHsd background to generate mice heterozygous for the galanin knockout allele. These mice became the founders of the new colony. In order to generate large numbers of animals for behavioral studies, KO × KO and WT × WT offspring of HET × HET mating pairs were bred together. GKO or wild type mice were then generated from several GKO × GKO or WT × WT mating pairs. Experimental testing occurred when mice were between the ages of 4-6 months. Mice were matched for age and sex for all experiments. All animal studies were conducted in accordance with guidelines from the National Institutes of Health and approved by the Yale Animal Care and Use Committee.
Morphine-induced locomotor activation
Male mice (5-8 months of age) were habituated to injection and the locomotor boxes for three consecutive days. Each mouse was given two injections of 0.9% saline 15 min apart. Following the second injection, each mouse was immediately placed into a locomotor box for 40 min. Locomotor activity was measured using a video tracking system and converted to distance traveled (cm) using Optimax software. GKO and wild type mice received a saline vehicle injection followed 15 minutes later by either 0, 5, 10 or 20 mg/kg morphine in a randomized latin square design on days 4, 6, 8 and 10. All animals received two saline injections on days 5, 7, 9 and 11. On day 12, each mouse received an i.p. injection of 2 mg/kg galnon (synthesized as described in Saar, et al., 2002) followed by an injection of 5 mg/kg morphine 15 min later. On day 13, injection of 2 mg/kg galnon was followed by an injection of saline 15 min later. Significance was determined using repeated-measures ANOVA followed by Tukey's HSD post hoc test (p<0.05).
Morphine conditioned place preference
For place preference experiments, male GKO or wild type mice were used and were generated as described above. Animals were transported to the facility where behavioral training would take place at least one week prior to behavioral testing and were habituated to experimenter handling for a minimum of three days. Med Associates place conditioning boxes were modified for a non-biased CPP paradigm. Two conditioning chambers with retractable doors were separated by a grey and white neutral chamber with a white Plexiglas floor. One conditioning chamber had a wire mesh floor and walls with black and white vertical stripes. The second conditioning chamber had a grid floor and brown marbled walls with black diagonal stripes. The location of each animal was recorded by photocell beam breaks and time spent in each chamber was calculated using Med-PC IV software.
During all phases of the experiment, mice were carried in their homecage into the testing room, subjects were weighed and injected i.p. with 3.33 ml/kg of 0.9% saline vehicle or morphine sulfate (0, 0.25, 3.0, or 5.0 mg/kg) just prior to placement into the CPP apparatus. Doses of morphine were selected to avoid possible ceiling effects that might preclude observation of differences between genotypes. Baseline preferences were determined prior to training. Following vehicle injection, animals were placed inside the neutral chamber and allowed to explore both conditioning chambers for 15 min. Fourteen subjects were excluded from further testing based on a bias greater than 70% for one chamber. During the training phase of the experiment, mice received 2 training sessions per day over 3 days of training. During the AM session (beginning at approximately 1000 h), mice were isolated in one conditioning chamber for 30 min following vehicle injection (Saline-Paired). During the PM session (beginning at approximately 1400 h) animals were isolated in the opposite conditioning chamber following an injection of morphine (Drug-Paired). Control subjects of each genotype received saline injections (0 mg/kg morphine) prior to placement in either chamber. One of the chambers was randomly designated as the Drug-Paired chamber for the saline-treated animals for statistical purposes. Animals were counterbalanced for drug-paired chamber according to treatment dose, genotype, and baseline preference. On the day after completion of training, animals were again injected with vehicle, placed in the neutral, center, compartment of the CPP apparatus, and allowed access to both conditioning chambers for a period of 15 min. Baseline and CPP testing sessions took place at an intermediate time between the AM and PM training sessions (approximately 1200 h). Total time spent in each chamber during CPP testing was observed and change from baseline preference for the drug-paired chamber was used as a measure of morphine CPP.
A 2 × 2 × 4 (chamber × genotype × treatment) ANOVA, with conditioning chamber as a within-subject measure and genotype and treatment as between-subjects measures, was used to determine effects of galanin genotype and drug dosage on morphine conditioned place preference. Given an a priori hypothesis derived from locomotor data, post-hoc one-tailed t-tests were performed.
A second set of locomotor experiments was performed using the 0.25 mg/kg threshold dose of morphine shown to result in morphine CPP in GKO but not wild type mice. Male mice (6-8 months of age) received 3 daily 40 min sessions of locomotor testing. Mice received either 0.25 mg/kg morphine or saline immediately prior to placement in a clear plastic locomotor box. Locomotor activity was recorded by photocells that measured the number of beam breaks and recorded as beam breaks per minute by a Dell computer using Med-PC IV software (Med Associates, St. Albans, VT). On the third day, the brains were collected by rapid decapitation, and punches were taken from brain regions for biochemical analysis. Significance was determined using repeated-measures ANOVA followed by Tukey's HSD post hoc test (p<0.05).
Western Blot Analysis
GKO and wild type mice were administered saline or 2 mg/kg galnon 15 min before injection of saline or 10 mg/kg morphine. Male and female mice exactly balanced for genotype were used for western blotting studies and analyzed separately. Since no sex differences were observed, data were combined for the analyses presented here. Five min after injection, brains were harvested by rapid decapitation and tissue punches of VTA, substantia nigra (SN), NAc, amygdala, hippocampus, LC, cingulate cortex and caudate putamen were isolated and immediately frozen on dry ice within 10 min of decapitation. Cell lysis buffer (20 mM Hepes, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 5% Glycerol, 10mM pyrophosphate, 1 mM NaVO3, 1 mM PMSF, 5 μg/ml aprotinin, and 5 μg/ml leupeptin, pH 7.5, for CREB and pCREB immunoblots; 50 mM Tris pH7.4, 1 mM EDTA, 1 mM EGTA 1% SDS, 1 mM PMSF for ERK and pERK immunoblots) was added to each frozen tissue punch and immediately pulse sonicated for 5 seconds, then cleared by centrifugation for 15 min at 4° C. Lowry reagents (Biorad, Hercules, CA) were used to determine protein concentrations according to manufacturer's instructions. Five - 10 μg of protein for each sample was separated on 8.5 % or 10 % polyacrylamide gels by SDS-PAGE and transferred to nitrocellulose membranes. Blots were blocked in 5% BSA/TBS or 5% Milk/TBS-T for 1 hr, then incubated overnight at 4° C in primary antibody. Polyclonal antisera specific for ERK1/2 and polyclonal or monoclonal antisera specific for the phosphorylated form of ERK (Cell Signaling, Beverley, MA) were used at a dilution of 1:1000 and 1:2000, respectively. Polyclonal antisera specific for CREB or phosphorylated serine 133 on CREB (Cell Signaling, Beverley, MA) were used at dilutions of 1:1000. TH immunoreactivity was used to verify the accuracy and consistency of VTA tissue punches. Anti-TH polyclonal antibody (Chemicon, Temecula, CA) was used at a dilution of 1:2000. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) immunoreactivity was used as an internal standard, by incubating the blots either alone for 30 min at room temperature in anti-GAPDH monoclonal primary antibody (Advanced ImmunoChemical Inc., Long Beach, CA) diluted 1:2000 in 5% BSA/TBS following the overnight incubation with CREB, or TH primary antibodies, or overnight in a cocktail with the ERK or pERK antibodies. Blots were washed 3 times for 5 min with TBS (pH 7.4)/0.05% Tween (TBST), incubated with peroxidase-labeled anti-rabbit IgG 1:2000 (Vector Laboratories, Inc., Burlingame, CA), or IR Dye 800 conjugated anti-rabbit IgG (Rockland Inc., Gilbertsville, PA) and Alexa fluor 680 conjugated anti-mouse IgG (Molecular Probes, Eugene, OR) secondary antibodies for 1 hr at room temp and washed 5 times with TBS-T. Bands were visualized by enhanced chemiluminescence and exposure to Kodak Biomax MR or AR film. Immunoblots incubated with fluorescent-conjugated secondary antibodies were scanned using the LI-COR Odyssey imager (LI-COR Biosciences, Lincoln, NE). Protein loading was verified using Ponceau staining and GAPDH immunoblotting. Bands were quantified using NIH Image software (URL:http//rsb.info.nig.gov/nih-image), or the odyssey imaging software. Levels of protein phosphorylation were determined by calculating the ratio of phosphorylated band intensity to total protein band intensity. Data were normalized to the WT saline group to allow comparison across multiple blots by dividing each value by the average of the WT saline group and multiplying by 100. Significance was determined using ANOVA followed by the least significant difference post hoc test (p<0.05).
Results
Morphine-induced locomotor activity and conditioned place preference
Most drugs of abuse, including morphine, can increase locomotion when injected acutely (Wise, 1987). We therefore used locomotor activation as a behavioral measure of sensitivity to opiates in mice lacking the neuropeptide galanin and their wild type controls (F(7, 97)=9.082, p < 0.05; Fig. 1A). Wild type mice of the 129Ola/Hsd strain did not show a significant increase in locomotor activity following morphine injection at the doses we used (Fig 1A). Morphine can both increase and depress locomotor activity in different strains of mice (Eidelberg and Erspamer, 1975; Oliverio et al, 1975), and the lack of locomotor activation in wild type mice in the current study may represent a strain difference between 129Ola/Hsd mice and other strains, such as C57BL/6 mice, that are more susceptible to the locomotor activating effects of morphine. In contrast, GKO mice on the 129Ola/Hsd background showed a significant increase in locomotor activity following morphine injection as compared to saline injected controls (p < 0.05, Tukey HSD post hoc test; Fig. 1A).
Figure 1. Locomotor activity following acute morphine administration.
A) Galanin wild type (WT; n=10) and knockout (GKO; n=11) mice were administered an acute i.p. injection of saline followed by 0, 5, 10, or 20 mg/kg morphine 15 min later. Locomotor activity was significantly increased in GKO animals as compared to WT following acute morphine administration. Significance was determined using repeated-measures ANOVA followed by Tukey's HSD post hoc test (F(7, 97)=9.082, p<0.05). B) i.p. injection of 2 mg/kg galnon 15 min prior to morphine administration (5 mg/kg) significantly inhibited morphine-induced increases in locomotor activity of GKO animals. Knockout animals receiving 5 mg/kg morphine are the only group significantly different from baseline (*). Significance was determined using repeated-measures ANOVA followed by Tukey's HSD post hoc test (F(7,99)=6.153, p<0.05).
To further determine whether galanin signaling inhibits morphine-induced locomotor activity directly, galanin wild type and knockout mice were treated with galnon, a galanin receptor agonist. Galnon, 7-((9-fluorenylmethoxycarbonyl)cyclohexylalanyllysyl)amino-4-methylcoumarin, is a low molecular weight, non-peptide galanin receptor agonist that displaces [125I]-glanin from its binding sites and inhibits adenylyl cyclase activity through activation of galanin receptors (Saar et al, 2002; Wu et al, 2003). Galnon administration reversed the morphine-induced increase in locomotor activation seen in GKO mice (F(7, 99)=6.513, p < 0.05; Fig. 1B); however, galnon did not further reduce locomotor activity in wild type mice and had no effect on its own (p > 0.05, Tukey HSD post hoc test).
Conditioned place preference for morphine was evaluated in GKO and wild type mice based on an earlier finding that infusion of galanin into the brain attenuates morphine place preference in wild type C57BL/6mice (Zachariou et al, 1999). Consistent with the idea that galanin normally antagonizes opiate reward, GKO mice show increased sensitivity to morphine place preference compared to wild type mice. There was a significant interaction of genotype and drug treatment F(3, 150) = 3.378, p < 0.05). Post-hoc t tests showed that at the 0.25 mg/kg dose of morphine, GKO subjects showed a greater increase in preference for the drug-paired chamber than wild type animals as measured by a significantly larger change from baseline (p<0.05; Fig. 2A). There was no significant difference in change of preference for the drug-paired chamber between WT and GKO subjects. There was also no effect of treatment or genotype on baseline preference for the conditioning chambers (Fig. 2B). At the threshold dose for morphine place preference (0.25 mg/kg), neither wild type nor GKO mice showed locomotor activation (Fig 2D)
Figure 2. Morphine place preference.
Morphine conditioned place preference in wild type (WT) and galanin knockout mice (GKO). A) Change from baseline preference for the drug-paired chamber is shown for GKO and WT mice. GKO mice showed significantly greater morphine preference than WT mice at the 0.25 mg/kg dose of morphine (*p < 0.05). B) Baseline levels of total time spent in the drug-paired and saline-paired chambers. C) Total time spent in the drug-paired and saline-paired chambers is shown for mice after conditioned place preference training. Control animals of each genotype (0 mg/kg) received saline vehicle prior to placement in either chamber, with one chamber randomly designated the “Drug-Paired” chamber for statistical analysis. D) Galanin wild type (WT; n=17) and knockout (GKO; n=15) mice were administered an acute i.p. injection of saline or 0.25 mg/kg morphine. The total locomotor activity measured as beam breaks were averaged over three days. There were no significant differences in locomotor activity between genotypes that received either treatment. Error bars represent standard error about the mean.
Neurochemical changes downstream of morphine signaling
Galanin modulates the release of a number of neurotransmitters in brain areas involved in behavioral responses to drugs of abuse (Pieribone et al, 1995; Tsuda et al, 1998; Wang et al, 1999). We therefore evaluated the levels and activity of signaling molecules known to be activated by drugs of abuse in tissue punches from regions involved in modulation of drug reward, including the VTA and catecholaminergic projection areas. We measured changes in total levels or phosphorylation state of CREB and ERK1/2 in GKO mice to identify galanin-associated neurochemical changes resulting from acute morphine treatment.
At a dose of 10 mg/kg, morphine induced a significant increase in the phosphorylation state of ERK1/2 (WT-Sal vs. WT-Mor: F(3, 19) = 6.09; p < 0.01; WT-Sal vs. KO-Mor: F(3, 20) = 22.8; p < 0.01; Tukey HSD post hoc test; Fig. 3A), but not CREB (WT-Sal vs.WT-Mor: F(3, 19) = 2.96; p > 0.05; WT-Sal va. KO-Mor: F(3, 21) = 2.9; p > 0.5; Tukey HSD post hoc test; Fig. 3B), in the VTA of both galanin wild type and knockout animals. No changes in total ERK and CREB were observed at any dose and no changes in P-ERK or P-CREB were observed in animals of either genotype treated with 0.25 mg/kg morphine (not shown). Since inhibition of ERK1/2 phosphorylation in the VTA blocks the rewarding effects of morphine (Berhow et al, 1996; Ozaki et al, 2004), the observed increases in ERK1/2 activation may regulate morphine reward. A trend for slightly higher levels of ERK1/2 phosphorylation in GKO mice (WT-Mor vs. KO-Mor: F(3,19) = 2.4; p = 0.08) correlates with the tendency for these animals to show increased behavioral sensitivity to morphine. Together with studies showing that ERK activity is necessary for place preference (Girault et al, 2007; Salzmann et al, 2003; Valjent et al, 2001; Valjent et al, 2006a; Valjent et al, 2006b; Valjent et al, 2004) and that galanin reduces morphine CPP (Zachariou et al, 1999), these data suggest that galanin might regulate morphine reward via regulation of ERK signaling in the VTA. It is also possible that ERK activity in the VTA regulates the ability of galanin to modulate morphine-induced locomotor activation.
Figure 3. Changes in levels and phosphorylation state of CREB and ERK in the VTA following administration of acute morphine alone or Galnon prior to morphine.
Galanin wild type and knockout mice were administered an acute i.p. injection of 10 mg/kg morphine alone or were administered morphine 15 min after an i.p. injection of Galnon at a dose of 2 mg/kg and sacrificed 5 min after the morphine injection. A, B) Tissue punches from the ventral tegmental area (VTA) were immunoblotted for ERK and phospho-ERK and CREB and phospho-CREB. * p value < 0.05 with respect to saline treated WT controls. WT-Saline, n=4; GKO-Saline, n=4; WT-Morphine, n=8; GKO-Morphine, n=8, WT-Galnon+Morphine, n=8, GKO-Galnon+Morphine, n=9.
To identify changes in intracellular signaling that regulate locomotor behavior, we evaluated the ability of galnon to reverse neurochemical changes resulting from morphine treatment in GKO mice. ERK activation in the VTA of galnon-injected animals was significantly lower than animals that received morphine alone (KO-Mor vs. KO-Gal+Mor: F(3, 21) = 5.8; p < 0.01; Tukey HSD post hoc test; Fig 3A); therefore, galnon attenuated the morphine-induced ERK activation in the VTA of both wild type and GKO animals.
ERK phosphorylation in the NAc in response to treatment with drugs of abuse is thought to be important for locomotor activation and reward (Valjent et al, 2004). Consistent with the propensity for GKO mice to show morphine-induced locomotor activation and an augmentation of morphine CPP, levels of phosphorylated ERK1/2 were significantly increased in the NAc of GKO mice (WT-Sal vs. KO-Mor: p < 0.01; F(3,19) = 9.56; Tukey HSD post hoc test) but not in wild type mice following morphine exposure (WT-Sal vs. WT-Mor: p > 0.05; F(3, 19) = 1.88; Tukey HSD post hoc test; Fig. 4A). The lack of morphine-induced ERK1/2 phosphorylation in the NAc of wild type mice might explain the absence of morphine-induced locomotor activation in mice of the 129Ola/Hsd strain. Total ERK, total CREB and CREB phosphorylation levels were all unchanged in the NAc of wild type and GKO mice following morphine administration (Fig. 4A, B).
Figure 4. Changes in levels and phosphorylation state of CREB and ERK in the NAc following administration of acute morphine alone or Galnon prior to morphine administration.
Galanin wild type and knockout mice were administered an acute i.p. injection of 10 mg/kg morphine alone or were administered morphine 15 min after an i.p. injection of Galnon at a dose of 2 mg/kg and decapitated 5 min after the morphine injection. A, B) Tissue punches from the nucleus accumbens (NAc) were immunoblotted for ERK and phospho-ERK and CREB and phospho-CREB. * p value < 0.05 with respect to saline treated WT controls. WT-Saline, n=4; GKO-Saline, n=4; WT-Morphine, n=8; GKO-Morphine, n=8, WT-Galnon+Morphine, n=8, GKO-Galnon+Morphine-n=9.
In the amygdala, both wild type and GKO mice treated with 10 mg/kg morphine exhibited a significant increase in ERK1/2 phosphorylation (WT-Sal vs. WT-Mor: F(3; F =, 21) = 6.56; p<0.01; WT-Sal vs. KO-Mor: F(3, 20) = 9.6; p < 0.01; Tukey HSD post hoc test; Fig. 5A) that was reversed by systemic galnon administration prior to morphine injection. In contrast, CREB phosphorylation was significantly increased by 150% in the amygdala of GKO mice (WT-Sal vs. KO-Mor: F(3; F, 20) = 19.67; p < 0.01; Tukey HSD post hoc test), but not regulated in wild type, mice following acute morphine injection (WT-Sal vs. WT-Mor: F(3, 20) = 1.9; p > 0.05; Tukey HSD post hoc test; Fig. 5B). Furthermore, a single injection of galnon did not attenuate CREB phosphorylation in the amygdala of GKO mice. At a threshold dose of 0.25 mg/kg morphine, there was no CREB activation in the amygdala of wild type or GKO mice (Fig. 5C).
Figure 5. Changes in levels and phosphorylation state of CREB and ERK in the amygdala following administration of acute morphine alone or Galnon prior to morphine administration.

A, B) Tissue punches from the amygdala were immunoblotted for CREB and phospho-CREB and ERK and phospho-ERK in galanin wild type and knockout mice administered an acute i.p. injection of 10 mg/kg morphine alone or administered morphine 15 min after an i.p. injection of 2 mg/kg Galnon and sacrificed 5 min after the morphine injection. C) CREB and phospho-CREB signaling in mice injected with 0.25 mg/kg morphine, assessed for locomotor activation on three successive days, and killed on the third day 5 min after morphine injection, immediately after the last locomotor test; * p value < 0.05 with respect to saline treated WT controls. WT-Saline, n=4; GKO-Saline, n=3; WT-Morphine, n=8; GKO-Morphine, n=8, WT-Galnon+Morphine, n=8, GKO-Galnon+Morphine, n=9.
There were no significant changes in levels of total or phosphorylated ERK or CREB in the SN, hippocampus, LC, caudate putamen, or cingulate cortex after acute morphine treatment in either genotype (F's < 1.0).
Discussion
Galanin null mutant mice were more sensitive to morphine than their wild-type counterparts in tests of locomotor activation and morphine CPP. GKO mice, but not WT animals exhibited increased locomotor activation following acute morphine treatment. Galnon co-administration reversed the morphine-induced increase in locomotor activity, suggesting that morphine locomotor activation in GKO mice is not due to a developmental difference in these animals, but that galanin acutely inhibits the psychomotor stimulant properties of morphine in adulthood. Morphine place preference was also enhanced in GKO mice compared to wild type controls, supporting neurochemical data suggesting that galanin counters drug reward (Zachariou et al, 1999). GKO mice also showed morphine-dependent increases in pERK in the NAc and pCREB in the amygdala, areas of the brain that are critical for behavioral effects of morphine. In the VTA and amygdala both wildtype and GKO mice showed elevated levels of pERK that were reversible by galnon, but this effect was exaggerated in the VTA of GKO mice. Morphine-dependent increases in levels of pERK but not pCREB were reversible by systemic administration of galnon, suggesting that galanin acutely regulates changes in ERK signaling in the VTA, NAc, and amygdala. There was no effect of morphine on ERK or CREB signaling in the substantia nigra (SN), hippocampus, LC, cingulate cortex or caudate putamen, demonstrating that morphine-associated effects on ERK and CREB signaling were specific to the VTA, NAc and amygdala in these studies. These data support the hypothesis that galanin antagonizes opiate reinforcement and reward and identify ERK signaling in the VTA, NAc, and amygdala as a potential downstream target for galanin effects on opiate-dependent behavior.
Galanin, opioid and DA receptors are G-protein-coupled receptors (GPCRs) that modulate many signaling pathways including those involving ERK 1/2 (Belcheva et al, 2005; Belcheva et al, 2001; Hawes et al, 2006a; Loh and Smith, 1990; Williams et al, 2001). Chronic morphine exposure (Berhow et al., 1996) and stimulation of several GPCRs, including opioid receptors, leads to ERK activation in the VTA (Eitan et al, 2003) and inhibition of ERK activity in the VTA suppresses the rewarding effects of morphine (Ozaki et al, 2004). The current data supports these findings, showing significant morphine conditioned place preference and increases in phosphorylation of ERK1/2 in the VTA of both galanin wild type and knockout mice following acute administration of a rewarding dose of morphine. These data collected 5 min following 10 mg/kg morphine injection differ somewhat from earlier findings (Berhow et al, 1996) that reported no changes in ERK activation in the VTA 2 hours following a single injection of a higher dose of morphine, however they are consistent with data showing that ERK phosphorylation peaks approximately 5 min after drug administration (Valjent et al, 2004). Similarly, we observed no change in ERK signaling in the hippocampus or cingulate cortex at a 5 min time-point, likely due to the fact that activation of ERK signaling by morphine in these areas peaks at 20 min post injection (Valjent et al, 2004). Other studies show that inhibition of ERK during place conditioning blocks cocaine CPP (Valjent et al, 2006a). ERK induction at the 5 min time-point indicates that stimulation of ERK would occur during exposure to the training chamber during place conditioning. In addition to the finding that GKO mice showed enhanced morphine CPP at low doses of morphine, there was a tendency for GKO mice to show elevated levels of phosphorylated ERK in the VTA in comparison to their wild type counterparts, suggesting that galanin-associated regulation of ERK may modulate behavioral sensitivity to morphine.
Effects of morphine on ERK signaling and locomotor activation were reversed by the galanin agonist, galnon. Galnon only partially reversed morphine-induced elevations of phosphorylated ERK in the VTA of GKO and WT mice, suggesting that galanin exerts limited control over ERK signaling in the VTA. By contrast, in the NAc, a single galnon injection completely abolished morphine-induced ERK activation. Galnon is a galanin agonist that is thought to have some selectivity for GalR1 over GalR2 and GalR3 (Saar et al, 2002; Wu et al, 2003). Galnon can act as a galanin receptor agonist in vivo in behavioral paradigms modulated by the endogenous peptide galanin, including opiate withdrawal (Zachariou et al, 2003), feeding behavior (Abramov et al. 2004), seizures (Saar et al, 2002), and anxiety (Rajarao et al. 2007). However, it should be noted that galnon can also interact directly with some G-proteins (Flören et al, 2005), thus the action of galnon may not be limited to activation of GalR1. GalR1 is negatively coupled to adenylyl cyclase and activation of this receptor subtype can therefore decrease PKA-mediated signaling as well as neuronal excitability (Berthold et al, 1997). Thus, galnon-mediated activation of GalR1 could reverse the effects of morphine on ERK signaling either by decreasing the excitability of neurons in which these signaling pathways were activated, or by decreasing the contribution of cyclic-AMP mediated pathways in those neurons through activation of Gi. Interestingly, morphine-associated NAc activation of ERK and locomotor activation were specific to GKO mice. Unlike other strains that show robust morphine-dependent locomotor activation, the 129Ola/Hsd mice in these studies did not display locomotor hyperactivity in response to morphine administration. GKO animals on the 129Ola/Hsd background, however, showed robust locomotor activation in response to morphine. Together, these data suggest that galanin is a negative modulator of the behavioral and neurochemical signs of acute opiate administration in vivo.
Galanin may modulate responses to drugs of abuse through multiple transmitter systems, either independently or in combination, in the brain regions involved in these behaviors. DA release in the NAc is thought to be critical for locomotor activation and opiate reward (Di Chiara et al, 1988). Galanin decreases presynaptic DA release in striatal slices (Tsuda et al, 1998) and one possible explanation for the heightened sensitivity of GKO mice to morphine-induced locomotion and CPP is that knockout of the peptide may result in hyperactivation of DA signaling following opiate administration. Whereas mRNA levels for galanin receptors are low in the NAc, galanin receptor binding is quite prominent (Burgevin et al, 1995; Gustafson et al, 1996; Hawes et al, 2004; Kolakowski et al, 1998; Waters et al, 2000) indicating that localization of galanin receptors on DA terminals might regulate DA release. Galanin is also known to be a potent inhibitory modulator of basal acetylcholine (ACh) release in the striatum (Antoniou et al, 1997) and it has been demonstrated that M1-muscarinic receptors and high affinity nicotinic receptors regulate the secondary rewarding effects of cues paired with a primary reinforcer such as morphine (Brunzell et al, 2006; Carrigan and Dykstra, 2007). Lack of galanin may therefore increase availability of ACh, thus increasing the sensitivity of GKO mice to opiates. Finally, galanin has also been shown to decrease GABA release in the cortex and striatum (Antoniou et al, 1997; Ellis and Davies, 1994), potentially increasing excitability in these brain areas and contributing to morphine's behavioral effects. The effects of galanin on morphine-related behaviors may therefore modulate reward pathways through multiple neurotransmitter systems.
A dramatic increase in CREB phosphorylation was detected in the amygdala of GKO mice treated with 10 mg/kg morphine, but not wild type mice. CREB is phosphorylated and activated in the amygdala by several drugs of abuse including opiates (Konradi et al, 1994; Olson et al, 2006; Shaw-Lutchman et al, 2002). In addition, opiate withdrawal induced by naloxone can also increase CREB phosphorylation in cultured neurons and in vivo (Chartoff et al, 2003), an effect that is abolished by co-administration of galanin (Hawes et al, 2006b). The behavioral implications of the increased CREB phosphorylation in the amygdala in GKO mice are unclear since, unlike morphine-induced locomotor activation and ERK phosphorylation, which were reversed by galnon administration in GKO mice, CREB activation in the amygdala was not reversed following a single injection of galnon. It is possible that lack of galanin during development may result in changes in the amygdala that make CREB activation in this brain area more sensitive to opiate administration. Alternatively, it is possible that galanin is important for drug-associated learning that occurs during early exposure to morphine. Galanin can block memory consolidation processes (Kinney et al, 2003) and the amygdala is critical for cue-associated learning (Robbins and Everitt, 1996). A low dose of morphine that resulted in morphine CPP did not affect phosphorylation of CREB in GKO mice, however. Thus, although activation of CREB in the amygdala may contribute to drug-associated learning, it is not essential for associating contextual cues with reward.
The data presented here provide strong evidence demonstrating that galanin normally acts as a negative regulator of the acute behavioral response to morphine. In addition, together with evidence indicating that phosphorylation of ERK is necessary for drug reinforcement (Ozaki et al, 2004; Valjent et al, 2001; Valjent et al, 2006a; Valjent et al, 2006b; Valjent et al, 2004; Valjent et al, 2005), these data suggest that galanin may modulate drug-dependent behaviors via activation of ERK in brain areas such as the VTA, NAc and amygdala. Thus, galanin agonists, may prove useful in attenuating the rewarding properties of opiates.
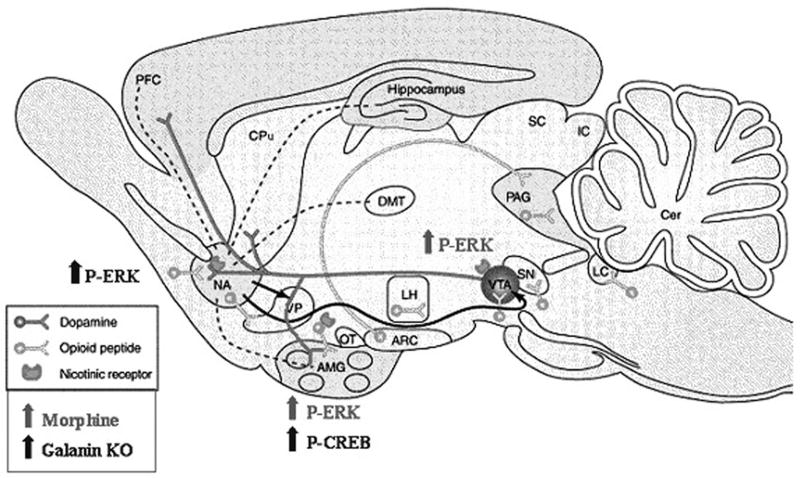
Figure 6. Summary of neurochemical changes.
Significant changes in ERK and CREB phosphorylation that are due to morphine and independent of galanin are indicated in grey. Significant neurochemical changes that are induced by morphine and are altered in galanin knockout mice, and thus likely to be modulated by galanin, are indicated in black. Ventral tegmental area (VTA), nucleus accumbens (NAc), amygdala (AMG), ventral portion of the caudate-putamen (CPu), substantia nigra (SN), prefrontal cortex (PFC), and locus coeruleus (LC).
Acknowledgments
This work was supported by grants DA15425 and DA00436 from the National Institutes of Health.
Footnotes
The authors declare that over the last 3 years MRP has received compensation from Pfizer for scientific consultation unrelated to the present work, and none of the other authors has any conflicts of interest.
Literature Cited
- Abramov U, Floren A, et al. Regulation of feeding by galnon. Neuropeptides. 2004;38(1):55–61. doi: 10.1016/j.npep.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Antoniou K, Kehr J, Snitt K, Ogren SO. Differential effects of the neuropeptide galanin on striatal acetylcholine release in anaesthetized and awake rats. Br J Pharmacol. 1997;121(6):1180–1186. doi: 10.1038/sj.bjp.0701233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, et al. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280(30):27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva MM, Szucs M, Wang D, Sadee W, Coscia CJ. mu-Opioid receptor-mediated ERK activation involves calmodulin-dependent epidermal growth factor receptor transactivation. J Biol Chem. 2001;276(36):33847–33853. doi: 10.1074/jbc.M101535200. [DOI] [PubMed] [Google Scholar]
- Berthold M, Kahl U, Jureus A, Kask K, Nordvall G, Langel Ű, et al. Mutagenesis and ligand modification studies on galanin binding to its GTP-binding-protein-coupled receptor GalR1. Eur J Biochem. 1997;249(2):601–606. doi: 10.1111/j.1432-1033.1997.00601.x. [DOI] [PubMed] [Google Scholar]
- Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16(15):4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR. beta2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology. 2006;184(3-4):328–338. doi: 10.1007/s00213-005-0099-z. [DOI] [PubMed] [Google Scholar]
- Burgevin MC, Loquet I, Quarteronet D, Habert-Ortoli E. Cloning, pharmacological characterization, and anatomical distribution of a rat cDNA encoding for a galanin receptor. J Mol Neurosci. 1995;6(1):33–41. doi: 10.1007/BF02736757. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28(8):436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carrigan KA, Dykstra LA. Behavioral effects of morphine and cocaine in M1 muscarinic acetylcholine receptor-deficient mice. Psychopharmacology (Berl) 2007;191(4):985–993. doi: 10.1007/s00213-006-0671-1. [DOI] [PubMed] [Google Scholar]
- Chang CK, Wang NL, Lin MT. Inhibition of the dopamine system in rat amygdala attenuates the picrotoxin-induced locomoter hyperactivity and hypertension. Clin Exp Pharmacol Physiol. 2004;31(5-6):284–288. doi: 10.1111/j.1440-1681.2004.03994.x. [DOI] [PubMed] [Google Scholar]
- Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu Rev Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]
- Chao JR, Ni YG, Bolanos CA, Rahman Z, DiLeone RJ, Nestler EJ. Characterization of the mouse adenylyl cyclase type VIII gene promoter: regulation by cAMP and CREB. Eur J Neurosci. 2002;16(7):1284–1294. doi: 10.1046/j.1460-9568.2002.02186.x. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, Konradi C, Carlezon WA., Jr Dopamine-dependent increases in phosphorylation of cAMP response element binding protein (CREB) during precipitated morphine withdrawal in primary cultures of rat striatum. J Neurochem. 2003;87(1):107–118. doi: 10.1046/j.1471-4159.2003.01992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg E, Erspamer R. Dopaminergic mechanisms of opiate actions in brain. J Pharmacol Exp Ther. 1975;192(1):50–57. [PubMed] [Google Scholar]
- Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Keith D, Jr, et al. Brain region-specific mechanisms for acute morphine-induced mitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. J Neurosci. 2003;23(23):8360–8369. doi: 10.1523/JNEUROSCI.23-23-08360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis Y, Davies JA. The effect of neuropeptides on the release of neurotransmitter amino acids from rat striatum. Neuropeptides. 1994;26(1):65–69. doi: 10.1016/0143-4179(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Flören A, Sollenberg U, Lundstrom L, Zorko M, Stojan J, Budihna M, et al. Multiple interaction sites of galnon trigger its biological effects. Neuropeptides. 2005;39(6):547–558. doi: 10.1016/j.npep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61(5):641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7(1):77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Smith KE, Durkin MM, Gerald C, Branchek TA. Distribution of a rat galanin receptor mRNA in rat brain. Neuroreport. 1996;7(4):953–957. doi: 10.1097/00001756-199603220-00025. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Narasimhaiah R, Picciotto MR. Galanin and galanin-related peptide modulate neurite outgrowth via PKC-mediated activation of ERK. Eur J Neurosci. 2006a;23(11):2937–2946. doi: 10.1111/j.1460-9568.2006.04828.x. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Narasimhaiah R, Picciotto MR. Galanin attenuates cyclic AMP regulatory element-binding protein (CREB) phosphorylation induced by chronic morphine and naloxone challenge in Cath.a cells and primary striatal cultures. J Neurochem. 2006b;96(4):1160–1168. doi: 10.1111/j.1471-4159.2005.03613.x. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Picciotto MR. Characterization of GalR1, GalR2, and GalR3 immunoreactivity in catecholaminergic nuclei of the mouse brain. J Comp Neurol. 2004;479(4):410–423. doi: 10.1002/cne.20329. [DOI] [PubMed] [Google Scholar]
- Hikida T, Kitabatake Y, Pastan I, Nakanishi S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci U S A. 2003;100(10):6169–6173. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW, Starosta G, Crawley JN. Central galanin administration blocks consolidation of spatial learning. Neurobiol Learn Mem. 2003;80(1):42–54. doi: 10.1016/s1074-7427(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Kolakowski LF, Jr, O'Neill GP, Howard AD, Broussard SR, Sullivan KA, Feighner SD, et al. Molecular characterization and expression of cloned human galanin receptors GALR2 and GALR3. J Neurochem. 1998;71(6):2239–2251. doi: 10.1046/j.1471-4159.1998.71062239.x. [DOI] [PubMed] [Google Scholar]
- Konradi C, Cole RL, Heckers S, Hyman SE. Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci. 1994;14(9):5623–5634. doi: 10.1523/JNEUROSCI.14-09-05623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh HH, Smith AP. Molecular characterization of opioid receptors. Annu Rev Pharmacol Toxicol. 1990;30:123–147. doi: 10.1146/annurev.pa.30.040190.001011. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10(3):201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Oliverio A, Castellano C, Eleftheriou BE. Morphine sensitivity and tolerance: a genetic investigation in the mouse. Psychopharmacologia. 1975;42(3):219–224. doi: 10.1007/BF00421259. [DOI] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311(5763):1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Narita M, Ozaki M, Khotib J, Suzuki T. Role of extracellular signal-regulated kinase in the ventral tegmental area in the suppression of the morphine-induced rewarding effect in mice with sciatic nerve ligation. J Neurochem. 2004;88(6):1389–1397. doi: 10.1046/j.1471-4159.2003.02272.x. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Chartoff EH, Carlezon WA, Jr, Zou J, Zhang H, Kreibich AS, et al. CREB gene transcription factors: role in molecular mechanisms of alcohol and drug addiction. Alcohol Clin Exp Res. 2005;29(2):176–184. doi: 10.1097/01.alc.0000153550.31168.1d. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Xu ZQ, Zhang X, Grillner S, Bartfai T, Hökfelt T. Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience. 1995;64(4):861–874. doi: 10.1016/0306-4522(94)00450-j. [DOI] [PubMed] [Google Scholar]
- Rajarao SJ, Platt B, et al. Anxiolytic-like activity of the non-selective galanin receptor agonist, galnon. Neuropeptides. 2007 doi: 10.1016/j.npep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Rezayof A, Zatali H, Haeri-Rohani A, Zarrindast MR. Dorsal hippocampal muscarinic and nicotinic receptors are involved in mediating morphine reward. Behav Brain Res. 2006;166(2):281–290. doi: 10.1016/j.bbr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6(2):228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Saar K, Mazarati AM, Mahlapuu R, Hallnemo G, Soomets U, Kilk K, et al. Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proc Natl Acad Sci U S A. 2002;99(10):7136–7141. doi: 10.1073/pnas.102163499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann J, Marie-Claire C, Le Guen S, Roques BP, Noble F. Importance of ERK activation in behavioral and biochemical effects induced by MDMA in mice. Br J Pharmacol. 2003;140(5):831–838. doi: 10.1038/sj.bjp.0705506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, et al. Regional and cellular mapping of cAMP response element-mediated transcription during naltrexone-precipitated morphine withdrawal. J Neurosci. 2002;22(9):3663–3672. doi: 10.1523/JNEUROSCI.22-09-03663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Funada M, Narita M, Misawa M, Nagase H. Morphine-induced place preference in the CXBK mouse: characteristics of mu opioid receptor subtypes. Brain Res. 1993;602(1):45–52. doi: 10.1016/0006-8993(93)90239-j. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Tsuda S, Nishio I, Masuyama Y, Goldstein M. Effects of galanin on dopamine release in the central nervous system of normotensive and spontaneously hypertensive rats. Am J Hypertens. 1998;11(12):1475–1479. doi: 10.1016/s0895-7061(98)00168-x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Caboche J, Vanhoutte P. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: a molecular substrate for learning and memory? Mol Neurobiol. 2001;23(2-3):83–99. doi: 10.1385/MN:23:2-3:083. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006a;103(8):2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006b;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19(7):1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102(2):491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Wild KD, Shank RP, Lee DH. Galanin inhibits acetylcholine release from rat cerebral cortex via a pertussis toxin-sensitive G(i)protein. Neuropeptides. 1999;33(3):197–205. doi: 10.1054/npep.1999.0024. [DOI] [PubMed] [Google Scholar]
- Waters SM, Krause JE. Distribution of galanin-1, -2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience. 2000;95(1):265–271. doi: 10.1016/s0306-4522(99)00407-8. [DOI] [PubMed] [Google Scholar]
- Widnell KL, Self DW, Lane SB, Russell DS, Vaidya VA, Miserendino MJ, et al. Regulation of CREB expression: in vivo evidence for a functional role in morphine action in the nucleus accumbens. J Pharmacol Exp Ther. 1996;276(1):306–315. [PubMed] [Google Scholar]
- Williams JA, Su HS, Bernards A, Field J, Sehgal A. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science. 2001;293(5538):2251–2256. doi: 10.1126/science.1063097. [DOI] [PubMed] [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35(1-2):227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- Wu WP, Hao JX, Lundström L, Wiesenfeld-Hallin Z, Langel Ű, Bartfai T, et al. Systemic galnon, a low-molecular weight galanin receptor agonist, reduces heat hyperalgesia in rats with nerve injury. Eur J Pharmacol. 2003;482(1-3):133–137. doi: 10.1016/j.ejphar.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Wynick D, Small CJ, Bloom SR, Pachnis V. Targeted disruption of the murine galanin gene. Ann N Y Acad Sci. 1998;863:22–47. doi: 10.1111/j.1749-6632.1998.tb10681.x. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Brunzell DH, Hawes J, Stedman DR, Bartfai T, Steiner RA, et al. The neuropeptide galanin modulates behavioral and neurochemical signs of opiate withdrawal. Proc Natl Acad Sci U S A. 2003;100(15):9028–9033. doi: 10.1073/pnas.1533224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Parikh K, Picciotto MR. Centrally administered galanin blocks morphine place preference in the mouse. Brain Res. 1999;831(1-2):33–42. doi: 10.1016/s0006-8993(99)01476-6. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Bananej M, Khalilzadeh A, Fazli-Tabaei S, Haeri-Rohani A, Rezayof A. Influence of intracerebroventricular administration of dopaminergic drugs on morphine state-dependent memory in the step-down passive avoidance test. Neurobiol Learn Mem. 2006a;86(3):286–292. doi: 10.1016/j.nlm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Farahmandfar M, Rostami P, Rezayof A. The influence of central administration of dopaminergic and cholinergic agents on morphine-induced amnesia in morphine-sensitized mice. J Psychopharmacol. 2006b;20(1):59–66. doi: 10.1177/0269881105057003. [DOI] [PubMed] [Google Scholar]