Abstract
The first total syntheses of piericidin A1 and B1 are disclosed and unambigously establish the relative and absolute stereochemistry of the natural products by an approach that will facilitate the synthesis of a series of analogues. Central to the approach is an inverse electron demand Diels–Alder reaction of a N-sulfonyl-1-aza-1,3-butadiene with tetramethoxyethene followed by Lewis acid-promoted aromatization used to assemble the functionalized pyridine core. Additional key elements in the convergent approach include the use of an anti-aldol reaction to install the C9 and C10 relative and absolute stereochemistry, a modified Julia olefination for formation of the C5–C6 trans double bond with convergent assemblage of the side chain, and a penultimate heterobenzylic Stille cross-coupling reaction of the pyridyl core with the fully elaborated side chain.
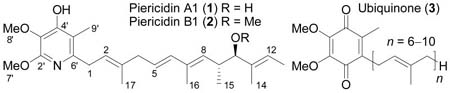
The piericidins are an important class of biologically active natural products isolated from Streptomyces mobaraensis and pactam of which piericidin A1 (1) is the prototypical member.1 Piericidin A1 is one of the most potent inhibitors of the mitochondrial electron transport chain protein NADH-ubiquinone reductase (complex I, Ki = 0.6–1.0 nM) defined to date, and has contributed extensively to the elucidation of the enzyme properties.1 It has been suggested that the 4-hydroxypyridine of 1 (as the pyridone tautomer) mimics the quinone of ubquinone (coenzyme Q, 3) with the side chain of 1 mimicking the prenyl chain and this apparent structural relationship was confirmed through their competitive binding against complex I.2–4
Despite an array of additional important biological activity, no total synthesis of a member of this now large class of natural products has been disclosed. In efforts directed at this family, the preparation of the fully elaborated pyridine ring system substituted with simplified side chains has been reported,5 as well as an asymmetric synthesis of the C6–C13 segment of the side chain bearing the most recent stereochemical assignment (9R,10R).6 Notably, the originally assigned absolute stereochemistry7 of the side chain substituents of 1 has been challenged, reassigned, and remained to be determined.8,9
Herein we detail the first total synthesis of piericidin A1 and B1 by an approach that establishes the absolute stereochemistry of the natural products and will facilitate the synthesis of a series of key analogues. Central to the approach is an inverse electron demand Diels–Alder reaction between a N-sulfonyl-1-aza-1,3-butadiene10 and tetramethoxyethene11 followed by Lewis acid-promoted aromatization used to assemble the functionalized pyridine core.12 Additional key elements in the convergent approach include the use of an asymmetric anti-aldol reaction to install the C9 and C10 relative and absolute stereochemistry, a modified Julia olefination for formation of the C5–C6 trans double bond with convergent assemblage of the side chain, and a penultimate heterobenzylic Stille cross-coupling reaction of the pyridyl core with the fully elaborated side chain.
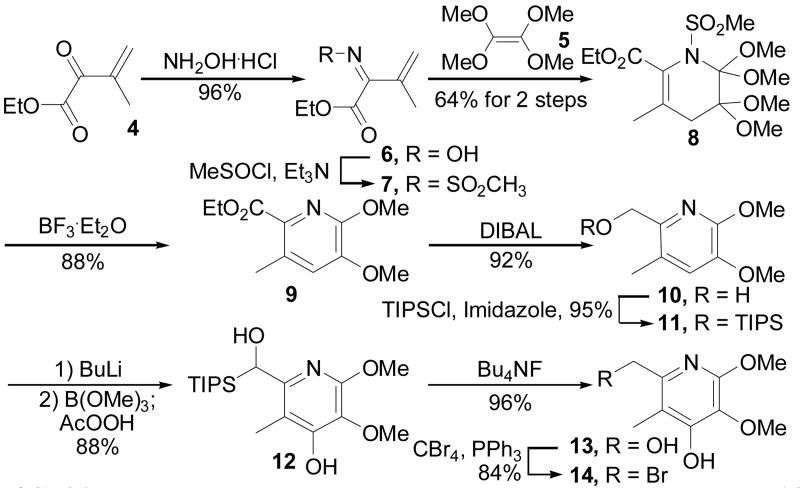
The key N-sulfonyl-1-aza-1,3-butadiene 7 was accessed through a two step sequence initiated by oxime formation of the ketone 413 (NH2OH–HCl, EtOH, 25 °C, 18 h, 96%), Scheme 1. Treatment of the oxime 6 with Et3N and methylsulfinyl chloride led to formation of the O-methanesulfinate and in situ homolytic rearrangement to give sulfonylimine 7 (CH3SOCl, Et3N, CH2Cl2, 0 °C, 20 min).14 Treatment of 7 with tetramethoxyethene11 (5, 18 h, toluene, 50 °C, 64% for 2 steps from 6) smoothly afforded the [4 + 2] cycloadduct 8. Characteristic of the unusual reactivity of such electron-deficient N-sulfonylazadienes substituted with an additional C2 electron-withdrawing substituent,12a,b the Diels–Alder reaction of 7 with the electron-rich dienophile 5 was found to proceed at or near room temperature, even though 5 is tetrasubstituted and sterically demanding. Efforts to induce aromatization of 8 under a variety of basic conditions were not successful, whereas the use of the Lewis acid BF3·OEt2 cleanly affected this transformation (CH2Cl2, 0 °C, 1 h, 88%). Reduction of the ethyl ester 9 with DIBAL (CH2Cl2, 0 °C, 1 h, 92%) followed by protection of the resulting alcohol 10 as its TIPS ether provided 11 (TIPSCl, imidazole, DMF, 25 °C, 18 h, 95%). It was envisioned that directed lithiation of the remaining site on the pyridine ring followed by a borylation/oxidation sequence would permit incorporation of the remaining oxygen substituent at C4′.15 Thus, treatment of 11 with excess base followed by trimethylborate (5 equiv of BuLi, 6 equiv of B(OMe)3, −78 °C, 1 h, 88%), and oxidative cleavage of the resulting aryl boronate ester unexpectedly provided the C-silyated species 12 resulting from both the desired C4′ hydroxylation and a competitive reverse Brook rearrangement of the benzylic TIPS ether under the strongly basic conditions.16 The use of fewer equivalents of base results in the migrated, non-oxidized product, and the use of alternative silyl ether protecting groups (TBS and TBPDS) or the free alcohol 10 itself resulted in lower yields of the diol 13. The deprotection of 12 proceeded cleanly and, interestingly, through the O-silyated intermediate S116 (Bu4NF, THF, 30 min, 25 °C, 80%) resulting from a Brook rearrangement, to give 13 (Bu4NF, THF, 36 h, 25 °C, 96% overall). Conversion of 13 to the heterobenzylic bromide 14 (CBr4, PPh3, CH2Cl2, 25 °C, 84%) provided a surprisingly stable partner for the Stille cross-coupling.
Scheme 1.
Synthesis of the Pyridine Core
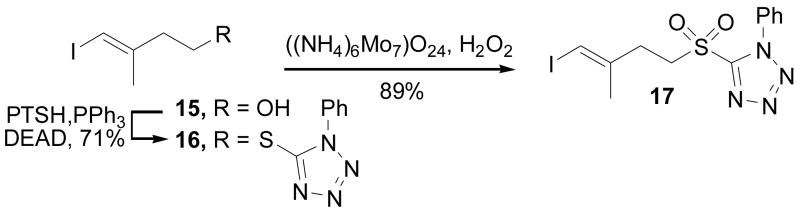
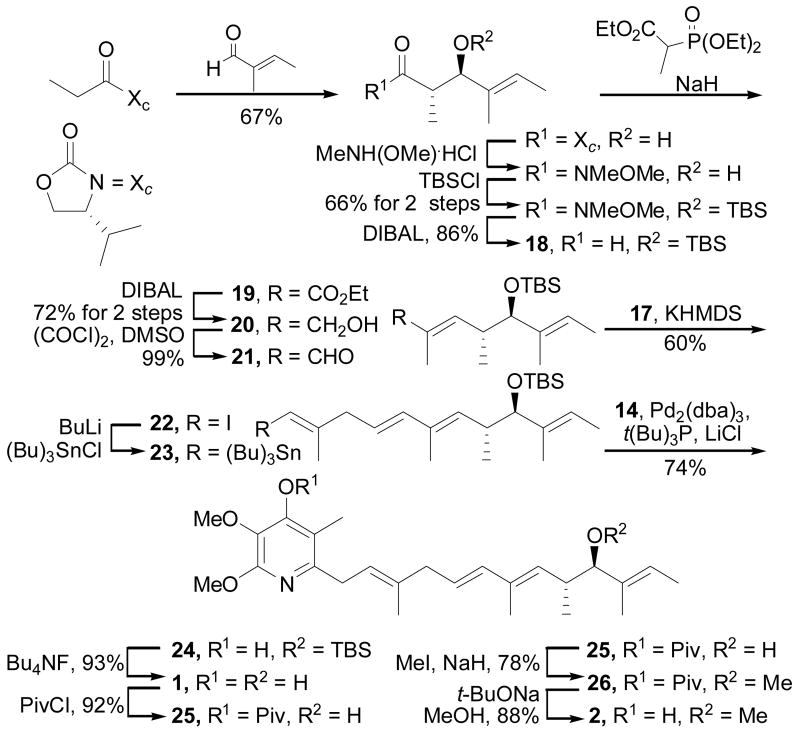
Synthesis of the side chain C2–C5 segment consisted of the preparation of the vinyl iodide 17 appropriately substituted for Julia–Kocienski olefination17 coupling with the C6–C13 side chain aldehyde 21, accessible from the known alcohol 20.6 This was accomplished through the reaction of alcohol 1518 with 1-phenyl-1H-tetrazole-5-thiol (PTSH) under Mitsunobu conditions (DEAD, PPh3, THF, 0 °C, 30 min, 71%) to provide thioether 16, which was cleanly oxidized to the sulfone 17 with ammonium molybdate (H2O2, EtOH–THF, 25 °C, 6 h, 89%), Scheme 2.19 In order to address limitations in the reported synthesis of 18 (diastereomer separation),6 a recently disclosed and more effective asymmetric anti-aldol reaction was employed to access aldehyde 18,20 which was converted to alcohol 20 as described6 and oxidized under Swern conditions to give 21 ((COCl)2, DMSO, −78 °C, 99%), Scheme 3. A modified Julia coupling between 17 and 21 cleanly provided 22 (KHMDS, DME, −78 °C, 18 h, 60%) with the trans alkene isomer as the only detected product. Lithium-halogen exchange of the vinyl iodide 22 upon treatment with n-BuLi, followed by treatment of the vinyllithium with tribuyltin chloride provided the vinyl stannane 23 for the Stille coupling with 14.
Scheme 2.
Synthesis of 17
Scheme 3.
Synthesis of Piericidin A1 and B1
Coupling of 14 with 23 was found to require elevated temperatures, high loadings of the Pd2(dba)3/(tBu)3P catalyst system employed by Fu,21 and LiCl as an additive to achieve good conversions, and when applied to the coupling provided 24 in superb conversions (Pd2(dba)3, (tBu)3P, LiCl, dioxane, 70 °C, 18 h, 74%) without protection of the pyridyl phenol. A final deprotection of the TBS ether 24 (Bu4NF, THF, 50 °C, 12 h, 93%) provided piericidin A1 identical in all respects with properties reported for the natural product (Scheme 3). This included the sign and magnitude of the reported optical rotation for 1 ([α]D25 +1.8 (c 0.1, MeOH) vs lit22 [α]D25 +1.0 (c 0.1, MeOH)). However, the magnitude of this rotation value was viewed as insufficient to assign the absolute configuration of 1. In order to more confidently address this assignment, the conversion of 1 of piericidin B1 (2), which exhibits a larger optical rotation value, was also conducted.23 Thus, selective protection of the pyridine hydroxyl of 1 as its pivolate ester 25 (PivCl, HSO4NBu4, aqueous 5 N NaOH–CH2Cl2, 25 °C, 92%), followed by O-methylation of the secondary alcohol 25 (NaH, MeI, DMF, 25 °C, 1 h, 78%), and finally pivolate hydrolysis of 26 (t-BuONa, MeOH, 60 °C, 3 h, 88%) provided 2. Synthetic piericidin B1 proved identical in all respects with properties reported for natural 2, including its optical rotation ([α]D25 –7.3 (c 0.2, MeOH) vs lit23 [α]D18 –6.5 (c 3.2, MeOH)), thereby further confirming the absolute configuration assignment for 1 and 2.
Throughout these studies and the handling of 1, 2, 12, 13, 14, and 24, only the 4-hydroxypyridine tautomer, and not the 4-pyridone tautomer, was observed even in protic solvents. Provocatively, this suggests that the ability of 1 to bind and inhibit NADH-ubiquinone reductase (complex I) may result from mimicry of reduced coenzyme Q (hydroquinone) and rather than 3 itself. The synthesis of a series of analogues based on the natural products are underway and will probe such questions.
Supplementary Material
Supporting Information Available: Full experimental details are provided in the supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We gratefully acknowledge the financial support of the National Institutes of Health (CA 42056) and the Skaggs Institute for Chemical Biology. MJS is a Skaggs Fellow.
References
- 1.Takahashi N, Suzuki A, Tamura S. J Am Chem Soc. 1965;87:2066. doi: 10.1021/ja01087a050. [DOI] [PubMed] [Google Scholar]
- 2.Esposti MD. Biochim Biophys Acta. 1998;1364:222. doi: 10.1016/s0005-2728(98)00029-2. [DOI] [PubMed] [Google Scholar]
- 3.Friedrich T, Van Heek P, Leif H, Ohnishi T, Forche E, Kunze B, Jansen R, Trowitzsch-Kienast W, Hofle G, Reichenbach H, Weiss H. Eur J Biochem. 1994;219:691. doi: 10.1111/j.1432-1033.1994.tb19985.x. [DOI] [PubMed] [Google Scholar]
- 4.Okun JG, Lummen P, Brandt U. J Biol Chem. 1999;274:2625. doi: 10.1074/jbc.274.5.2625. [DOI] [PubMed] [Google Scholar]
- 5.Schmidtchen FP, Rapoport H. J Am Chem Soc. 1977;99:7014. doi: 10.1021/ja00463a041. [DOI] [PubMed] [Google Scholar]
- 6.Cox CM, Whiting DA. J Chem Soc Perkin Trans 1. 1991:1901. [Google Scholar]
- 7.Takahashi N, Suzuki A, Kimura Y, Miyamoto S, Tamura S. Tetrahedron Lett. 1967;8:1961. doi: 10.1016/s0040-4039(00)90764-0. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida S, Shiraishi S, Fujita K, Takahashi N. Tetrahedron Lett. 1975;16:1863. [Google Scholar]
- 9.Jansen R, Hofle G. Tetrahedron Lett. 1983;24:5485. [Google Scholar]
- 10.(a) Boger DL, Corbett WL, Wiggins JM. J Org Chem. 1990;55:2999. [Google Scholar]; (b) Boger DL, Corbett WL, Curran TT, Kasper AM. J Am Chem Soc. 1991;113:1713. [Google Scholar]; (c) Boger DL. Tetrahedron. 1983;39:2869. Reviews: [Google Scholar]; Boger DL. Chem Rev. 1986;86:781. [Google Scholar]; Boger DL. Chemtracts: Org Chem. 1996;9:149. [Google Scholar]
- 11.Scheeren JW, Staps RJFM, Nivard RJF. Rec Trav Chim. 1973;92:11. [Google Scholar]
- 12.(a) Boger DL, Cassidy KC, Nakahara S. J Am Chem Soc. 1993;115:10733. [Google Scholar]; (b) Boger DL, Hüter O, Mbiya K, Zhang M. J Am Chem Soc. 1995;117:11839. [Google Scholar]; (c) Boger DL, Hong J. J Am Chem Soc. 1998;120:1218. [Google Scholar]; (d) Boger DL, Blagg BSJ. Tetrahedron. 2002;58:6343. [Google Scholar]
- 13.Rambaud M, Bakasse M, Duguay G, Villieras J. Synthesis. 1988:564. [Google Scholar]
- 14.Brown C, Hudson RF, Record KAF. J Chem Soc Perkin Trans 2. 1978:822. [Google Scholar]
- 15.Trecourt F, Mallet M, Mongin F, Queguiner G. J Org Chem. 1994;59:6173. [Google Scholar]
- 16.Wright A, West R. J Am Chem Soc. 1974;96:3214. [Google Scholar]; See Supporting Information for the isolation, structure, and characterization of S1.
- 17.Blakemore PR, Cole WJ, Kocienski PJ, Morley A. Synlett. 1998:26. [Google Scholar]
- 18.Ndibwani A, Lamothe S, Guay D, Plante R, Soucy P, Goldstein S, Deslongchamps P. Can J Chem. 1993;71:695. [Google Scholar]
- 19.Ahmed A, Hoegenauer EK, Enev VS, Hanbauer M, Kaehlig H, Ohler E, Mulzer J. J Org Chem. 2003;68:3026. doi: 10.1021/jo026743f. [DOI] [PubMed] [Google Scholar]
- 20.Nakao Y, Yoshida WY, Takada Y, Kimura J, Yang L, Mooberry SL, Scheuer PJ. J Nat Prod. 2004;67:1332. doi: 10.1021/np049949f. [DOI] [PubMed] [Google Scholar]
- 21.Littke AF, Schwarz L, Fu GC. J Am Chem Soc. 2002;124:6343. doi: 10.1021/ja020012f. [DOI] [PubMed] [Google Scholar]
- 22.Urakawa A, Sasaki T, Yoshida K, Otani T, Lei Y, Yun W. J Antibiot. 1996;49:1052. doi: 10.7164/antibiotics.49.1052. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi N, Suzuki A, Kimura Y, Miyamoto S, Tamura S, Mitsui T, Fukami J. Agric Biol Chem. 1968;32:1115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: Full experimental details are provided in the supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.