Abstract
Background
Over the past two decades, high false alarm (FA) rates have remained an important yet unresolved concern in the Intensive Care Unit (ICU). High FA rates lead to desensitization of the attending staff to such warnings, with associated slowing in response times and detrimental decreases in the quality of care for the patient. False arrhythmia alarms are commonly due to single channel ECG artifacts and low voltage signals, and therefore it is likely that the FA rates may be reduced if information from other independent signals is used to form a more robust hypothesis of the alarm’s etiology.
Methods
A large multi-parameter ICU database (PhysioNet’s MIMIC II database) was used to investigate the frequency of five categories of false critical (“red” or “life-threatening”) ECG arrhythmia alarms produced by a commercial ICU monitoring system, namely: asystole, extreme bradycardia, extreme tachycardia, ventricular tachycardia and ventricular fibrillation/tachycardia. Non-critical (“yellow”) arrhythmia alarms were not considered in this study. Multiple expert reviews of 5,386 critical ECG arrhythmia alarms from a total of 447 adult patient records in the MIMIC II database were made using the associated 41,301 hours of simultaneous ECG and arterial blood pressure (ABP) waveforms. An algorithm to suppress false critical ECG arrhythmia alarms using morphological and timing information derived from the ABP signal was then tested.
Results
An average of 42.7% of the critical ECG arrhythmia alarms were found to be false, with each of the five alarm categories having FA rates between 23.1% and 90.7%. The FA suppression algorithm developed was able to suppress 59.7% of the false alarms, with FA reduction rates as high as 93.5% for asystole and 81.0% for extreme bradycardia. FA reduction rates were lowest for extreme tachycardia (63.7%) and ventricular-related alarms (58.2% for ventricular fibrillation/tachycardia and 33.0% for ventricular tachycardia). True alarm (TA) reduction rates were all 0%, except for ventricular tachycardia alarms (9.4%).
Conclusions
The FA suppression algorithm reduced the incidence of false critical ECG arrhythmia alarms from 42.7% to 17.2%, where simultaneous ECG and ABP data were available. The present algorithm demonstrated the potential of data fusion to reduce false ECG arrhythmia alarms in a clinical setting, but the non-zero TA reduction rate for ventricular tachycardia indicates the need for further refinement of the suppression strategy. To avoid suppressing any true alarms, the algorithm could be implemented for all alarms except ventricular tachycardia. Under these conditions the FA rate would be reduced from 42.7% to 22.7%. This implementation of the algorithm should be considered for prospective clinical evaluation. The public availability of a real-world ICU database of multiparameter physiologic waveforms, together with their associated annotated alarms is a new and valuable research resource for algorithm developers.
Keywords: annotated database, blood pressure, false alarms, false alarm reduction, intensive care unit, life-threatening alarms, signal quality
1. Introduction
False alarms in the Intensive Care Unit (ICU) can lead to a disruption of care, impacting both the patient and the clinical staff through noise disturbances, desensitization to warnings and slowing of response times [1], leading to decreased quality of care [2,3]. ICU alarms produce sound intensities above 80 dB that can lead to sleep deprivation [1,4,5], inferior sleep structure [6,7,8], stress for both patients and staff [9,10,11,12,13] and depressed immune systems [14]. There are also indications that the incidence of re-hospitalization is lower if disruptive noise levels are decreased during a patient’s stay [15]. Furthermore, such disruptions have been shown to have an important effect on recovery and length of stay [2,10]. In particular, cortisol levels have been shown to be elevated (reflecting increased stress) [12,13], and sleep disruption has been shown to lead to longer stays in the ICU [5]. ICU false alarm (FA) rates as high as 86% have been reported, with between 6% and 40% of ICU alarms having been shown to be true but clinically insignificant (requiring no immediate action) [16]. In fact, only 2% to 9% of alarms have been found to be important for patient management [17].
Previous investigations into reducing false alarms in data recorded from critically ill patients are relatively few, and were performed on small data sets. Mäkivirta et al. [18] implemented a recursive two-stage median filter for heart rate trends which provided improved smoothing at the expense of increased error in heart rate estimation. The first (3-point, 15 s) filter removed only brief transients, and the second longer (15-point) filter removed more persistent artifacts. Makivirta’s approach reduced FA frequency from 88% to 51% in data from 10 post-cardiac surgery patients. Sittig and Factor [19] developed a multi-state Kalman filter approach to identifying artifacts and reducing alarms, but only tested the system on simulated data. Koski et al [20] used 134 hours of data from 15 patients to develop a knowledge-based system for reducing false alarms on post-operative patients, achieving an increase in specificity from 20% to 74%. However, none of these studies used a large, representative database for training or testing. GE Medical (Waukesha, WI) is currently awaiting FDA 510(k) approval for their ‘Intellirate’ algorithm, which uses a range of simultaneously available pulsatile signals in the ICU (such as the pulse oximeter and arterial blood pressure waveforms) to help verify electrocardiogram (ECG)-based alarms. However, little has been published concerning the Intellirate algorithm, including details of the relatively small set of data on which the algorithm was tested. Schapira and Van Ruiswyk, in a poster presentation [21], reported an evaluation of GE’s algorithm. The algorithm employed by the monitors was shown to have a sensitivity of 94% and a positive predictive value of 74%. After applying an unspecified fusion algorithm that used the information in all the recorded channels the sensitivity remained unchanged, but the positive predictive value increased to 86%. Unfortunately, no per-alarm category analysis was given based upon alarm type, and only 151 alarms in total were used. In a previous work, we analyzed a public database of ICU data which contained 89 distinct critical (life-threatening) ECG arrhythmia alarms (defined as asystole, extreme bradycardia, extreme tachycardia, ventricular tachycardia and ventricular fibrillation/tachycardia) recorded from a total of 21 subjects and 800 hours of ICU waveform data [22]. A total of 25% of the 89 critical ECG arrhythmia alarms were found to be false. An ABP analysis strategy (that involved checking to see if the morphology and timing of the ABP was commensurate with the issued alarm) was successful in suppressing all the false alarms in this study, without suppressing any true alarms. However, given the small size of the dataset, it is unlikely that the FA suppression rate would remain at 100% on a larger set of data, and some true alarm suppression would likely be inevitable.
To avoid erroneous triggers of critical ECG ICU alarms, noisy sections of data could be rejected using signal quality measures. Furthermore, intelligent multi-lead ECG analysis (as is employed by most ICU monitors) and the use of data derived from an independent cardiac-cycle signal might facilitate the rejection of false arrhythmia alarms. The corroboration of alarms using information extracted from a signal highly correlated with the ECG, (such as a pulsatile waveform) that uses an independent sensor to monitor the cardiac cycle, might be able to suppress a large number of false ECG alarms in the ICU. The ABP waveform signal is generated by an independent transducer located away from the torso, exhibits different noise characteristics from an ECG waveform, and is unlikely to contain ECG-related artifacts (except in the case of large body movements of the patient that affect both sensors simultaneously). Therefore, by using information derived from ABP and ECG waveforms, it is likely that true ECG alarms can be effectively corroborated and false ECG alarms suppressed. In the study presented in this article, a new multi-parameter ICU database (PhysioNet’s MIMIC II database) [23, 24, 25] was used to investigate the frequency of true and of false critical ECG arrhythmia alarms generated by patient monitors in real ICU settings. No second-level “yellow” alarms were considered in this study. The methods presented here are broken down into two pieces of work. Firstly, procedures to identify and annotate critical ECG arrhythmia alarms are detailed. Secondly, a strategy is presented for suppressing false critical ECG arrhythmia alarms using an algorithm that exploits morphological and timing information derived from the ABP waveform. Methods for optimizing the algorithm are discussed. Results are then presented in three sections: 1) false and true alarm rates of the annotated data, 2) optimized parameters values for the FA suppression algorithm using the training set, and 3) the performance of the FA suppression algorithm. Weaknesses of, and possible improvements to the algorithm are discussed.
2. Methods
2.1. Data Sources
The Multi-Parameter Intelligent Monitoring for Intensive Care II (MIMIC II) database was assembled primarily to facilitate the development and evaluation of ICU decision support systems [23, 24, 25]. The database currently includes more than 2,000 records containing multiparameter physiologic waveforms and accompanying data which span approximately 10,000 patient-days. Each record contains up to four channels of continuously monitored waveforms (usually two leads of ECG, arterial BP, and pulmonary arterial pressure where available), as well as monitor-generated alarms. Data was obtained under an IRB-approved protocol from adult patients (ages 18 – >90 years, mean 68.3 years), in 48 medical, surgical, and coronary intensive care beds at an urban tertiary-level hospital. All waveform data and alarms were collected using Philips CMS bedside patient monitors (Philips Medical Systems, Andover, MA). Although multi-lead arrhythmia analysis was available in these monitors, it is important to note that the clinical staff at the data collection site chose to use single lead arrhythmia analysis. Waveforms were stored at 125 Hz with 8 bit resolution. The original ECG sampling rate was 500 Hz, and the ECG was then compressed to 125Hz using a turning point algorithm to preserve ECG peaks [26]. A subset of records were selected from the MIMIC II database that fulfilled two criteria: 1) a critical ECG arrhythmia alarm was issued at some time during the ICU stay, and 2) one channel of ECG and an ABP waveform were present at the time of the arrhythmia alarm.
2.2. Alarm Definitions
In a modern ICU virtually all bedside monitors generate two classes of alarms: A “yellow” alarm for notification of something abnormal, and a “red” alarm for notification of a critical or life-threatening event. The “yellow” alarms are typically not very loud and last for 5 or 6 seconds. However, the critical or “red” alarms have a much louder and distinctive tone that remain on until they are “acknowledged” by the care giver, usually a nurse. In this study we considered only critical “red” ECG alarms, which comprise approximately 4% to 8% of the ECG alarms in our database.
Critical arrhythmia alarms issued by the bedside monitors as a result of ECG signal processing were defined by the manufacturer according to the current ANSI/AAMI EC13 Cardiac Monitor Standards [27] as follows: (1) Asystole alarms were triggered by a default asystolic pause of 4 seconds that was user-adjustable between 2.5 and 4 seconds. (2) Extreme bradycardia was defined to be a heart rate (HR) less than 40 BPM. (3) Extreme tachycardia was defined to be a HR greater than 140 BPM, adjustable up to 200 BPM for an adult population. (4) VTach was defined as a run of ventricular beats at a rate of at least 100 BPM, lasting 5 or more beats. (5) VTach/VFib was defined as a fibrillatory waveform lasting for at least 4 seconds. Table 1 details the alarm definitions and thresholds for the monitors used in this study. Note that each triggered alarm also documented the currently valid user-defined threshold settings where applicable.
Table 1.
Alarm definitions and thresholds (for Adults).
| Alarm Type | Default Heart Rate (BPM) Criteria | Alarm Adjustable Range | Typical Time Delay to Alarm (sec) | Time Delay For Alarm AAMI-EC-13 Cardiotach Standard (sec) |
|---|---|---|---|---|
| Asystole | No QRS for 4 sec | 2.5 – 4.0 sec | 5 sec† | < 10 sec |
| Extreme Bradycardia | < 40 | Larger of 40 or (Low HR Limit – 20) | 5 sec† | < 10 sec |
| Extreme Tachycardia | > 140 | Smaller of 200 or (High HR Limit + 20) | 6 sec† | < 10 sec |
| VTach | Run of 5 or more Ventricular Beats with HR > 100 | 3 to 99 (Run) 15 – 300 (HR) | N/A | N/A |
| VTach/VFib | Fibrillatory waveform for 4 sec or more | N/A | 5 sec† | < 10 sec |
N/A = not applicable.
indicates that criterion meets AAMI-EC-13 Cardiotach Standard [29].
2.3. “Gold Standard” Alarms: Annotation & Adjudication
Since no large annotated dataset of alarms is publicly available, a new set of “gold standard” alarms was required to support the development and testing of false alarm rejection strategies. Patient records which met the required criteria (described above), were selected from the MIMIC II database, yielding 496 adult patient records with a total of 45,370 hours of simultaneous ECG & ABP waveforms containing 8,636 alarms.
Eleven volunteers were recruited to manually review the alarms. The volunteers consisted of two main groups; first, a group of experienced researchers (one physician with several decades of experience, and four signal processing experts, each with over a decade of experience analyzing such data), and second, a group of six graduate students, all with graduate level training in cardiac electrophysiology [28]. The dataset was carefully reviewed by two annotators working independently and in different locations. The reviewers were able to view all the ECG and ABP waveforms surrounding each alarm (with a controllable window size), using a standard open-source tool (‘WAVE’, available from PhysioNet.org [29]). The default view provided all available bedside monitor signals 30 seconds either side of the alarm. Reviewers could expand and shrink both the time and amplitude scales at their discretion to provide more detailed information or to add context to the alarm. The reviewers were instructed to mark each alarm as true, false, or ambiguous (if they were not completely certain). The reviewers’ annotations were recorded by the annotation software. The two passes were then digitally compared for each individual alarm. Two sets were produced: 1) a set of 6,402 matched alarms where both reviewers agreed on the state of the alarm as true or false, and 2) a set of 2,234 mismatched alarms, where the two reviewers either disagreed, or at least one of them was uncertain of the state of the alarm. The mismatched set was reviewed by one experienced physician or one experienced research engineer to provide a final adjudication. The entire matched set was also reexamined by a graduate student to ensure consistency, with any anomalies fed back to the research engineers or physician. During the adjudication process, any uncertainty was directed to the experienced physician for resolution. Throughout the iterative process, alarms without associated physiological waveforms (due to disconnections), and alarm repetitions referring to the same event, were removed. Furthermore, 49 patients who had active intra-aortic balloon pumps (IABP) were excluded, since their ABP waveforms did not appear as “physiologically normal”. The final “gold standard” alarm set comprised 5,386 alarms from 447 patients during a total of 41,301 hours. Hence, on average, there were approximately 3 critical ECG arrhythmia alarms per patient per day. Table 2 details the relative frequency of each alarm category and their associated true and false rates, as judged by the annotators.
Table 2.
Gold standard database of N=5386 critical ECG arrhythmia alarms: relative frequency of true and false alarms on a per-alarm basis. Average true alarm rate = 57.3%.
| Alarm Type | All Alarms | True Alarms | False Alarms | |||||
|---|---|---|---|---|---|---|---|---|
| Total Alarms | % of all alarms | N | % of all alarms that are true | % of specific alarm type that are true | N | % of all alarms that are false | % of specific alarm type that are false | |
| Asystole | 579 | 10.8% | 54 | 1.0% | 9.3% | 525 | 9.7% | 90.7% |
| Extreme Bradycardia | 717 | 13.3% | 507 | 9.4% | 70.7% | 210 | 3.9% | 29.3% |
| Extreme Tachycardia | 1877 | 34.8% | 1444 | 26.8% | 76.9% | 433 | 8.0% | 23.1% |
| VTach | 1900 | 35.3% | 1015 | 18.8% | 53.4% | 885 | 16.4% | 46.6% |
| VTach/VFib | 313 | 5.8% | 64 | 1.2% | 20.4% | 249 | 4.6% | 79.6% |
| All | 5386 | 3084 | 57.3% | 2302 | 42.7% | |||
2.4. Algorithm Architecture
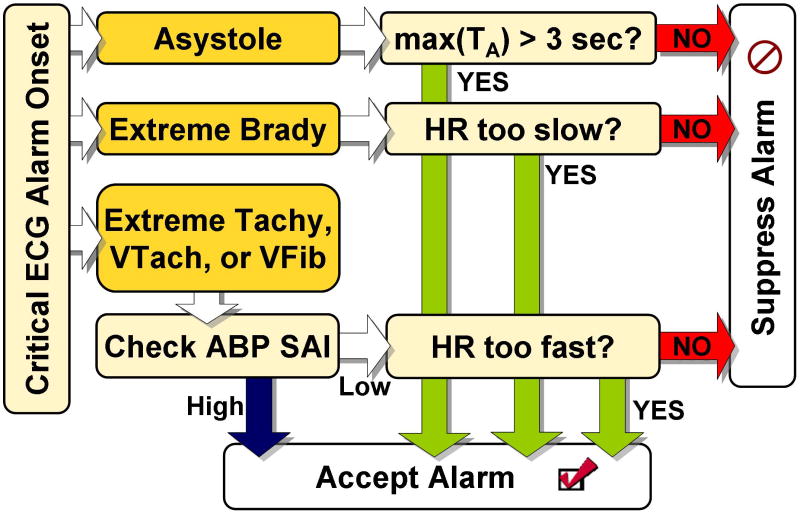
The algorithm described here was designed to be used as a post-processing module that could filter a bedside monitor’s critical alarm output in real-time. The logic flow, depicted in Figure 1, consisted of using evidence from the ABP waveform to accept or suppress an ECG-based alarm. At the onset of each critical ECG arrhythmia alarm, a 17-second ABP waveform segment was extracted, including 13 seconds prior to the alarm onset and 4 seconds after the alarm. The AAMI standards [27] require that asystole and rate-limit arrhythmia alarms must be triggered within 10 seconds of the onset of the event. Given that each alarm was triggered within 5 to 6 seconds of the onset of the event, an additional 4 second delay from the processing still satisfied the AAMI requirements.
Figure 1.
Flowchart outlining the major logical steps of the FA reduction algorithm.
After notification of each alarm, the algorithm first determined whether the signal quality of the ABP was high enough to enable a decision concerning the validity of the alarm to be made (except in the case of asystole or bradycardia, where the algorithm searched for the absence of beats). This filter used the signal abnormality index (SAI) of Sun et al. [30] and the beat detection algorithm of Zong et al [31, 32]. The SAI value (‘0’ for a good beat and ‘1’ for an abnormal beat) was calculated by comparing intervals, gradients and amplitudes of the blood pressure waveform to pre-defined thresholds. If more than a given number of beats, M, (which could be optimized differently for different alarm types) in the 17-second analysis window were considered abnormal, then the ABP signal was deemed unsuitable for further processing and by default, the arrhythmia alarm was accepted as true. If a sufficient number of beats were considered normal, each arrhythmia alarm was processed as detailed below.
2.4.1 Asystole Processing
An asystole alarm was issued by the bedside monitor if a beat-to-beat interval longer than TA seconds (the variable asystole pause interval) was found for the single lead being monitored. To decide on the truth of each asystole alarm the ABP waveform was used to compute first, the largest pulse-to-pulse interval within the analysis window (in case the asystole resolves itself within the window) and second, the last pulse-to-window end interval (i.e. the time interval between the last detected pulse onset and the end of the analysis window, in case the asystole was sustained beyond the end of the analysis window). If the larger of the two intervals was greater than TA, the asystole alarm was accepted; otherwise it was suppressed.
2.4.2 Extreme Bradycardia Processing
To determine the validity of an extreme bradycardia alarm, NB of the longest pulse-to-pulse intervals extracted from the ABP waveform in the analysis window were used to estimate the mean heart rate (by using the mean interval between consecutive high quality beats). If the mean HR was above the monitor’s HR threshold by at least EB BPM, the corresponding extreme bradycardia alarm was suppressed.
2.4.3 Extreme Tachycardia Processing
The mean HR was computed based on the NT shortest pulse-to-pulse intervals in the ABP waveform within the analysis window. There were three requirements for alarm suppression: 1) there must be less than or equal to MT abnormal ABP beat(s) (determined by the SAI algorithm), 2) the duration of the MT abnormal beat(s), if any exist, must be less than a total of TT seconds, and 3) the mean HR (calculated from NT beats) must be lower than ET BPM below the monitor’s adjusted threshold. The condition of permitting MT abnormal beat(s) lasting a total of less than TT seconds was designed to decrease the method’s sensitivity to spurious noise, and to allow suppression of alarms with a marginally abnormal ABP waveform. (In this context, marginal means at least one and less than six beats in the 17-second window were labeled as abnormal by the SAI algorithm.)
2.4.4 Ventricular Tachycardia
The mean HR was computed based on the NVT shortest pulse-to-pulse intervals in the ABP waveform within the analysis window. A VTach alarm was suppressed if both of the following conditions held: 1) the ABP waveform contained less than or equal to MVT abnormal beats as defined by the SAI algorithm, and 2) the mean HR (calculated over NVT beats) was below a variable threshold, RVT BPM.
2.4.5 Ventricular Fibrillation/Tachycardia
The mean HR was computed based on the NVF shortest pulse-to-pulse intervals in the ABP waveform within the analysis window. A ventricular fibrillation alarm was suppressed if both of the following conditions held: 1) the ABP waveform displayed abnormal behavior (as judged by the SAI algorithm) for less than TVF seconds and, 2) the mean HR (calculated over NVF beats) was below a variable threshold, RVF BPM.
2.5. Algorithm Development: Training and Test Data Sets
The data in this study were divided into a test set and a training set of roughly equal sizes. Optimization of each of the 13 parameters, (TA, EB, ET, NB, NT, NVT, NVF, TT, TVF, MT, MVT, RVT and RVF) described above (in section 2.4) was performed over the training set, between the limits listed in Table 3. The test set was used to estimate the algorithm’s performance on ‘unseen’ data. The alarms were distributed amongst the training and test groups on a per-patient basis, balancing them according to the frequency of arrhythmia alarms. The patient records were rank-ordered with respect to frequency of alarms and then divided into training (n=267) and test (n=180) groups.
Table 3.
Parameter ranges used in training.
| Parameter ▸
Alarm Type ▾ |
Maximum pulse-to-pulse length (s) | HR error margin (BPM) | Number of beats for computing HR | Duration of Bad Beats | Number of Abnormal Beats | Maximum HR (BPM) |
|---|---|---|---|---|---|---|
| Asystole | TA ={1…6} | N/A | N/A | N/A | N/A | N/A |
| Extreme Bradycardia | N/A | EB={0…20} | NB ={1…10} | N/A | N/A | N/A |
| Extreme Tachycardia | N/A | ET={0…20} | NT ={1…10} | TT ={0…6} | MT ={1…5} | N/A |
| VTach | N/A | N/A | NVT ={1…10} | N/A | MVT ={1…5} | RVT ={80..150} |
| VTach/VFib | N/A | N/A | NVF ={1…10} | TVF ={0…6} | N/A | RVF ={90..175} |
N/A = not applicable. All ranges span all integer values between the max and min values shown, except for maximum HR, which increments every 5 BPM.
The distribution of the 5,386 distinct critical ECG arrhythmia alarms for each group (and each alarm) is detailed in Table 4, together with their respective false alarm rates. As can be observed from the table, each group was roughly equally balanced for each type of alarm, although there were few true asystole and VTach/VFib alarms in each group. The imbalance between the FA rates in the training and test sets for the extreme bradycardia group indicated that the true and false alarm rates for extreme bradycardia (but not the other critical alarm types in this study) were highly subject specific, particularly with respect to the ratio of the true to false alarms.
Table 4.
Distribution of alarms in training, test, and combined sets.
| Training (n=267) | Test (n=180) | Combined training and test sets (n=447) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarm Type | FALSE | TRUE | TOTAL | FA RATE (%) | FALSE | TRUE | TOTAL | FA RATE (%) | FALSE | TRUE | TOTAL | FA RATE (%) |
| Asystole | 281 | 35 | 316 | 88.9 | 244 | 19 | 263 | 92.8 | 525 | 54 | 579 | 90.7 |
| Brady | 143 | 207 | 350 | 40.9 | 67 | 300 | 367 | 18.3 | 210 | 507 | 717 | 29.3 |
| Tachy | 256 | 816 | 1072 | 23.9 | 177 | 628 | 805 | 22.0 | 433 | 1444 | 1877 | 23.1 |
| VTach | 484 | 517 | 1001 | 48.4 | 401 | 498 | 899 | 44.6 | 885 | 1015 | 1900 | 46.6 |
| VT/VF | 137 | 39 | 176 | 77.8 | 112 | 25 | 137 | 81.8 | 249 | 64 | 313 | 79.6 |
| Total alarms | 1301 | 1614 | 2915 | 44.6 | 1001 | 1470 | 2471 | 40.5 | 2302 | 3084 | 5386 | 42.7 |
2.6 Algorithm Optimization
Due to the low number of algorithm parameters required for processing each alarm type and the relatively small search-space required, complex optimization schemes (such as gradient descent or Newton-based methods) were not required. Furthermore, in some cases there were large areas of optimality where either the extremes or the centroids of the parameter domains were appropriate. Since an asymmetric optimization of several parameters using two cost functions sequentially was required, (the minimum TA suppression rate, and then the maximum FA suppression rate), a slightly unconventional approach was employed. Generally, for the optimization of one parameter, a receiver-operator curve (FA suppression rate versus one minus the TA suppression rate) is plotted. However, such an approach would assume that a trade-off between FA and TA rates is acceptable. For critical ECG arrhythmia alarms, non-zero TA suppression rates are unacceptable. Therefore, the search was restricted to parameter values that resulted in the lowest TA suppression rate. Figure 2 illustrates this approach. As the parameter TA (the minimum length of the asystole) was increased from 1 to 3 seconds, the FA suppression rate increased steadily to 92.5%. For TA > 3s, the FA suppression rate continues to rise, but with a rapidly increasing suppression rate of true asystoles; an unacceptable scenario. In this case the largest value of TA (3 sec.) that gave a FA suppression rate of zero was chosen.
Figure 2.
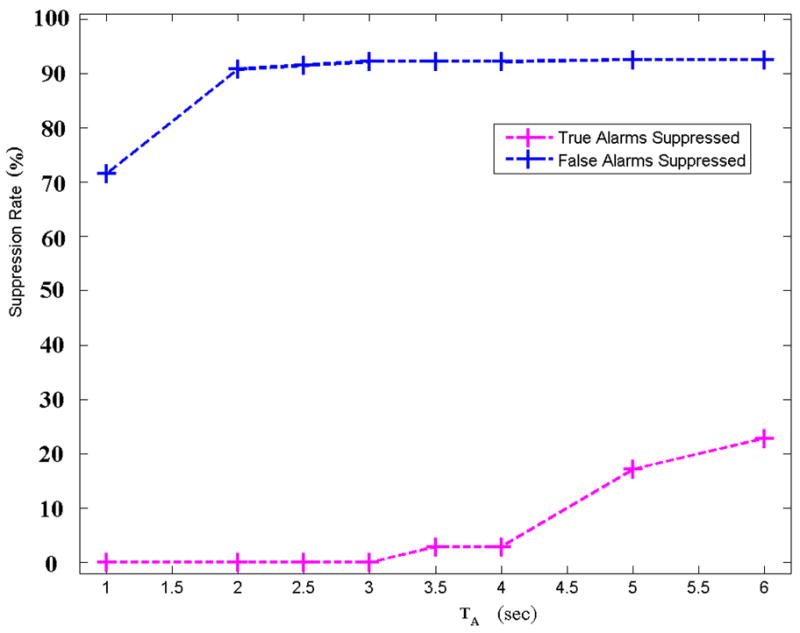
False and true alarm rate for asystole as a function of the single variable, TA (the minimum length of the asystole). Note that TA indicates an ABP pulse-to-pulse interval and that the true alarm suppression rate is zero for values of the threshold TA ≤3s.
Optimal parameter threshold values were determined by simply repeating the FA suppression algorithm over all possible combinations of relevant parameter values (within the ranges detailed in Table 3). Parameter values that provided the minimal TA suppression rate were noted for each alarm type. After identifying a subset of parameter values yielding a minimal TA suppression rate, the parameter values giving the maximal FA suppression rate were ultimately chosen. Figure 3 illustrates extreme bradycardia parameter optimization, a two-dimensional (two-parameter) problem and the second least complicated scenario for the five alarm categories. In this case the objective was to optimize the same two cost functions (minimum TA and maximum FA suppression rates), but for two variables; EB (the maximum negative error allowed between the HR calculated by the bedside monitor and the ABP waveform-derived HR) and NB (the number of beats used to calculate the HR). FA suppression rates are marked by circles and TA suppression rates are marked by squares. Additionally, points where the TA suppression rate is zero are marked by stars.
Figure 3.
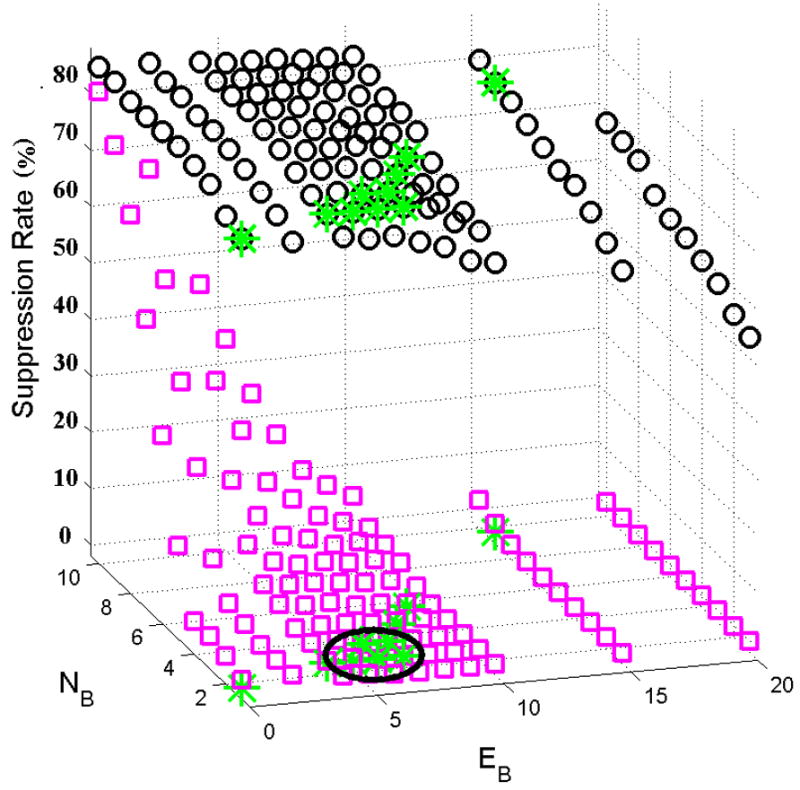
False (O) and true (□) alarm suppression rate for extreme bradycardia as a function of the two variables, EB (the maximum negative error allowed between the HR calculated by the bedside monitor and the ABP-derived HR) and NB (the number of beats used to calculate the HR). Points where the TA suppression rate is zero are marked by stars. The circled area is the parameter subset where the FA suppression rate is largest given a TA suppression rate of zero, and where small changes in the parameters will not change the performance substantially.
Similar approaches were taken for the other arrhythmia alarm categories, although their dimensionality was much higher and therefore not amenable to graphical illustration. After determining the optimal values for each parameter using the training data, the algorithm was applied to the test data.
3. Results
3.1. Human Annotation: Critical ECG Alarm Distribution in the ICU
For the 447 patients studied, there were 5,380 ECG life-threatening alarms, representing approximately 4% to 8% of all the ECG alarms in our database. As can be seen in Table 2, extreme tachycardia and VTach were the most frequent critical ECG arrhythmia alarms, totaling respectively 34.8% and 35.3% of all alarms. Extreme bradycardia and asystole were approximately one third as frequent, comprising respectively 13.3% and 10.8% of all alarms. The alarms caused by ventricular fibrillation were the least frequent, comprising only 5.8% of the total critical ECG arrhythmia alarms.
The 579 asystole alarms were almost as frequent as the 717 extreme bradycardia alarms. However, the asystole alarms had the highest FA rate (90.7%), while the bradycardia FA rate was relatively low (29.3%). We found that only 54 of the asystole alarms were true (1.0% of all critical alarms in this study) compared to 507 true extreme bradycardia alarms (9.4% of all the alarms). Simple rate-related alarms (extreme bradycardia and extreme tachycardia) were the most accurate (with TA rates of 70.7% and 76.9%). However, true extreme bradycardia events were almost three times less frequent than true extreme tachycardia (9.4% versus 26.8%). Extreme tachycardia and VTach comprised 70.1% of the overall arrhythmia alarms, and 45.6% of the true arrhythmia alarms. VTach/VFib was by far the least frequent overall arrhythmia alarm, (only 5.8% of the alarms in the dataset) but had a high associated FA rate (79.6%). The true VTach/VFib alarms were therefore almost as infrequent (1.2% of all alarms in the dataset) as true asystole alarms. The overall FA rate for the data used in this study was found to be 42.7%, with asystole, extreme tachycardia and VTach being the major contributors to the FA rate.
3.2. Algorithm optimization
Table 5 lists the optimal parameter values found during algorithm training. In some cases, a broad range of parameters was possible, in which case either the center of the parameter ranges, or the most logical upper or lower limits were chosen. (For example, when computing HR it is logical to use as many beats as possible, within the optimal range, to obtain the best estimate. When choosing the threshold for bradycardia, it is logical to choose the optimal value that most closely maps to the clinical threshold for bradycardia.)
Table 5.
Optimal parameters found during training.
| Parameter ▸
Alarm Type ▾ |
Maximum pulse-to-pulse length (s) | HR error margin (BPM) | Number of intervals for computing HR | Duration of Bad Beats (s) | Number of Abnormal Beats Allowed | Maximum HR (BPM) |
|---|---|---|---|---|---|---|
| Asystole | TA = 3 | N/A | N/A | N/A | N/A | N/A |
| Extreme Bradycardia | N/A | EB = 7 | NB = 3 | N/A | N/A | N/A |
| Extreme Tachycardia | N/A | ET = 20 | NT = 1 | TT = 4 | MT = 5 | N/A |
| VTach | N/A | N/A | NVT =1 | N/A | MVT = 0 | RVT = 80 |
| VTach/VFib | N/A | N/A | NVF = 7 | TVF =2 | N/A | RVF = 150 |
N/A = not applicable.
Figure 2 illustrates that an optimal value of TA=3 sec gave a FA suppression rate of zero for asystole alarms. Figure 3 illustrates the more complicated scenario for bradycardia FA suppression optimization, where two parameters (EB and NB) can be varied. The circled area is the subset of points where the FA suppression rate is largest given a TA suppression rate of zero, and where small changes in parameter values do not change the performance substantially. An 80% FA suppression rate is possible (with a zero TA suppression rate) for a non-unique set of values for EB and NB. In this case the center of the region is used, giving EB=6 and NB=3. Note that decreasing EB or decreasing NB can improve the FA suppression rate by at most 2% (to 82%) but at the great cost of elevating the TA suppression rate from 0% to as high as 80%.
3.3. Algorithm performance
Surprisingly, the performance of the arrhythmia alarm suppression algorithm was better on the test set than on the training set, and hence a slight asymmetry in the quality of the signals must exist between the test and training sets. Normally, we would have expected a better performance on the training set, and would have reported the (generally poorer results) on the test set. Since in this case the test set essentially inflates the performance of the algorithm we were testing, we decided to report also the lower performance statistics provided by averaging the results from both the training and testing sets.
Table 6 details the FA and TA suppression performance of the algorithm on the training and test sets, and also on the combined training and test sets. FA suppression rates for the combined set ranged between 58.2% and 93.5% for all arrhythmia alarm types except for VTach. The last two columns of Table 6 provide the FA rates before and after suppression for the combined set. The asystole FA rates were reduced from 90.7% to 5.5%. Extreme bradycardia and tachycardia FA rates were reduced from 29.3% and 23.1% to 5.5% and 8.4% respectively. VTach/VFib FA rates were reduced from 79.6% to 33.1%. The false VTach alarm suppression rate was the lowest of all alarm categories tested, with a reduction in the FA rate from 46.6% to 30.8%, at the cost of suppressing 9.4% of the true VTach alarms (14.5% in the training data and 4.0% in the test data). No true alarms were suppressed for any other critical alarm group in this study. The overall FA rate was reduced from 42.7% to 17.2%.
Table 6.
False and true alarm suppression results with resultant average FA alarm rates.
| TRAINING SET (n=267) | TEST SET (n=180) | COMBINED TRAINING AND TEST SETS (n=447) | ||||||
|---|---|---|---|---|---|---|---|---|
| Alarm Type | Suppression Rates | Suppression Rates | FA Rates | |||||
| FA | TA | FA | TA | FA | TA | Before suppression | After suppression | |
| Asystole | 92.5% | 0.0% | 95.0% | 0.0% | 93.5% | 0.0% | 90.7% | 5.5% |
| Brady | 79.7% | 0.0% | 83.6% | 0.0% | 81.0% | 0.0% | 29.3% | 5.5% |
| Tachy | 59.4% | 0.0% | 70.1% | 0.0% | 63.7% | 0.0% | 23.1% | 8.4% |
| VTach | 28.3% | 14.5% | 38.7% | 4.0% | 33.0% | 9.4% | 46.6% | 30.8% |
| VT/VF | 57.7% | 0.0% | 58.9% | 0.0% | 58.2% | 0.0% | 79.6% | 33.1% |
| ALL | 57.0% | 4.7% | 63.2% | 1.4% | 59.7% | 2.4% | 42.7% | 17.2% |
4. Discussion
In the present study, for patients with invasive ABP monitoring, false critical ECG arrhythmia alarm rates in the ICU were found to be, on average, 42.7%, with individual rates varying between 23.1% and 90.7%. The literature reports FA rates in ICU data (for both “red” and “yellow” conditions) between 40% and 90% [16–21]; results which are consistent with those presented in this study. The false asystole alarm rates (and FA rates for all the critical ECG arrhythmia alarms) in our data may have been higher than they needed to be. Firstly, the critical care units from which these data were recorded chose to standardize arrhythmia analysis on only one selected lead of ECG even though the monitors were capable of using multilead arrhythmia analysis. Hence the arrhythmia alarms included in the MIMIC-II database do not reflect the optimal performance of the vendor’s arrhythmia algorithms. In addition, most false asystole alarms were caused by low amplitude QRS complexes in the ECG (less than 150 microvolts), which could not be reported as valid beats according to the current ANSI/AAMI EC13 Cardiac Monitor Standards [27].
The false alarm suppression strategy explored in this study proved remarkably effective at suppressing false arrhythmia alarms in ICU data, reducing the average FA rate from 42.7% to 17.2%. The algorithm was particularly successful in reducing FA rates for asystole, extreme bradycardia, and extreme tachycardia, with zero suppression of true alarms. The algorithm achieved more moderate reductions in FA rates for VTach and VTach/VFib, and only at the expense of suppressing 9.4% of true VTach alarms. To avoid suppressing any true alarms, the algorithm could be implemented for all alarms except VTach. In this case, the average FA rate would be reduced from 42.7% to 22.7%.
The algorithm’s requirement for simultaneous ECG and ABP signals is a condition that is not always satisfied in the ICU since not all patients require invasive ABP monitoring. Only 63.8% of patients in the MIMIC II database had invasive ABP monitoring during part of their ICU stay, and hence the algorithm described in this paper will not affect FA rates for the other 36.2% of patients. Furthermore, it is likely that the FA rates in patients not requiring ABP monitoring are higher, reflecting their more active behavior.
Future work will focus on extracting information from the ECG and other pulsatile waveforms (such as the pulse oximeter and pulmonary arterial pressure) to improve the FA reduction rate on a broader patient population. Additionally, information from multiple leads of ECG is required to reduce the number of suppressed true VTach alarms to a negligible amount, and increase the number of false VTach alarms one can suppress. Such an approach is likely to require a combination of signal quality indices [33] and additional signal processing methods applied to the ECG and other cardiovascular signals. Other improvements should include a method for automatically identifying intra-aortic balloon pumps, and developing signal quality indices for the pulse oximeter waveform (to allow the incorporation of this signal into this FA suppression framework).
5. Conclusions
The study described in this paper demonstrated that a FA suppression algorithm that used only one extra channel of non-ECG information (the ABP waveform) and some simple logic allowed for the identification and suppression of the majority of false critical ECG arrhythmia alarms. The algorithm demonstrated the potential of using multiple physiologic waveforms for reducing false alarms in the clinical setting. An extension of the algorithm could be applied to physiological monitoring in a general sense (to other signals, other alarms categories, and in other settings, such as the operating room) and would only be limited by the number of related cardiovascular signals and their respective signal qualities. Specific extensions to the algorithm should include a fusion of data from multiple ECG leads and from other pulsatile waveforms, such as that derived from the pulse oximeter. The analysis of the pulse oximeter waveform is important for the subset of patients that are not being monitored with an invasive ABP line. The analysis of multiple ECG leads will be of particular use in dealing with the “yellow” second-level alarms.
The demonstrated improvement in alarm performance described in this study should motivate monitoring vendors to process multiple physiologic waveforms within their own alarm algorithm architectures. In fact, the present algorithm should be deployed in a controlled small scale clinical study to assess its impact on reducing false critical arrhythmia alarms in the ICU. To avoid suppressing any true positive alarms the algorithm could be run for all alarm types except for VTach, where the suppression strategy needs further refinement.
Since the annotated database used in this work is publicly available [25], it is hoped that other research groups and device manufacturers will improve both on the algorithms described here, and on the quality and quantity of the annotated data (such as a subset of the “yellow alarms”). It would be useful to identify possible errors (or points of contention) in the annotations, and to identify arrhythmic events that were missed by the original monitors. It is likely, given previous studies [21], that around 200 to 300 such false negative events are hidden within the data used here. If current device manufacturers run their arrhythmia algorithms on this data, some of the missing events may be identified. Ultimately, collaborative efforts are needed to develop new multi-parameter annotated databases that can serve as “gold-standards” to support the development and evaluation of novel monitoring algorithms and to provide high quality metrics for regulatory bodies.
Acknowledgments
This work was performed as part of a Bioengineering Research Partnership funded by the U.S. National Institute of Biomedical Imaging and Bioengineering (NIBIB) and the National Institutes of Health (NIH) under Grant Number R01-EB001659, and also in part by Philips Medical Systems. Anton Aboukhalil was also partially supported by a postgraduate scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC). The content of this document is solely the responsibility of the authors and does not necessarily represent the official views of the NIBIB, the NIH, Philips Medical Systems or the NSERC. The authors thank the numerous database developers and annotators who contributed to the construction of the alarms database, including Omar Abdala, Thomas Heldt, Caleb Hug, Sherman Jia, Tin Htet Kyaw, Li-Wei Lehman, Benjamin Moody, George Moody, Greg Raber, Andrew Reisner, Ali Saeed, Dewang Shavdia, Mauricio Villarroel, Ying Zhang and Wei Zong. The authors also thank the anonymous reviewers for their helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chambrin MC. Review: Alarms in the intensive care unit: how can the number of false alarms be reduced? Critical Care. 2001 Aug;5(4):184–8. doi: 10.1186/cc1021. Epub 2001 May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donchin Y, Seagull FJ. The hostile environment of the intensive care unit. Curr Opin Crit Care. 2002 Aug;8(4):316–20. doi: 10.1097/00075198-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Imhoff M, Kuhls S. Alarm algorithms in critical care monitoring. Anesth Analg. 2006 May;102(5):1525–37. doi: 10.1213/01.ane.0000204385.01983.61. [DOI] [PubMed] [Google Scholar]
- 4.Meyer TJ, Eveloff SE, Bauer MS, Schwartz WA, Hill NS, Millman RP. Adverse environmental conditions in the respiratory and medical ICU settings. Chest. 1994 Apr;105(4):1211–16. doi: 10.1378/chest.105.4.1211. [DOI] [PubMed] [Google Scholar]
- 5.Parthasarathy S, Tobin MJ. Sleep in the intensive care unit. Intensive Care Med. 2004 Feb;30(2):197–206. doi: 10.1007/s00134-003-2030-6. [DOI] [PubMed] [Google Scholar]
- 6.Johnson AN. Neonatal response to control of noise inside the incubator. Pediatr Nurs. 2001 Nov-Dec;27(6):600–5. [PubMed] [Google Scholar]
- 7.Slevin M, Farrington N, Duffy G, Daly L, Murphy JF. Altering the NICU and measuring infants’ responses. Acta Paediatr. 2000 May;89(5):577–81. doi: 10.1080/080352500750027899. [DOI] [PubMed] [Google Scholar]
- 8.Zahr LK, de Traversay J. Premature infant responses to noise reduction by earmuffs: effects on behavioral and physiologic measures. J of Perinatol. 1995 Nov-Dec;15(6):448–55. [PubMed] [Google Scholar]
- 9.Baker CF. Discomfort to environmental noise: heart rate responses of SICU patients. Crit Care Nurs Q. 1992 Aug;15(2):75–90. doi: 10.1097/00002727-199208000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Cropp AJ, Woods LA, Raney D, Bredle DL. Name that tone. The proliferation of alarms in the intensive care unit. Chest. 1994 Apr;105(4):1217–20. doi: 10.1378/chest.105.4.1217. [DOI] [PubMed] [Google Scholar]
- 11.Novaes MA, Aronovich A, Ferraz MB, Knobel E. Stressors in ICU: patients’ evaluation. Intensive Care Med. 1997 Dec;23(12):1282–5. doi: 10.1007/s001340050500. [DOI] [PubMed] [Google Scholar]
- 12.Topf M, Thompson S. Interactive relationships between hospital patients’ noise induced stress and other stress with sleep. Heart Lung. 2001 Jul-Aug;30(4):237–43. doi: 10.1067/mhl.2001.116592. [DOI] [PubMed] [Google Scholar]
- 13.Morrison WE, Haas EC, Shaffner DH, Garrett ES, Fackler JC. Noise, stress, and annoyance in a pediatric intensive care unit. Crit Care Med. 2003 Jan;31(1):113–9. doi: 10.1097/00003246-200301000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Berg S. Impact of reduced reverberation time on sound-induced arousals during sleep. Sleep. 2001 May 1;24(3):289–92. doi: 10.1093/sleep/24.3.289. [DOI] [PubMed] [Google Scholar]
- 15.Hagerman I, Rasmanis G, Blomkvist V, Ulrich R, Eriksen CA, Theorell T. Influence of intensive coronary care acoustics on the quality of care and physiological state of patients. Int J Cardiol. 2005 Feb 15;98(2):267–70. doi: 10.1016/j.ijcard.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Lawless ST. Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994 Jun;22(6):981–5. [PubMed] [Google Scholar]
- 17.Tsien CL, Fackler JC. Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997 Apr;25(4):614–9. doi: 10.1097/00003246-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Mäkivirta A, Koski E, Kari A, Sukuvaara T. The median filter as a preprocessor for a patient monitor limit alarm system in intensive care. Comput Methods Programs Biomed. 1991 Feb-Mar;34(2–3):139–44. doi: 10.1016/0169-2607(91)90039-v. [DOI] [PubMed] [Google Scholar]
- 19.Sittig DF, Factor M. Physiologic trend detection and artifact rejection: a parallel implementation of a multi-state Kalman filtering algorithm. Comput Methods Programs Biomed. 1990 Jan;31(1):1–10. doi: 10.1016/0169-2607(90)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Koski EM, Sukuvaara T, Mäkivirta A, Kari A. A knowledge-based alarm system for monitoring cardiac operated patients-assessment of clinical performance. Int J Clin Monit Comput. 1994 May;11(2):79–83. doi: 10.1007/BF01259556. [DOI] [PubMed] [Google Scholar]
- 21.Schapira RM, Van Ruiswyk J. Reduction in alarm frequency with a fusion algorithm for processing monitor signals. Meeting of the American Thoracic Society. 2002 Session A56, Poster H57. Available online at: http://www.abstracts2view.com/atsall/
- 22.Clifford GD, Aboukhalil A, Sun JX, Zong W, Janz BA, Moody GB, Mark RG. Using the blood pressure waveform to reduce critical false ECG alarms. Comput Cardiol. 2006;33:829–32. [Google Scholar]
- 23.Saeed M, Lieu C, Raber G, Mark RG. MIMIC II: a massive temporal ICU patient database to support research in intelligent patient monitoring. Comput Cardiol. 2002;29:641–4. [PubMed] [Google Scholar]
- 24.Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000 Jun 13;101(23):e215–20. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 25.The MIMIC II Database. Available online at: http://www.physionet.org/physiobank/database/mimic2db/
- 26.Jalaleddine SMS, Hutchens CG, Strattan RD, Coberly WA. ECG data compression techniques – a unified approach. IEEE Trans Biomed Eng Apr. 1990;37(4):329–43. doi: 10.1109/10.52340. [DOI] [PubMed] [Google Scholar]
- 27.American National Standard (ANSI/AAMI EC13:2002) Cardiac monitors, heart rate meters, and alarms. Arlington, VA: Association for the Advancement of Medical Instrumentation; 2002. [Google Scholar]
- 28.Mark RG. HST.542J/2.792J/20.371J/6.022J Quantitative Physiology: Organ Transport Systems. Open CourseWare, M.I.T.; Spring. 2004. Available online at: http://ocw.mit.edu/OcwWeb/Health-Sciences-and-Technology/HST-542JSpring-2004/CourseHome/index.htm. [Google Scholar]
- 29.Moody GB. WAVE User’s Guide. (5) Available online at: http://physionet.org/physiotools/wug/
- 30.Sun JX, Reisner AT, Mark RG. A signal abnormality index for arterial blood pressure waveforms. Comput Cardiol. 2006;33:13–6. [Google Scholar]
- 31.Zong W, Heldt T, Moody GB, Mark RG. An open-source algorithm to detect onset of arterial blood pressure pulses. Comput Cardiol. 2003;30:259–62. [Google Scholar]
- 32.Zong W, Moody GB, Mark RG. Reduction of false arterial blood pressure alarms using signal quality assessment and relationships between the electrocardiogram and arterial blood pressure. Med Biol Eng Comput. 2004 Sep;42(5):698–706. doi: 10.1007/BF02347553. [DOI] [PubMed] [Google Scholar]
- 33.Li Q, Mark RG, Clifford GD. Robust heart rate estimate fusion using signal quality indices and a Kalman filter IOP. Physiol Meas. 2008 Jan;29:15–32. doi: 10.1088/0967-3334/29/1/002. [DOI] [PMC free article] [PubMed] [Google Scholar]