Abstract
Addition of rapamycin to cultures of expanding natural CD4+CD25+Foxp3+ T regulatory cells (Tregs) helps maintain their suppressive activity, but the underlying mechanism is unclear. Pim 2 is a serine/threonine kinase that can confer rapamycin resistance. Unexpectedly, pim 2 was found to be constitutively expressed in freshly isolated, resting Tregs, but not in CD4+CD25− T effector cells. Introduction of Foxp3, but not Foxp3Δ2, into effector T cells induced pim 2 expression and conferred preferential expansion in the presence of rapamycin, indicating that Foxp3 can regulate pim 2 expression. Finally, we determined there is a positive correlation between Treg expansion and Foxp3 expression in the presence of rapamycin. Together, these results indicate that Tregs are programmed to be resistant to rapamycin, providing further rationale for why this immunosuppressive drug should be used in conjunction with expanded Tregs.
INTRODUCTION
The ability of natural CD4+CD25+Foxp3+ T regulatory cells (Tregs) to suppress immune responses has generated interest in harnessing their therapeutic power to treat autoimmune disease and enable transplants (1,2). Successful therapeutic application of Tregs will likely require significant ex vivo expansion. The inability to isolate pure Treg populations coupled with their relative ex vivo proliferative disadvantage has made expansion of functional Tregs isolated from peripheral blood problematic (1). A considerable breakthrough occurred when Roncarolo and colleagues observed that addition of rapamycin to expanding murine Treg cultures significantly and consistently increased the yield of Foxp3 expressing cells with suppressive activity (3). Similar findings have been described in human Treg culture systems. Furthermore, in vivo administration of rapamycin preferentially preserves Treg function (4-6).
The mechanism by which rapamycin maintains the suppressive activity of expanding Tregs is unclear. One study demonstrated that Tregs were resistant to rapamycin-induced apoptosis and thus selectively expanded in the presence of rapamycin (7) while another study suggested that rapamycin induced a transient Treg phenotype in T effector cells (8). Resolving this controversy has important clinical implications. If rapamycin only temporally endows T effector cells with regulatory activity, as the latter study suggests, then its clinical utility is questionable. In contrast, if rapamycin does preferentially select for Tregs at the expense of effector cells, then it would be an important component of Treg culture systems for adoptive T cell therapy.
Pim 2 is a transciptionally regulated serine/threonine kinase discovered as a proviral integration site for the Moloney Murine Leukemia virus (9,10). Functionally, pim 2 has considerable overlap with Akt, and by extension, mTOR. Akt and pim 2 share many common downstream targets including Bad and 4E Binding Protein-1 (11). In effector lymphocytes, pim 2 expression is tightly regulated by cytokine induced JAK/STAT pathways and its expression rapidly disappears upon cytokine removal (12). In murine lymphocytes and cell lines, pim 2 can mediate resistance to rapamycin (13). Here, we show that pim 2 is regulated in a fundamentally different way in Tregs. Foxp3, the master regulator of Tregs, induces pim 2 expression in Tregs. This permits constitutive pim 2 expression in resting Tregs, conferring a replicative advantage in cultures containing rapamycin. These results argue that natural, Foxp3 expressing T cells are indeed selected for in the presence of rapamycin and thus the use of rapamycin in expanding Treg cultures is a promising way to enable adoptive Treg cell therapy.
MATERIALS AND METHODS
Cell isolation, artificial APC preparation cell expansion, and cell stimulation
Primary human CD4+ T cells from healthy donors were purified by negative selection as previously described (14). Tregs were purified by CD25+ or CD127-CD25+ selection using magnetic beads as per manufacturer suggestions (Miltenyi Biotech). CD4 T cells were expanded with αCD3/αCD28 Ab coated beads (15) or by co-culture with irradiated, αCD3 Ab loaded, lentiviral vector transduced K562 aAPCs expressing CD64 and CD86 as described previously (16). T cells were cultured in the presence of rhIL-2 (300 U/ml, Chiron) and, where indicated, rapamycin (100 ng/ml, Calbiochem). To determine relative cell expansion the numbers of cells at the end of culture was divided by the number of cells that initiated the culture. 50 ng of phorbol 12-myristate 13-acetate (PMA) (Sigma) and 500 ng of Ionomycin (Calbiochem) were added to the cells prior to performing intracellular cytokine staining.
Flow Cytometric analysis
Surface staining for CD4 and CD25 (BD Pharmingen) was performed according to manufacturer's recommendations. Intracellular staining for Foxp3 was performed using the FOXP3 Fix/Perm kit (Biolegend) and for IL-2 (BD Pharmingen) was performed using the Caltag Fix&Perm kit (Invitrogen) as per the manufacturer's recommendations. All flow cytometry was analyzed uisng FACSCalibur (BD Biosciences) and FlowJo software (Tree Star).
RNA extraction and RT-PCR
RNA was purified, reverse transcribed, amplified and analyzed as previously described (14) using the ABI Prism 7900HT (Applied Biosystems). Primers and probes to detect 28 S ribosomal RNA and pim 2 were designed using Primer Express software (Applied Biosystems) and are available upon request.
Cell lysis, SDS-PAGE, and Western blotting
Cell lysis, electrophoresis and immunoblotting were performed as described previously (15). Anti human pim 2 (C-20) and anti human actin (I-19) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA)
Production of lentiviral vectors and transduction of CD4+CD25− T cells
GFP, YFP 2A Foxp3 and YFP 2A Foxp3Δ2 were cloned upstream of the EF-1a promoter in a previously described lentiviral vector (15) so that all transduced cell populations could be detected by FL1. The 2A sequence used to allow co-expression of YFP and Foxp3 was GSGEGRGSLLTCGDVEENPGP. High titer vector was used to transduce T cells as previously described (15).
RESULTS AND DISCUSSION
Natural, Foxp3-expressing Tregs Constitutively Express Pim 2
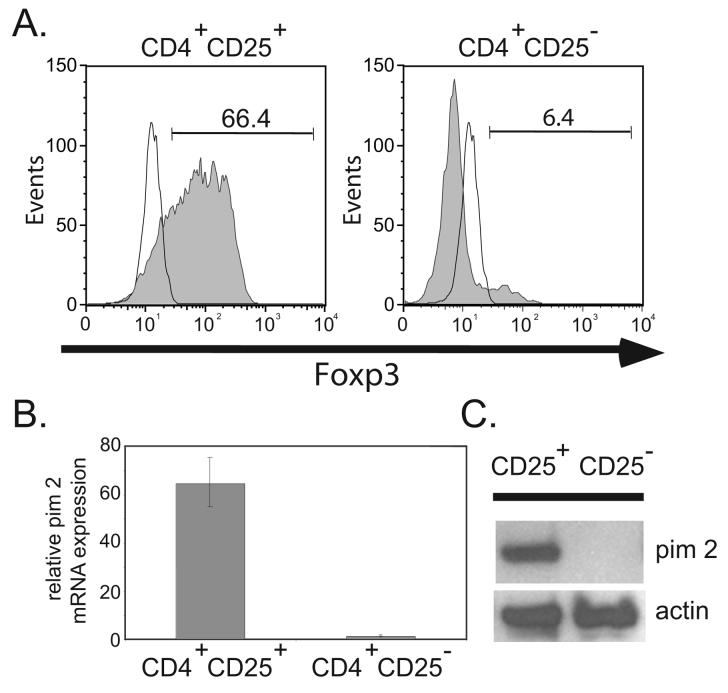
Pim 2 expression confers rapamycin resistance to murine T cells (14). We first confirmed that human pim 2 also confers rapamycin resistance. To do this, we transduced primary human CD4 T cells with a lentiviral vector expressing pim 2 and noted that pim 2 promoted the expansion of CD4 T cells in the presence and absence of rapamycin (data not shown), making it an attractive target to study in Tregs. Studies have shown that addition of rapamycin at the initiation of a Treg culture preserves the suppressive function of the expanded cells (3,5,8). This suggests that if pim 2 confers rapamycin resistance in Tregs, it should be expressed in resting Tregs. To investigate this, we purified Tregs and conventional T cells from freshly isolated leukaphersis products and examined the populations for pim 2 mRNA and protein expression. Measurement of Foxp3 expression by flow cytometry confirmed that we successful enriched for Tregs (Fig. 1A). Surprisingly, pim 2 mRNA (Fig. 1B) and protein (Fig. 1C) were readily found in the CD4+CD25+ T cells, and virtually undetectable in the CD4+CD25− population. This was unexpected, because both pim 2 mRNA and protein are highly labile (12), making it well suited for its role as an environmental sensor. Indeed, the first insights into pim 2 function were revealed in a DNA microarray screen searching for transcripts acutely regulated by cytokine withdrawal (12). Further studies demonstrated that multiple cytokines and growth factors can induce pim 2 expression via JAK/STAT signaling pathways (11,17). In fact, pim 2 expression has not been described in primary cells in the absence of growth factors and cytokines. Given the number of microarrays used to find differences between conventional and Tregs, it is surprising that pim 2 expression in these cells is not more widely appreciated. One study did observe that resting Tregs express ∼2 fold more pim 2 than resting CD4+CD25− T cells (18) whereas other studies that compared murine Tregs with effector CD4+ T cells did not observe any differences (19,20). Our data suggests that there is a cytokine-independent pathway to induce pim 2 expression in Tregs. Alternatively, these results could reflect differences in how Tregs and T effectors respond to minute levels of cytokine or antigen stimulation.
Figure 1. Natural, Foxp3 expressing Tregs Constitutively Express Pim 2.
A. Diagram of Foxp3 expression vectorsFreshly purified CD4+CD25+ Tregs (left panel) and CD4+CD25− T effector cells (right panel) were stained with anti-Foxp3 Ab (shaded histograms) or an isotype control Ab (open histograms) and analyzed by flow cytometry. B. 28S RNA-normalized mRNA levels of pim 2 were measured from freshly isolated CD4+CD25+ and CD4+CD25− T lymphocyte populations as determined by quantitative RT-PCR analysis. The data plotted as the mean ± SD of triplicate determinations from an individual donor. C. Western blot detection of pim 2 and actin (loading control) from freshly isolated CD4+CD25+ and CD4+CD25− T cell populations shown in A. Lysates correspond to 5 × 105 cell equivalents. Data is representative of 3 independent experiments.
Foxp3 but not Foxp3Δ2 Expression Induces Pim 2 and Confers Rapamycin Resistance
Foxp3 expression is the hallmark of Tregs and ectopic expression in CD4+ T effector cells results in repression of IL-2 production and upregulation of Treg cell surface markers, including CTLA-4, GITR, and CD25(21). One striking difference between human and murine Tregs is the approximately equal expression of full length and a truncated, exon 2 deleted form of Foxp3 (Foxp3Δ2) in human cells. To date, the functional significance of Foxp3Δ2 has not been elucidated. One study suggested that co-expression of both isoforms slightly increases suppressive activity (21), but the mechanism underlying this observation remains unclear. Thus, we investigated whether both Foxp3 isoforms induced pim 2 in human CD4 T cells. CD4+ CD25− T cells were activated with CD3/28 coated beads and transduced with GFP, YFP-2A-Foxp3 or YFP-2A-Foxp3Δ2 expression vectors (Figs. 2A, 2B). Since suppression of IL-2 production is indicative of Foxp3 activity (22), we measured IL-2 production by Foxp3 or Foxp3Δ2–expressing cells (Fig. 2C). Upon stimulation with PMA and ionomycin, 60% of untransduced cells produced IL-2. Thus, the ratio of IL-2 producing to IL-2 non-producing cells was 1.5. This ratio was inverted in both Foxp3 and Foxp3Δ2-expressing cells (0.43 and 0.47, respectively). Additionally, both Foxp3 and Foxp3Δ2-expressing cells inhibited IL-2 production from non-transduced cells in the same culture (0.48 and 0.28, respectively), as previously reported (21). These data suggests that our Foxp3 and Foxp3Δ2 expression vectors are produce functional Foxp3 isoforms.
Figure 2. Foxp3, but not Foxp3Δ2 Expression, Induces Pim 2 and Confers Rapamycin Resistance.
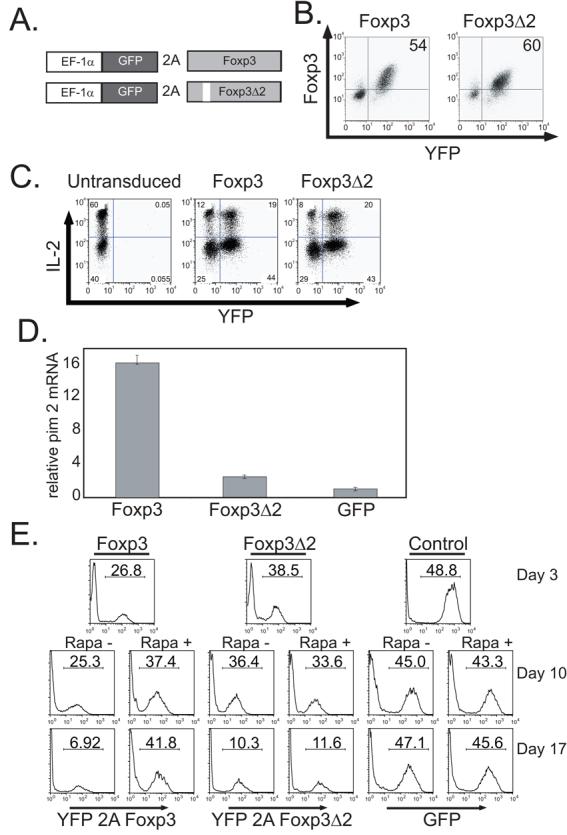
A. Diagram of constructs used in this study. B. CD4+CD25− T cells were activated with CD3/CD28 Ab coated beads and transduced with lentiviral vectors expressing either YFP-2A-Foxp3 or YFP-2A-Foxp3Δ2. The correlation between Foxp3 and YFP expression was determined by flow cytometry. C. YFP-2A-Foxp3, YFP-2A-Foxp3Δ2 and untransduced CD4+CD25− T cells were re-stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 4 hours and intracellular IL-2 expression was detected by flow cytometry. D. 28S-normalized mRNA levels of pim 2 from either YFP-2A-Foxp3, YFP-2A-Foxp3Δ2, or GFP transduced CD4+CD25− T cells shown in A. was determined by quantitative RT-PCR analysis. The cells were harvested 17 days after transduction. The data are plotted as the mean ± SD of triplicate determinations from one donor. E. CD4+CD25− T cells were activated with CD3/CD28 Ab coated beads and transduced with lentiviral vectors expressing YFP-2A-Foxp3, YFP-2A-Foxp3Δ2 or GFP. 3 days post-transduction the percentage of transduced cells was determined and half the culture was placed in rapamycin (100 ng/ml) containing medium. After an additional 7 (middle panels) or 14 (bottom panels) days of culture, the percentage of transduced cells was measured by flow cytometry. Data is representative of 3 independent experiments.
Next, we examined the ability of both Foxp3 isoforms to induce pim 2 in human CD4+ T cells. We observed that Foxp3, but not Foxp3Δ2, can induce pim 2 in CD4+CD25− T cells (Fig. 2D). To demonstrate that Foxp3 expression leads to preferential T cell expansion in the presence of rapamycin, we transduced primary human CD4+ CD25− T cells with Foxp3, Foxp3Δ2 or control GFP expression vectors as described above. After three days of expansion, we split the cultures so that the cells were expanded either in the presence or absence of rapamycin for an additional 14 days. In the absence of rapamycin, both Foxp3 and Foxp3Δ2 expressing cells were at a replicative disadvantage relative to their untransduced counterparts and were diluted out (Fig. 2E). Foxp3-transduced cells expanded in the presence of rapamycin were enriched in these cultures. In contrast, Foxp3Δ2-expressing cells were not enriched in the presence of rapamycin and were equally diluted out as they were in the absence of rapamycin. We did not observe induction of Foxp3 expression in GFP-transduced control cells (data not shown), suggesting that rapamycin selects for Foxp3 expressing cells rather than inducing Foxp3 expression. These data demonstrate that both Foxp3 isoforms can suppress proliferation of primary human CD4+ CD25− T cells. However, only the full length Foxp3 isoform induces pim 2 expression allowing for preferential expansion in the presence of rapamycin.
Foxp3 interacts with multiple partners, including both NFAT (23) and Runx1(24), suggesting that the molecular mechanism by which it regulates pim 2 expression is likely to be complex. However, recent data addressing the differential effects of mTOR inhibition on Tregs and effector cells may provide some clues(6). These studies illustrate that Treg activation leads to prolonged STAT5 phosphorylation rather than PI3K/AKT/mTOR activation as compared to CD4+CD25− T cells. Since STAT5 phosphorylation can also induce pim 2 expression (25), this might be one mechanism by which Tregs upregulate pim 2. While providing some insight, this propensity of Tregs to activate STAT5 does not fully explain constitutive pim 2 expression. Nor does it fully delineate the relationship between Foxp3, STAT5 and pim 2.
Treg expansion in the presence of rapamycin correlates with Foxp3 expression
Our data up to this point confirm previous work that forced Foxp3 overexpression hinders the expansion of T cells (21). More importantly, our results indicate that in the presence of rapamycin, Foxp3 expression aids in the expansion of T cells by upregulating pim 2. Thus, we predicted that the degree of expansion of freshly isolated natural Tregs would positively correlate with Foxp3 expression if cultured with rapamycin. Similarly, in the absence of rapamycin, we would expect the opposite to hold true. To test this, we expanded enriched Treg cells (40-80% Foxp3 positive) isolated from healthy donors using a previously described K562 cell-based artificial antigen presenting cell (aAPC). These KT64 86 aAPCs express CD64 to load an anti-CD3 agonist antibody and CD86 to engage CD28 (16). Enriched Tregs from nine donors were expanded by anti-CD3 loaded KT64 86 cells at a 2:1 ratio (Treg:aAPC) in the absence of rapamycin. Tregs from the same nine donors plus an additional six (15 total) were similarly expanded in the presence of rapamycin. Both groups were cultured for 14-20 days, after which regression analysis was performed to determine correlation between Foxp3 expression and relative expansion. Representative data for one donor are shown in Fig. 3A, B. Here, we observed enrichment for Foxp3 positive cells in the presence of rapamycin and dilution of Foxp3 positive cells in the absence of rapamycin. It is important to emphasize that Tregs are not immune to all of the effects of rapamycin nor do they expand as well as effector cells ex vivo (Fig. 3B). Rather, Tregs, because of their Foxp3 mediated pim 2 expression, are less sensitive to the immunosuppressive effects of rapamycin and preferentially expand in the presence of rapamycin. Additionally, as others have shown (3-5,7), Tregs expanded for extended periods in the presence of rapamycin retain suppressive ability whereas Tregs grown in its absence lose suppressive function (data not shown). In rapamycin containing Treg cultures, we observed a positive correlation (R=.438) between Foxp3 expression and the degree of expansion (Fig. 3C). Similarly, Tregs cultured without rapamycin exhibited a negative correlation (R=.349) between Foxp3 expression and the magnitude of expansion (Fig. 3D). Taken together, these studies illustrate that pim 2 expression by Tregs grants a growth advantage in the presence of rapamycin. These data also strengthens the argument for the use of combination therapies employing both rapamycin and Tregs to suppress unwanted immune responses.
Figure 3. Natural T regulatory cell expansion in the presence of rapamycin correlates with Foxp3 expression.
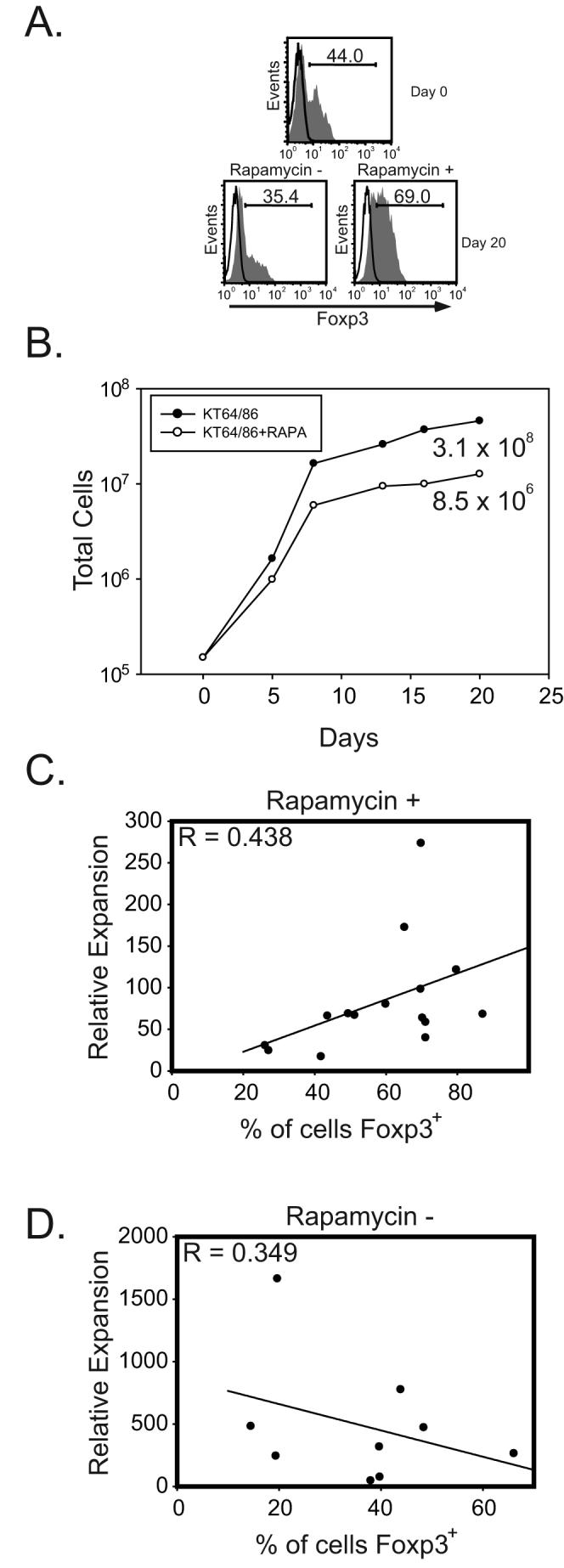
A. Tregs were enriched by CD25 selection and stained for Foxp3 pre- and post-expansion with irradiated, anti-CD3 Ab loaded KT64 CD86 aAPCs. B. Overall relative expansion of T cells from day 0 to day 20 in the presence or absence of rapamycin (100ng/ml). A. and B. are from the same experiment and are representative of data collected using 9 different donors. C. Enriched Tregs were expanded with rapamycin (100 ng/ml) and the percentage of Foxp3 expressing cells at the end of culture was determined by flow cytometry. Data are compiled from 15 independent experiments. D. Enriched Tregs were expanded without rapamycin and the percentage of Foxp3 expressing cells at the end of culture was determined by flow cytometry. Data are compiled from 9 independent experiments. Correlation between Foxp3 expression and relative cell expansion was determined by nonlinear regression analysis.
In summary, pim 2 is constitutively expressed in Tregs in a Foxp3 dependent manner and this expression allows for a selective growth advantage in the presence of rapamycin. These data further demonstrate that only full length Foxp3 protein can induce pim 2, while the equally expressed Foxp3Δ2 is unable to do so. Thus, it is reasonable to conclude that factors that interact with exon 2 in Foxp3 are necessary to induce pim 2 expression. Our data demonstrate that functional Tregs are indeed selected for by rapamycin. Furthermore, employing rapamycin for the selective ex vivo expansion of Tregs for adoptive T cell immunotherapy is an attractive strategy.
ACKNOWLEDGEMENTS
We are grateful to members of the JDRF Collaborative Center for Cell Therapy for helpful suggestions and discussions; Drs. Richard Carroll, and Gwen Binder for proofreading the manuscript; Dr. Steve Ziegler for providing the Foxp3 cDNAs, Abraham Chacko for help in creating the Foxp3 lentiviral vectors, the Penn CFAR Immunology Core for providing primary human T cells;
SB received support from T32CA101968. Supported by JDRF Collaborative Center for Cell Therapy, JDRF Autoimmunity Center on Cord Blood Therapy for Diabetes, R01CA105216, and R01AI057838.
REFERENCES
- 1.Bluestone JA. Regulatory T-cell therapy: is it ready for the clinic? Nat Rev Immunol. 2005;5:343–349. doi: 10.1038/nri1574. [DOI] [PubMed] [Google Scholar]
- 2.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 3.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 4.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 5.Coenen JJA, Koenen HJPM, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107:1018–1023. doi: 10.1182/blood-2005-07-3032. [DOI] [PubMed] [Google Scholar]
- 6.Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, Hou JZ, Negrin RS. Differential impact of mTOR inhibition on CD4+CD25+Foxp3+ regulatory T cells as compared to conventional CD4+ T cells. Blood. 2007 doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective Survival of Naturally Occurring Human CD4+CD25+Foxp3+Regulatory T Cells Cultured with Rapamycin. J Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 8.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M. Rapamycin-Mediated Enrichment of T Cells with Regulatory Activity in Stimulated CD4+ T Cell Cultures Is Not Due to the Selective Expansion of Naturally Occurring Regulatory T Cells but to the Induction of Regulatory Functions in Conventional CD4+ T Cells. J Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 9.Allen JD, Verhoeven E, Domen J, d. van V, Berns A. Pim-2 transgene induces lymphoid tumors, exhibiting potent synergy with c-myc. Oncogene. 1997;15:1133–1141. doi: 10.1038/sj.onc.1201288. [DOI] [PubMed] [Google Scholar]
- 10.van der Lugt NM, J. D. E. V. K. L. H. v. d. G. J. A. a. A. B. Proviral tagging in Eu-myc transgenic mice lacking the Pim-1 proto-oncogene leads to compensatory activation of Pim-2. The EMBO Journal. 1995;14:2536–2544. doi: 10.1002/j.1460-2075.1995.tb07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White E. The pims and outs of survival signaling: role for the Pim-2 protein kinase in the suppression of apoptosis by cytokines. Genes Dev. 2003;17:1813–1816. doi: 10.1101/gad.1123103. [DOI] [PubMed] [Google Scholar]
- 12.Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 2003;17:1841–1854. doi: 10.1101/gad.1105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox CJ, Hammerman PS, Thompson CB. The Pim kinases control rapamycin-resistant T cell survival and activation. J. Exp. Med. 2005;201:259–266. doi: 10.1084/jem.20042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley JL, Blair PJ, Musser JT, Abe R, Tezuka K, Tsuji T, June CH. ICOS Costimulation Requires IL-2 and Can Be Prevented by CTLA-4 Engagement. J Immunol. 2001;166:4943–4948. doi: 10.4049/jimmunol.166.8.4943. [DOI] [PubMed] [Google Scholar]
- 15.Chemnitz JM, Lanfranco AR, Braunstein I, Riley JL. B and T Lymphocyte Attenuator-Mediated Signal Transduction Provides a Potent Inhibitory Signal to Primary Human CD4 T Cells That Can Be Initiated by Multiple Phosphotyrosine Motifs. J Immunol. 2006;176:6603–6614. doi: 10.4049/jimmunol.176.11.6603. [DOI] [PubMed] [Google Scholar]
- 16.Suhoski MM, Golovina TN, Aqui NA, Tai VC, Varela-Rohena A, Milone MC, Carroll RG, Riley JL, June CH. Engineering Artificial Antigen-presenting Cells to Express a Diverse Array of Co-stimulatory Molecules. Mol Ther. 2007;15:981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aho TLT, Lund RJ, Ylikoski EK, Matikainen S, Lahesmaa R, Koskinen PJ. Expression of human pim family genes is selectively up-regulated by cytokines promoting T helper type 1, but not T helper type 2, cell differentiation. Immunology. 2005;116:82–88. doi: 10.1111/j.1365-2567.2005.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Learn CA, Fecci PE, Schmittling RJ, Xie W, Karikari I, Mitchell DA, Archer GE, Wei Z, Dressman H, Sampson JH. Profiling of CD4+, CD8+, and CD4+CD25+CD45RO+FoxP3+ T cells in patients with malignant glioma reveals differential expression of the immunologic transcriptome compared with T cells from healthy volunteers. Clin. Cancer Res. 2006;12:7306–7315. doi: 10.1158/1078-0432.CCR-06-1727. [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, Sakaguchi S. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int. Immunol. 2006;18:1197–1209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 21.Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, Ziegler SF, Roncarolo MG, Levings MK. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J. Clin. Invest. 2005;115:3276–3284. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziegler SF. FOXP3: of mice and men. Annu. Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 Controls Regulatory T Cell Function through Cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 24.Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, Miyachi Y, Tsukada T, Sakaguchi S. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 25.Mizuki M, Schwable J, Steur C, Choudhary C, Agrawal S, Sargin B, Steffen B, Matsumura I, Kanakura Y, Bohmer FD, Muller-Tidow C, Berdel WE, Serve H. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood. 2003;101:3164–3173. doi: 10.1182/blood-2002-06-1677. [DOI] [PubMed] [Google Scholar]