Abstract
Five commercial botanical products [Shilianhua (SLH) tablets, Shiu Huo pills, Fenulyn, Bitter Melon and Glucose Metabolic Support)], available in the US market, with reported claims for regulation of metabolism were screened for their effect on body weight gain in high-fat diet (HFD)-induced obese mice. Pilot results suggested that Shilianhua (SLH) tablets attenuated body weight gain, whereas Shiu Huo pills and Fenulyn tended to promote weight gain in the mice on the high fat diet. To investigate the bioactive components in the SLH tablet, the wild SLH plant (Sinocrassula indica Berge) was collected from China, and used to make a variety of extracts including aqueous extract (SLH-A), ethanol extract (SLH-E) and subfraction F100. In the study of metabolic activities, the extracts were administrated through food intake by incorporating them into the diet. A rigorous evaluation of the extracts on body weight was conducted in two animal models. SLH-A and SLH-E were tested in dietary obese mice, while F100 together with SLH-E was tested in KK-Ay mice, a genetic diabetic model. In the 12–16 week study, body weight was not significantly altered by the SLH extracts in the two animal models. The results suggest that neither the total extract nor the purified components from the SLH plant have a clear effect in the regulation of body weight. The weight reduction observed with the “over the counter” SLH tablet in the pilot studies may be secondary to other components in the tablet, but not from the SLH extract.
Introduction
Many “over the counter” (OTC) supplements have been marketed as being effective to promote glucose metabolism in humans, and consumption of dietary supplements by the general public has increased in the US to facilitate the management of blood glucose (1). Among these dietary supplements, botanical extracts are extremely popular components and have been promoted to enhance the therapeutic activities and reduce the side effects of synthetic drugs. However, clinical efficacy and mechanism of action of many botanicals have not been well characterized. In this study, we evaluated the weight regulation activities of several botanical products that are marketed in the US and make claims for metabolic regulation. Reduction of body weight is known to decrease blood glucose through improvement of insulin sensitivity. Specifically, five commercial products, based on reported claims, were chosen for evaluation in this study. Specifically, they were Shilianhua (SLH) tablets, Shiu Huo pills, Fenulyn, Bitter Melon and Glucose Metabolic Support. The efficacies of these products in the management of body weight were tested in the dietary obese mice by oral administration.
The commercial SLH tablet contains several botanical products including SLH (Sinocrassula Berger), Spirulina, Lycium berries, Soy, fiber, Guar gum, et al. SLH (Sinocrassula indica) is also patented to reduce blood glucose in U.S., Japan and China. Shilianhua is the Chinese name for houseleek, which is widely distributed in the world. In addition, it is consumed as tea in Taiwan and Japan. To study bioactivities of the SLH plant, we purified extracts from the wild Sinocrassula indica, and examined their efficacy in the regulation of body weight in obese mice. The SLH extract was also divided into four sub-fractions and one fraction (F100) was tested in mice for weight regulation. The result suggests that the SLH extracts have no activities in the regulation of body weight.
Materials and Methods
1. Mouse models and treatment
Dietary obesity was generated in the male C57BL/6J mice with a high-fat diet (HFD) as described elsewhere (2). Male C57BL/6J mice and KK-Ay (KK.Cg-Ay mutant) mice were purchased from the Jackson laboratory, and housed singly in the study. The C57BL/6J mice were fed on a HFD (58% calories as fat, Research Diets D12331) at 5 weeks of age to induce obesity. KK-Ay mice were fed a defined low-fat diet (Research Diets D12329) throughout the experiment. The control group was fed the defined diet, and the treatment group was fed the same diet containing one of following products, SLH tablets (SLH, 5.2 mg/kg/d), Shiu Huo pills (SHP, 100 mg/kg/d), Fenulyn (Fen, 150 mg/kg/d), Bitter Melon (BM, 150 mg/kg/d) and Glucose Metabolic Support (GMS, 200 mg/kg/d). In addition, animals had diets supplemented with SLH fractions (see preparation and purification below) consisting of SLH aqueous extract (SLH-A; 0.4% w/w), SLH ethanol extract (SLH-E; 1% w/w) or F100 at 0.05% (w/w). Body weight was measured weekly. Food intake was measured twice for one week intervals during weeks 3 and 6.
2. Preparation of the aqueous extract (SLH-A)
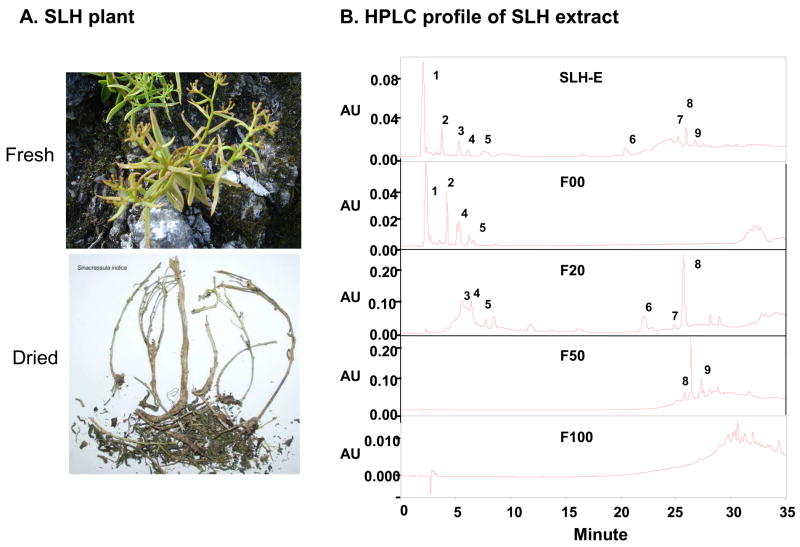
The photos of fresh and dried SLH plants (Sinocrassula indica) are shown in Fig. 1A. The extract of SLH used in this study was prepared from the aerial parts of wild SLH plant. The plant was collected from the vast arid and mountainous areas in Guizhou Province, southwestern China where SLH has been used as a medicinal herb by the local residents for hundreds of years. The SLH sample was certified by a taxonomist at the Institute of Medicinal Plant Development (IMPLAD), a Chinese authority in the identification and authentication of Traditional Chinese Herbs and Medicinal Plants. The fresh aerial part of SLH was air-dried under shade to reduce moisture content to approximately 8% w/w. The dry material was ground into 6-mm or smaller pieces. The grounded material was first soaked in deionized water at 1:8 w/v ratio for 60 min at room temperature, and then extracted twice at 50°C in a rotary extractor for 6 hrs. The water-soluble extract was separated from the solids (structural components of fibers, cellulose, semi-cellulose, debris of cells) by first centrifuging at 3500 rpm with an Allegra™ 6KR Centrifuge (Beckman Coulter, Palo Alto CA) and then filtering with Whatman #4 paper. The liquid product was then concentrated in a rotary evaporator of 20-L capacity (Buchi Rotavapor R-220, Flawi Switzerland), and followed by freeze drying (Labconco Co., Kansas City, Mo) into an aqueous extract powder (SLH-A) which accounted for 29.8% w/w of the raw herb.
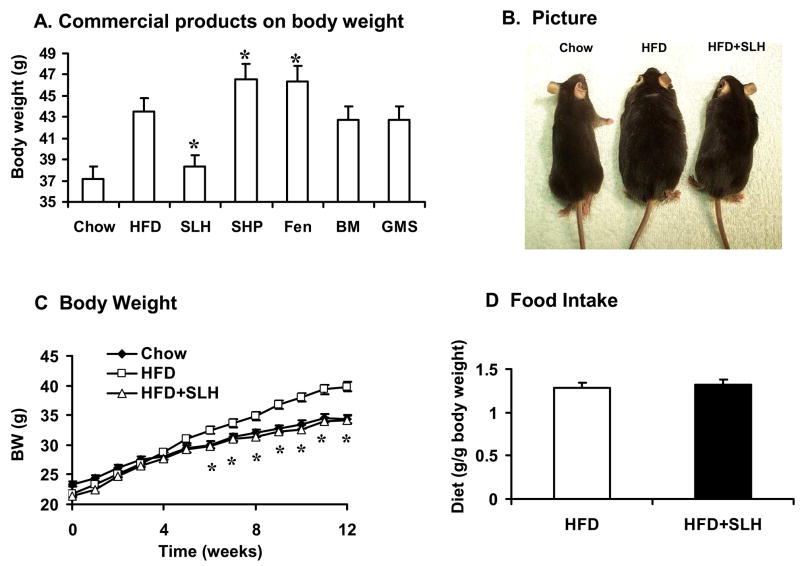
Fig. 1. Regulation of body weight by commercial botanical products.
Five commercial botanical products including SLH, SHP, Fen, BM and GMS were tested in the prevention of dietary induced obesity in mice fed on high fat diet (HFD). (A) SLH tablets reduced gain in body weight significantly when compared to the other commercial products. The body weight was examined at the end of 12 weeks on HFD. (B) Picture of mice at 12 weeks on HFD and SLH tablets. (C) Time course of body weight gain on HFD and SLH tablets. (D) Food intake (g) per body weight (g) over 4 weeks from 12–6 wks on HFD. Each point presents mean ± SE (n = 11). Compared with HFD control: * P < 0.01
2. Preparation of the ethanol extract (SLH-E)
The aqueous extract (about 1.3 Kg) was further fractionated to yield a concentrated SLH extract. It was dissolved into 15 L of deionized water, loaded into a 15 kg macroporous adsorbent polymer resin column (L493; Sigma Chemical Company, St. Louis, MO, USA), washed with 5 L of water, and then eluted with 20 L 95% ethanol, which were evaporated to obtain SLH ethanol extract (SLH-E).
3. Preparation of sub-fractions of SLH
The ethanol in 95% ethanol elutes was removed by evaporation under reduced pressure. SLH was fractionated using a HPLC system with C18 column (Sigma Chemical Company, St. Louis, MO, USA) that was eluted with water, 20% MeOH, 50% MeOH and 100% MeOH in a sequence to obtain four sub-fractions, i.e., F00, F20, F50 and F100. F100 was found to be the most active subfraction in the inhibition of inflammatory responses (data not shown) and was therefore selected as the subfraction for in vivo assessment on body weight gain along with the SLH-E and SLH-A extracts.
4. Characterization of SLH-E and sub-fractions
Chemical fingerprint of the SLH extracts was developed using a Waters HPLC system consisting of Waters Delta 600 pump (Waters Co., Milford, MA), an Waters 717 plus autosampler, and Waters 2996 Photodiode Array Detector (190 to 800 nm) (Figure 1B). The system is controlled by computer and the data was analyzed with the Empower software system (Waters). The mobile phase consisted of HPLC-grade methanol and water run through a gradient elution from 3:97 (MeOH: H2O) to 10:90 (MeOH: H2O) for the first 6 minutes, followed by a gradient elution to 80:20 (MeOH: H2O) for 14 minutes and gradient elution to 100: 0 (MeOH: H2O) for 5 minutes, kept for 5 minutes, and then equilibrated to 3:97 for 15 minutes before next sample was injected.
5. Statistical Analysis
In the bar figures, a mean value and standard error of multiple data points or samples were used to represent the final result. Student’s t test or one-way ANOVA was used in the statistical analysis of the data with a significance level of P < 0.05.
Results
Commercial SLH tablet attenuates diet-induced weight gain
The effects of five commercial products on body weight were investigated in dietary obese mice. The products were administrated through food intake by incorporating them into the high-fat diet. Among the five products, the SLH tablets reduced gain in body weight on HFD (Fig. 2A and 2B). At end of 12 weeks, the body weight was 38 g in the chow diet group (lean control) and 43 g in the HFD group (13% increase, P<0.05). With SLH supplementation, the body weight was 38 g in mice on HFD, which was identical to that of the chow diet group (Fig. 2C). With SHP or Fen supplementation, the body weight was 5% (P<0.05) higher than that of the HFD group. These data suggest that SLH tablet attenuated body weight gain whereas the SHP and Fen tablets promoted body weight gain in mice on HFD. This anti-obesity effect of SLH was not a result of alteration in food intake as there was no significant difference in the food intake in the control (HFD) and experimental (SLH) groups (Fig. 2D). This pilot study suggested that the SLH tablet may contain bioactive compounds that attenuates HFD-induced obesity in mice.
Fig. 2. SLH extracts.
(A) Picture of wild SLH plant (Sinocrassula indica) used in this study. The plant was collected in the Guizhou Province, southwestern China. (B) Chromatographic fingerprints of SLH and its fractions. Nine major components were observed in the chromatographic fingerprinting profile of SLH-E. To enrich the metabolic activity, SLH-E was fractionated into four fractions in HPLC on the basis of polarity of components. These were F00, F20, F50 and F100. The fingerprint profile shows that components 1, 2, 4, and 5 were located in the F00 fraction; components 3, 5, 6, and 8 were included in the fraction F20; components 8 and 9 were contained in the fraction F50; and the least polar components were retained in the fraction F100.
Chemical fingerprints of SLH extracts
Since the major component of the SLH tablet is the extract of the Shilianhua plant, we made an effort to study the bioactivity in the plant, Sinocrassula indica. A variety of extracts were isolated from the plant and their chemical finger prints were analyzed with HPLC. Nine major components were observed in the chromatographic fingerprinting profile of SLH-E (Fig. 1B). SLH-E was fractionated into four fractions in HPLC on the basis of polarity of components. These were identified as F00, F20, F50 and F100. The fingerprint profile suggests that components 1, 2, 4, and 5 were located in the F00 fraction; components 3, 5, 6, and 8 were included in the fraction F20; components 8 and 9 were contained in the fraction F50; and the least polar components were retained in the fraction F100. F100 was used as an organic extract of SLH since it was collected through elution with 100% MeOH.
Purified SLH extracts had no effects on body weight of mice
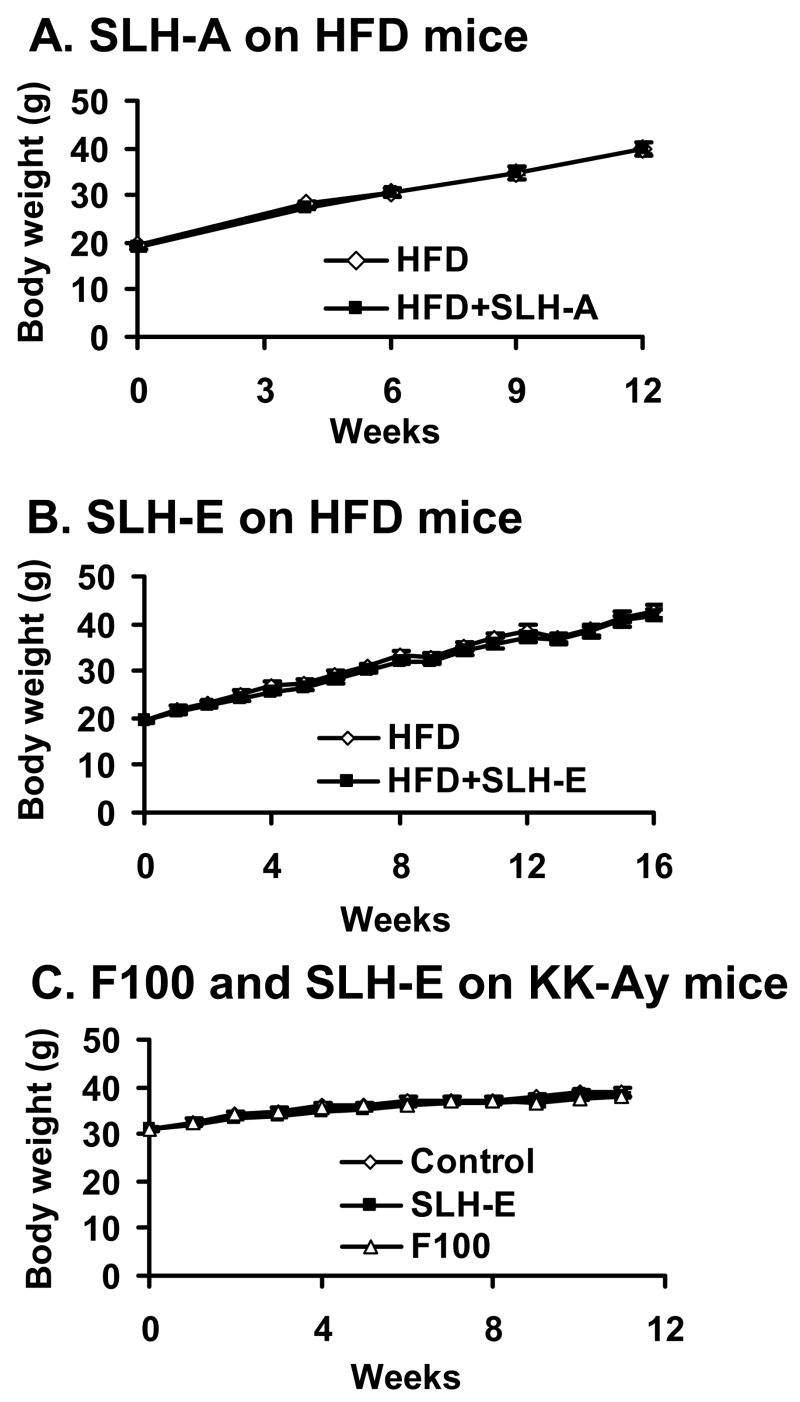
The purified SLH extracts were studied in the mouse model of obesity in identical experiments as assessed with the commercial products. First, SLH-A was tested in the dietary obese mouse model through oral administration (dietary supplementation). The dosage of extract was 0.4% (w/w) in the diet, which is equivalent to 500–700 mg/Kg body weight per day. Both in the control and in the SLH-A treated mice, body weight was increased in the mice in a time-dependent manner on HFD. No difference was observed between the SLH group and control group in body weight (Fig. 3A). Since SLH-A had no obvious actions in the dietary obese mouse model, we tested SLH-E in the HFD mice. The dose of SLH-E was increased to 1% (w/w) in the diet. No change in body weight was observed for SLH-E (Fig. 3B). Finally, SLH-E was also tested in KK-Ay diabetic mice that develop hyperglycemia and insulin resistance on low fat diet. F100, a subfraction of SLH-E, was also tested in the study. SLH-E and F100 were administrated orally through diet supplementation at 2% and 0.05% w/w in the low-fat diet, respectively. In the study, body weight was not significantly changed by SLH-E or F100 compared with the control mice (Fig. 3C), suggesting that the extracts of SLH were unable to regulate body weight in the diabetic KK-Ay mice.
Fig. 3. SLH had no effects on body weight of mice.
(A) Aqueous extract of SLH (SLH-A) had no effects on HFD induced obese mice (n = 9). (B) Ethanol extract of SLH (SLH-E) had no effects on HFD induced obese mice (n=11). (C) F100 and SLH-E had no effects on KK-Ay mice (n=9).
Discussion
This study represents a very rigorous assessment of OTC supplements in the regulation of body weight. In this study, the commercial SLH tablet prevented body weight gain whereas SHP and Fen promoted body weight gain in the dietary obese mice. However, when the major component of the SLH tablet was tested in the same animal model, no significant activity was observed in the regulation of body weight. Thus, this work strongly suggests that the SLH extract does not have an effect to regulate body weight.
The plant SLH is a shrub in the Crassulaceae family that grows in the southwestern part of China including Yunnan, Guangxi, and Guizhou Provinces. It had been used as an herb for hundreds of years in Southwest China and SLH is also patented to reduce blood glucose in U.S., Japan and China. As we have described, when compared to the five commercial products we tested, only the SLH tablet was found to have a potent effect on regulation of metabolism. Thus, to identify the bioactive components for the activity for this commercial product, the SLH plant was obtained and used to make a variety of extracts including SLH-A, SLH-E, F00, F20, F50 and F100. Among them, the aqueous extract, i.e. SLH-A, was the crude extract that contained all bioactive components except the fibers, cellulose and debris of the cells that are felt not to be bioactive. The ethanol extract, i.e. SLH-E, is a 95% ethanol extract of the plant and much more concentrated than SLH-A when assessing activity of inhibition of inflammatory response (data not shown). Compared with SLH-A, SLH-E contains more small-molecule compounds and fewer components with large molecules like polysaccharides and peptides, which are suggested to be inactivated in the gut after oral administration. In the subfractions of SLH, F100 was the organic extract with the lowest polarity in the SLH-E. F100 contains small molecules, such as saponin and alkaloid, which are water-insoluble and with bioactivity in general. F100 is the most active subfraction in the inhibition of inflammation response (data not shown). In this study, SLH-A, SLH-E and F100 were tested in dietary obese mice and KK-Ay mice, which are models for obesity and type 2 diabetes. However, no anti-obesity effect was observed for these SLH extracts in the mice. The results indicate that SLH has no bioactivities in the regulation of body weight.
If the SLH plant does not have an activity in the regulation of body weight, what was responsible for the weight control activity of the SLH tablet in the pilot studies? First, the bioactivity of SLH tablet may be due to the components other than SLH extracts. In addition to the SLH plant extract, the SLH tablet also contains products from four other plants, such as Spirulina maxima, Lycium berries, soy fiber and guar gum. Spirulina was reported to reduce blood glucose, alleviate dyslipidemia and fatty liver (3–5). Berry of Lyceum barbarum is a popular traditional Chinese herb that is used to treat aging-related disease. Berry of Lyceum barbarum was reported to have hypoglycemic, hypolipidemic and antioxidant effects in both type 1 and type 2 diabetes in animal models (6–9). Soy fiber is able to decrease postprandial blood glucose by regulation of glucagons, pancreatic polypeptide and somatostatin secretion (10, 11). Guar gum, a well-established water-soluble fiber, is known to reduce hypercholesterolemia, hyperglycemia and obesity (12–14). The combination of these botanical products in the SLH tablet may have constituted the antiobesity activity. However, to definitively state that the effect is from these extracts, the individual extracts also will have to be rigorously tested. In addition, it could also be argued that other components, e.g. pharmacological agents, may have been involved. OTC agents are not regulated in the same fashion, nor have the same quality control standards, as demanded by the FDA for prescription drugs. Thus, the claims made for products rarely are ever validated in placebo, controlled trials.
In summary, we conclude that the SLH plant as a total extract, or when separated into specific bioactive fractions, has no effects on regulation of body weight. The effect of weight reduction in the SLH tablet is clearly not due to SLH extract. The activity of SLH tablet may be from the other components or the synergetic effects of the five botanical components.
Acknowledgments
This study is supported by NIH grant 1P50AT002776-010002 and DK68036 to Ye J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dwyer JT, Allison DB, Coates PM. Dietary supplements in weight reduction. J Am Diet Assoc. 2005;105:S80–6. doi: 10.1016/j.jada.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 2.Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18:2024–034. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- 3.Huang ZX, Mei XT, Xu DH, Xu SB, Lv JY. Protective effects of polysacchride of Spirulina platensis and Sargassum thunbeergii on vascular of alloxan induced diabetic rats. Zhongguo Zhong Yao Za Zhi. 2005;30:211–15. [PubMed] [Google Scholar]
- 4.Parikh P, Mani U, Iyer U. Role of Spirulina in the Control of Glycemia and Lipidemia in Type 2 Diabetes Mellitus. J Med Food. 2001;4:193–99. doi: 10.1089/10966200152744463. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Hernandez A, Ble-Castillo JL, Juarez-Oropeza MA, Diaz-Zagoya JC. Spirulina maxima prevents fatty liver formation in CD-1 male and female mice with experimental diabetes. Life Sci. 2001;69:1029–037. doi: 10.1016/s0024-3205(01)01185-7. [DOI] [PubMed] [Google Scholar]
- 6.Li XM. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int J Biol Macromol. 2007;40:461–65. doi: 10.1016/j.ijbiomac.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Luo Q, Cai Y, Yan J, Sun M, Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76:137–49. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Guo H, Zhao R. Effect of Lycium barbarum polysaccharide on the improvement of antioxidant ability and DNA damage in NIDDM rats. Yakugaku Zasshi. 2006;126:365–71. doi: 10.1248/yakushi.126.365. [DOI] [PubMed] [Google Scholar]
- 9.Zhao R, Li Q, Xiao B. Effect of Lycium barbarum polysaccharide on the improvement of insulin resistance in NIDDM rats. Yakugaku Zasshi. 2005;125:981–88. doi: 10.1248/yakushi.125.981. [DOI] [PubMed] [Google Scholar]
- 10.Slavin J. Nutritional benefits of soy protein and soy fiber. J Am Diet Assoc. 1991;91:816–19. [PubMed] [Google Scholar]
- 11.Tsai AC, Vinik AI, Lasichak A, Lo GS. Effects of soy polysaccharide on postprandial plasma glucose, insulin, glucagon, pancreatic polypeptide, somatostatin, and triglyceride in obese diabetic patients. Am J Clin Nutr. 1987;45:596–01. doi: 10.1093/ajcn/45.3.596. [DOI] [PubMed] [Google Scholar]
- 12.Butt MS, Shahzadi N, Sharif MK, Nasir M. Guar gum: a miracle therapy for hypercholesterolemia, hyperglycemia and obesity. Crit Rev Food Sci Nutr. 2007;47:389–96. doi: 10.1080/10408390600846267. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins DJ, Wolever TM, Haworth R, Leeds AR, Hockaday TD. Guar gum in diabetes. Lancet. 1976;2:1086–087. doi: 10.1016/s0140-6736(76)90998-3. [DOI] [PubMed] [Google Scholar]
- 14.Sesmilo G, Coves MJ, Gomis R. Guar gum in the treatment of NIDDM. Diabetes Care. 1995;18:584–85. doi: 10.2337/diacare.18.4.584. [DOI] [PubMed] [Google Scholar]