Abstract
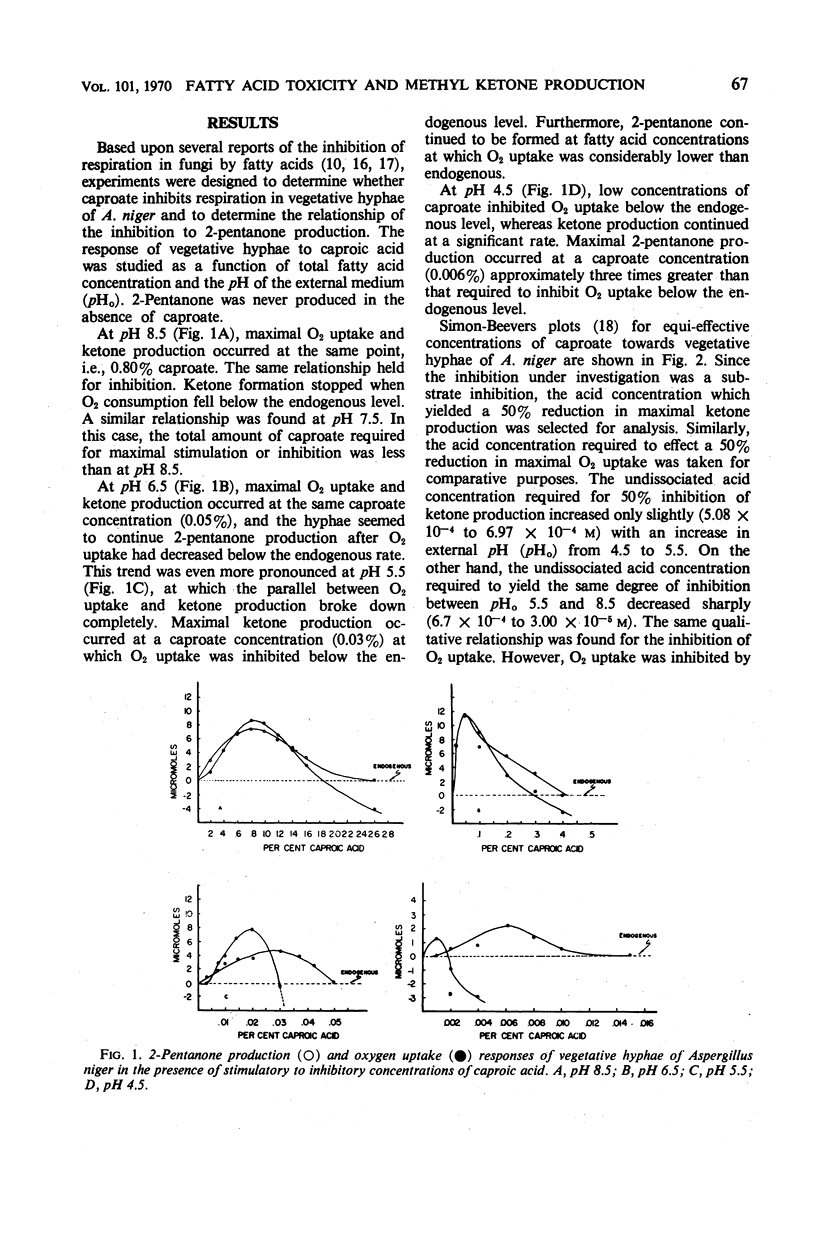
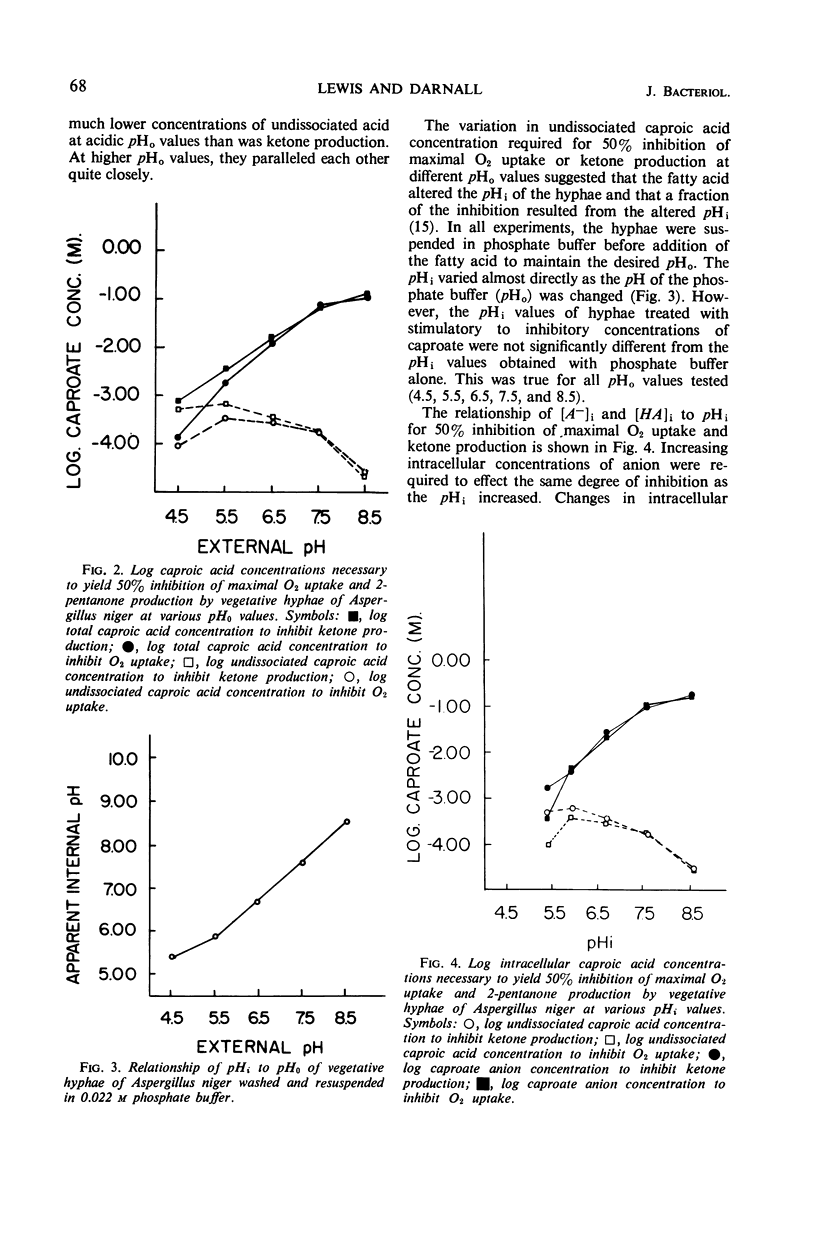
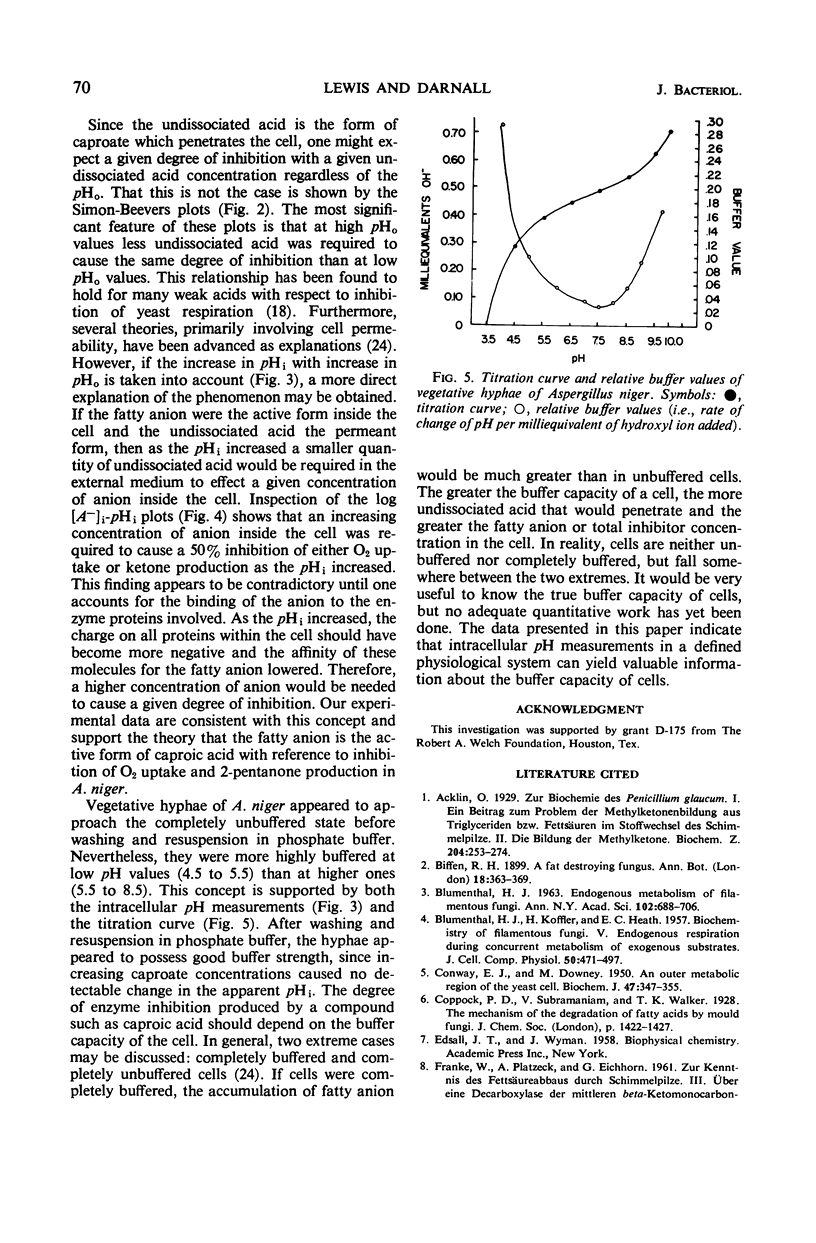
Vegetative hyphae of Aspergillus niger rapidly converted caproic acid into 2-pentanone. More caproic acid was required for maximal ketone production at alkaline as compared to acidic pH values. Further increases in caproate concentrations at each pH value tested (4.5, 5.5, 6.5, 7.5, and 8.5) resulted in inhibition of ketone production and O2 uptake. At alkaline pH values (8.5 and 7.5), oxygen uptake above the endogenous level and the production of 2-pentanone were parallel. This relationship did not hold at acidic pH values. At these pH values, ketone production continued (pH 6.5) or attained a maximum (pH 5.5 and 4.5) at caproate concentrations at which oxygen uptake was inhibited below endogenous levels. These data indicate that endogenous oxygen uptake was not inhibited by caproate at alkaline pH values at concentrations which did inhibit caproate oxidation and 2-pentanone production. Conversely, at acidic pH values, endogenous oxygen uptake was vigorously inhibited by caproate at concentrations at which exogenous fatty acid oxidation and 2-pentanone production were less affected. Simon-Beevers plots of these data showed that the undissociated acid was the permeant form of caproic acid. The fatty anion appeared to be the active or inhibitory form of caproate within the cell. Vegetative hyphae of A. niger were poorly buffered. Once the hyphae were washed and resuspended in phosphate buffer, they were well buffered towards inhibitory concentrations of caproic acid. These findings suggest that the primary mechanism(s) by which caproate inhibits oxygen uptake and ketone formation does not involve a change in the intracellular pH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUMENTHAL H. J. Endogenous metabolism of filamentous fungi. Ann N Y Acad Sci. 1963 Jan 21;102:688–706. doi: 10.1111/j.1749-6632.1963.tb13669.x. [DOI] [PubMed] [Google Scholar]
- BLUMENTHAL H. J., KOFFLER H., HEATH E. C. Biochemistry of filamentous fungi. V. Endogenous respiration during concurrent metabolism of exogenous substrates. J Cell Physiol. 1957 Dec;50(3):471–497. doi: 10.1002/jcp.1030500310. [DOI] [PubMed] [Google Scholar]
- CONWAY E. J., DOWNEY M. An outer metabolic region of the yeast cell. Biochem J. 1950 Sep;47(3):347–355. doi: 10.1042/bj0470347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOTYK A. Uptake of 2,4-dinitrophenol by the yeast cell. Folia Microbiol (Praha) 1962 Mar;7:109–114. doi: 10.1007/BF02927233. [DOI] [PubMed] [Google Scholar]
- Neal A. L., Weinstock J. O., Lampen J. O. Mechanisms of Fatty Acid Toxicity for Yeast. J Bacteriol. 1965 Jul;90(1):126–131. doi: 10.1128/jb.90.1.126-131.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMSON F. E., KATZ A. M., HARRIS D. L. Effects of acetate and other short-chain fatty acids on yeast metabolism. Arch Biochem Biophys. 1955 Feb;54(2):406–423. doi: 10.1016/0003-9861(55)90054-0. [DOI] [PubMed] [Google Scholar]
- Stokoe W. N. The rancidity of coconut oil produced by mould action. Biochem J. 1928;22(1):80–93. doi: 10.1042/bj0220080. [DOI] [PMC free article] [PubMed] [Google Scholar]



