HISTORY
Nerve growth factor (NGF) was discovered in the early 1950s due to its trophic (survival- and growth-promoting) effects on sensory and sympathetic neurons (Levi-Montalcini and Hamburger, 1951), In 1982, brain-derived neurotrophic factor (BDNF), the second member of the “neurotrophic” family of neurotrophic factors, was shown to promote survival of a subpopulation of dorsal root ganglion neurons, and subsequently purified from pig brain (Barde et al., 1982). Since then, other members of the neurotrophin family such as neurotrophin-3 (NT-3) (Maisonpierre et al., 1990) and neurotrophin-4/5 (NT-4/5) (Hallbook et al., 1991; Ip et al., 1992) have been described, each with a distinct profile of trophic effects on subpopulations of neurons in the peripheral and central nervous systems.
GENE AND PROTEIN STRUCTURE
The BDNF gene (in humans mapped to chromosome 11p) has four 5′ exons (exons I-IV) that are associated with distinct promoters, and one 3′ exon (exon V) that encodes the mature BDNF protein (Metsis et al., 1993; Timmusk et al., 1993). Eight distinct mRNAs are transcribed, with transcripts containing exons I-III expressed predominantly in brain and exon IV found in lung and heart (Timmusk et al., 1993).
BDNF shares about 50% amino acid identity with NGF, NT-3 and NT-4/5. Each neurotrophin consists of a noncovalently-1 linked homodimer and contains (1) a signal peptide following the initiation codon; and (2) a pro-region containing an N-linked glycosylation site. Initially produced as proneurotrophins, prohormone convertases such as furin cleave the proneurotrophins (M.W. ~30kDa) to the mature neurotrophin (M.W. ~14kDa) (Chao and Bothwell, 2002). Proneurotrophins have altered binding characteristics and distinct biologic activity in comparison with mature neurotrophins (Lee et al., 2001a,b). Neurotrophins also share a distinctive three-dimensional structure containing two pairs of antiparallel β-strands and cysteine residues in a cystine knot motif.
BDNF SIGNAL TRANSDUCTION
Each neurotrophin binds one or more of the tropomyosin-related kinase (trk) receptors, members of the family of receptor tyrosine kinases (Patapoutian and Reichardt, 2001). Ligand-induced receptor dimerization results in kinase activation; subsequent receptor autophosphorylation on multiple tyrosine residues creates specific binding sites for intracellular target proteins, which bind to the activated receptor via SH2 domains (Barbacid, 1994; Patapoutian and Reichardt, 2001). These include PLC-γ1 (phospholipase C), p85 (the noncatalytic subunit of PI-3 kinase) and Shc (SH2-containing sequence); activation of these target proteins can then lead to a variety of intracellular signalling cascades such as the Ras-MAP (mitogen-activated protein) kinase cascade and phosphorylation of cyclic AMP-response element binding protein (CREB) (Patapoutian and Reichardt, 2001; Segal, 2003).
TrkA binds NGF (with low-affinity binding by NT-3 in some systems); trkB binds BDNF and NT-4/5 with lower-affinity binding by NT-3; and trkC binds NT-3 (Barbacid, 1994). Trk receptors exist in both a full-length (trkB.FL) form as well as truncated (trkB.T1. trkB.T2) forms lacking the kinase domain (Eide et al., 1996; Fryer et al., 1997). Although most functions attributed to BDNF are associated with full-length trkB, several roles have been suggested for truncated receptors, including growth and development (Fryer et al., 1997; Yacoubian and Lo, 2000; Luikart et al., 2003) and negative modulation of trkB receptor expression and function (Eide et al., 1996; Haapasalo et al., 2001; Haapasalo et al., 2002). Expression of truncated trk receptors on astrocytes is upregulated following injury (Frisen et al.,1993) and may modulate neuronal vulnerability (Saarelainen et al., 2000a,b) and sequestration of BDNF in astrocytes (Biffo et al., 1995; Roback et al., 1995; Alderson et al., 2000). Recent studies have shown that BDNF activates glial calcium signalling by truncated trk receptors (Climent et al., 2000: Rose et al., 2003).
In addition, all of the neurotrophins bind to the p75 receptor, designated p75NTR. p75NTR, related to proteins of the tumor necrosis factor (TNFR) superfamily, has a glycosylated extracellular region involved in ligand binding, a transmembrane region, and a short cytoplasmic sequence lacking intrinsic catalytic activity (Chao and Hempstead, 1995; Dechant and Barde, 2002). Neurotrophin binding to p75NTR is linked to several intracellular signal transduction pathways, including nuclear factor-κB (NF-κB), Jun kinase and sphingo-myelin hydrolysis (Dechant and Barde, 2002). P75NTR signalling mediates biologic actions distinct from those of the trk receptors, notably the initiation of programmed cell death (apoptosis) (Casaccia-Bonnefil et al., 1996; Frade et al., 1996; Roux et al., 1999; Dechant and Barde, 2002). It has also been suggested that p75 may serve to determine neurotrophin binding specificity (Esposito et al., 2001; Lee et al., 2001a,b; Zaccaro et al., 2001).
BDNF GENE REGULATION
A multitude of stimuli have been described that alter BDNF gene expression in both physiologic and pathologic states (Lindholm et al., 1994). For example, light stimulation increases BDNF mRNA in visual cortex (Castrén et al., 1992), osmotic stimulation increases BDNF mRNA in the hypothalamus (Castrén et al., 1995; Dias et al., 2003), and whisker stimulation increases BDNF mRNA expression in somatosensory barrel cortex (Rocamora et al., 1996). Electrical stimuli that induce long-term potentiation (LTP) in the hippocampus, a cellular model of learning and memory, increase BDNF and NGF expression (Patterson et al., 1992; Castrén et al., 1993; Bramham et al., 1996). Even physical exercise has been shown to increase NGF and BDNF expression in hippocampus (Neeper et al., 1995). Interestingly, BDNF levels vary across the estrous cycle, which correlate with its effects on neural excitability (Scharfman et al., 2003).
Distinct BDNF 5′ exons are differentially regulated by stimuli such as neural activity. For example, exons I-III, but not exon IV, increase after kainic acid-induced seizures (Timmusk et al., 1993) or other stimuli that increase activity (Lauterborn et al., 1996; Tao et al., 2002). Protein synthesis is required for the effects of activity on exons I and II, but not III and IV, raising the possibility that the latter act as immediate early genes (Lauterborn et al., 1996; Castrén et al., 1998). The transcription factor CaRF activates transcription of exon III under the control of a calcium response element. CaRE1 (Tao et al., 2002). CREB, which can be stimulated by diverse stimuli ranging from activity to chronic antidepressant treatment (Nibuya et al., 1995, 1996; Shieh et al., 1998; Tao et al., 1998; Shieh and Ghosh, 1999), also modulates exon III transcription. Recent evidence also indicates that neural activity triggers calcium-dependent phosphorylation and release of methyl-CpG binding protein 2 (MeCP2) from BDNF promoter III to derepress transcription (Chen et al., 2003).
LOCALIZATION, TRANSPORT AND RELEASE
BDNF and trkB mRNA have a widespread distribution in the central nervous system (Merlio et al., 1993; Conner et al., 1997). BDNF and trkB protein immunoreactivity is also widespread (Conner et al., 1997; Yan et al., 1997a,b; Drake et al., 1999), Like BDNF mRNA, constitutive BDNF protein expression is particularly high in the hippocampus, where the mossy fibre axons of dentate granule cells display BDNF immunoreactivity (Conner et al., 1997).
Unlike the classical target-derived trophic factor model in which neurotrophins—such as NGF—are retrogradely transported, there is now abundant evidence that BDNF is also anterogradely transported in brain. First, BDNF protein is localized to nerve terminals (Conner et al., 1997), and pathway transection or axonal transport inhibition abrogates this terminal expression (Altar et al., 1997; Conner et al., 1997; Altar and DiStefano, 1998). Second, higher-resolution studies have shown that BDNF is associated with dense-core vesicles (Fawcett et al., 1997; Altar and DiStefano, 1998), which are the primary site for neuropeptide storage and release from nerve terminals. Third, further functional studies have supported the anterograde transport hypothesis (Fawcett et al., 1998, 2000). Fourth, pro-BDNF is shuttled from the trans-Golgi network into secretory granules, where it is cleaved by prohormone convertase 1 (PC1) (Farhadi et al., 2000).
In addition, emerging evidence suggests that both BDNF and trk receptors may undergo regulated intracellular transport. For example, seizures lead to redistribution of BDNF mRNA from hippocampal CA3 cell bodies to their apical dendrites (Bregola et al., 2000; Simonato et al., 2002). Trk signalling is now thought to include retrograde transport of intact neurotrophin-trk complexes to the neuronal cell body (Miller and Kaplan, 2001; Ginty and Segal, 2002).
Recent evidence indicates that neurotrophins are released acutely following neuronal depolarization (Griesbeck et al., 1999; Mowla et al., 1999; Goggi et al., 2003). In fact, direct activity-dependent pre- to post-synaptic transneuronal transfer of BDNF has recently been demonstrated using fluorescently-labelled BDNF (Kohara et al., 2001). The released form of BDNF is thought to be proBDNF (Mowla et al., 2001), raising the possibility of postsecretory proteolytic processing by membrane-associated or extracellular proteases in the modulation of BDNF action (Lee et al., 2001a,b).
BDNF AND DEVELOPMENT
BDNF has survival- and growth-promoting actions on a variety of neurons, including dorsal root ganglion cells (Acheson et al., 1995) and hippocampal and cortical neurons (Huang and Reichardt, 2001). Certain peripheral sensory neurons, especially those in vestibular and nodose-petrosal ganglia, depend on the presence of BDNF because BDNF homozygous (−/−) knockout mice lack these neurons (Huang and Reichardt. 2001). Unlike NGF, sympathetic neurons are not affected, nor are motor neurons. BDNF homozygous (−/−) knockout mice fail to survive past 3 weeks, but heterozygous BDNF knockout (+/−) mice are viable, and exhibit a variety of phenotypes, including obesity (Lyons et al., 1999; Kernie et al., 2000), decreased seizure susceptibility (Kokaia et al., 1995) and impaired spatial learning (Linnarsson et al., 1997). Interestingly, conditional postnatal BDNF gene deletion (Rios et al., 2001) and reduction in trkB expression (Xu et al., 2003) also cause obesity.
Physiologic regulation of BDNF gene expression may be very important in the development of the brain. For example, BDNF contributes to activity-dependent development of the visual cortex. Provision of excess BDNF (Cabelli et al., 1995) or blockade of BDNF signalling (Cabelli et al., 1997) leads to abnormal patterning of ocular dominance columns during a critical period of visual cortex development. This suggests a role for BDNF in axonal path-finding during development. BDNF also has powerful effects on dendritic morphology (McAllister et al., 1997; Murphy et al., 1998; Horch and Katz, 2002; Tolwani et al., 2002).
EFFECTS ON SYNAPTIC TRANSMISSION
The first studies of BDNF effects on synaptic transmission showed that BDNF increased the frequency of miniature excitatory postsynaptic currents (EPSCs) in Xenopus cultures (Lohof et al., 1993). Since then, numerous studies have examined the actions of BDNF. Overall, BDNF appears to strengthen excitatory (glutamatergic) synapses and weaken inhibitory (GABAergic) synapses. Schuman and colleagues demonstrated that exposure of adult rat hippocampal slices to BDNF led to a long-lasting potentiation of afferent input to hippocampal pyramidal cells (Kang and Schuman, 1995). Subsequent studies have supported a role of BDNF in LTP (Korte et al., 1995, 1996; Patterson et al., 1996; Kang, 1997; Xu et al., 2003). For example, incubation of hippocampal or visual cortical slices with trkB inhibitors inhibits LTP (Figurov et al., 1996), and hippocampal slices from BDNF knockout animals exhibit impaired LTP induction (Korte et al., 1995) which is restored by reintroduction of BDNF (Korte et al., 1996; Patterson et al., 1996).
Whether BDNF-induced synaptic potentiation occurs primarily by a presynaptic action (e.g. through enhancement of glutamate release) or postsynaptically (e.g. via phosphorylation of neurotransmitter receptors) is intensely debated (Schinder and Poo, 2000) (Fig. 1). A number of studies have provided evidence for a presynaptic locus (Xu et al., 2000; Tyler et al., 2002) (see also, Kafitz et al., (1999)), yet evidence for postsynaptic actions has also been obtained (Black, 1999; Thakker-Varia et al., 2001) (reviewed in Poo (2001)). Both pre- and postsynaptic trkB receptors in the hippocampus may be important (Drake et al., 1999).
FIGURE 1.
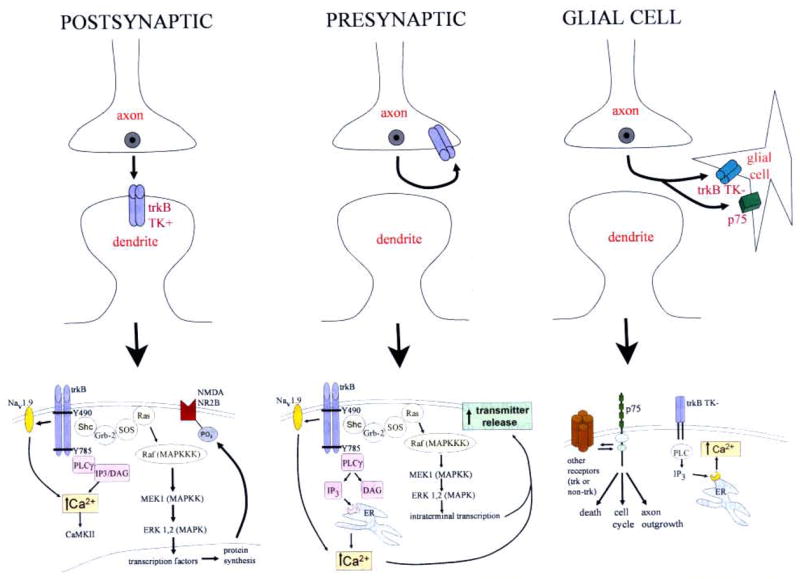
Multiple potential effects of local BDNF release at glutamatergic synapses. LEFT: Postsynaptic mechanisms. Top: BDNF released from dense core vesicles diffuses across the synaptic cleft to activate full-length trkB receptors (shown dimerized, trkB TK+) located at synapses on postsynaptic dendritic spines. Bottom: Postsynaptic signal transduction leads to protein phosphorylation, such as the NR2B subunit of the NMDA receptor, as well as other actions, leading to enhanced synaptic transmission. Note that the site of transcription could be the nucleus, as shown, or occur locally in the dendrite. CENTER: Presynaptic mechanisms. Top: BDNF activates, in an autocrine fashion, full-length trkB receptors on the plasma membrane of the axon terminal. Bottom: Presynaptic trkB activation leads to increased neurotransmitter release by several potential mechanisms. RIGHT: Synaptic modulation by glial cells. Top: When BDNF is released into the synaptic cleft, it may bind to receptors on juxtaposed glial cells, such as truncated trkB (trkB TK − ), possibly full-length trkB (not shown) or p75 receptors. Bottom: Activation of truncated trkB has the potential to modulate glial Ca2+ signalling, and p75 activation can initiate other pathways; both could ultimately lead to changes in synaptic transmission.
A role for BDNF in GABAergic synapses was first raised by studies showing that BDNF influences GABAergic neuronal phenotype (Marty et al., 1996). Subsequently, BDNF was shown to decrease inhibitory (GABAergic) synaptic transmission (Tanakaer et al., 1997; Frerking et al.. 1998; Wardle and Poo, 2003), perhaps in part via modulation of GABAA receptor phosphorylation (Jovanovic et al., 2004). Interestingly, BDNF may also regulate the efficacy of GABAergic synapses by direct downregulation of the neuronal K+-Cl− co-transporter, which would impair neuronal Cl− extrusion and weaken GABAergic inhibition (Rivera et al., 2002). Similarly, a recent paper found differential effects of BDNF on GABA-mediated currents in excitatory and inhibitory neuron subpopulations, selectively decreasing the efficacy of inhibitory neurotransmission by downregulation of Cl− transport (Wardle and Poo, 2003).
NEUROGENESIS
BDNF has also been found to enhance neurogenesis. For example, intraventricular infusion of BDNF or adeno-viral-induced BDNF activity increases the number of neurons in the adult olfactory bulb, striatum, septum and thalamus (Zigova et al., 1998; Benraiss et al., 2001; Pencea et al., 2001), which can be potentiated by concurrent inhibition of glial differentiation of subepen-dymal progenitor cells (Chmielnicki et al., 2004). Studies of cultured progenitor cells have elucidated some of the signalling mechanisms, which appear to involve trkB activation, followed by activation of the MAP kinase and PI3-kinase pathways (Barnabe-Heider and Miller, 2003) and downstream modification of basic helix-loop-helix transcription factors (Ito et al., 2003). Although some studies have concluded that the primary effect of BDNF is on proliferation (Katoh-Semba et al., 2002), other experiments suggest an important effect on survival (Lee et al., 2002). The effects of BDNF may depend on a previous history of ischemic damage (Larsson et al., 2002; Gustafsson et al., 2003).
LEARNING AND MEMORY
Since BDNF appears to be involved in activity-dependent synaptic plasticity, there is great interest in its role in learning and memory (Yamada and Nabeshima, 2003). The hippocampus, which is required for many forms of long-term memory in humans and animals, appears to be an important site of BDNF action. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning has been demonstrated (Hall et al., 2000), and function-blocking antibodies to BDNF (Alonso et al., 2002), BDNF knockout (Linnarsson et al., 1997), knockout of forebrain trkB signalling (Minichiello et al., 1999), or overexpression of truncated trkB (Saarelainen et al., 2000a,b) in mice impairs spatial learning. Another study demonstrated upregulation of BDNF in monkey parietal cortex associated with tool-use learning (Ishibashi et al., 2002). In humans, a valine to methionine polymorphism at the 5′ pro-region of the human BDNF protein was found to be associated with poorer episodic memory; in vitro, neurons transfected with met-BDNF-GFP exhibited reduced depolarization-induced BDNF secretion (Egan et al., 2003).
BDNF AND EPILEPSY
The discovery that limbic seizures increase NGF mRNA levels (Gall and Isackson, 1989) led to the idea that seizure-induced expression of neurotrophic factors may contribute to the lasting structural and functional changes underlying epileptogenesis (Gall et al., 1991; 1997; Jankowsky and Patterson, 2001). Recent in vitro and in vivo findings implicate BDNF in the cascade of electrophysiologic and behavioural changes underlying the epileptic state. BDNF mRNA and protein are markedly upregulated in the hippocampus by seizure activity in animal models (Ernfors et al., 1991; Isackson et al., 1991; Lindvall et al., 1994; Nibuya et al., 1995), and infusion of anti-BDNF agents (Binder et al., 1999b) or use of BDNF knockout (Kokaia et al., 1995) or truncated trkB-overexpressing (Lahteinen et al., 2002) mice inhibits epileptogenesis in animal models. Conversely, direct application of BDNF induces hyperexcitability in vitro (Scharfman, 1997; Scharfman et al., 1999), overexpression of BDNF in transgenic mice leads to spontaneous seizures (Croll et al., 1999), and intrahippocampal infusion of BDNF is sufficient to induce seizure activity in vivo (Scharfman et al., 2002) (but see, Reibel et al. (2000)), The hippocampus and closely associated limbic structures are thought to be particularly important in the pro-epileptogenic effects of BDNF (Binder et al., 1999a,b), and indeed increased BDNF expression in the hippocampus is found in specimens from patients with temporal lobe epilepsy (Mathern et al., 1997; Takahashi et al., 1999). It is hoped that understanding of the hyperexcitability associated with BDNF in epilepsy animal models may lead to novel anticonvulsant or antiepileptogenic therapies (Binder et al., 2001).
BDNF AND PAIN
BDNF also may play an important neuromodulatory role in pain transduction (Malcangio and Lessmann, 2003). BDNF is synthesized by dorsal horn neurons and markedly upregulated in inflammatory injury to peripheral nerves (along with NGF) (Fukuoka et al., 2001). BDNF acutely sensitizes nociceptive afferents and elicits hyperalgesia which is abrogated by BDNF inhibitors (Kerr et al., 1999; Thompson et al., 1999; Pezet et al., 2002). Central pain sensitization is an activity-dependent increase in excitability of dorsal horn neurons leading to a clinically intractable condition termed “neuropathic pain” in which normally nonpainful somatosensory stimuli (touch and pressure) become exquisitely painful (allo-dynia). Electrophysiological and behavioural data demonstrate that inhibition of BDNF signal transduction inhibits central pain sensitization (Kerr et al., 1999: Pezel et al., 2002).
BDNF AND NEURODEGENERATIVE DISEASES
The idea that degenerative diseases of the nervous system may result from insufficient supply of neurotrophic factors has generated great interest in BDNF as a potential therapeutic agent. Many reports have documented evidence of decreased expression of BDNF in neurological disease (Murer et al., 2001). Selective reduction of BDNF mRNA in the hippocampus has been reported in Alzheimer’s disease specimens (Phillips et al., 1991; Ferrer et al., 1999), although in an animal model upregulation appears to occur in plaque-related glial cells (Burbach et al., 2004). Decreased BDNF protein has been demonstrated in the substantia nigra in Parkinson’s disease (Howells et al., 2000). Interestingly, recent work has implicated BDNF in Huntington’s disease as well. Huntingtin, the protein mutated in Huntington’s disease, upregulates BDNF transcription, and loss of huntingtin-mediated BDNF transcription leads to loss of trophic support to striatal neurons which subsequently degenerate in the hallmark pathology of the disorder (Zuccato et al., 2001). A recent study has demonstrated that huntingtin normally inhibits the neuron restrictive silencer element (NRSE) involved in tonic repression of transcription from BDNF promoter II (Zuccato et al., 2003). In all of these disorders, provision of BDNF or increasing endogenous BDNF production may conceivably be therapeutic if applied in the appropriate spatiotemporal context (Spires et al., 2004).
BDNF AND NEUROPSYCHIATRIC DISEASE
BDNF signalling may also be involved in affective behaviours (Altar, 1999). Environmental stresses such as immobilization that induce depression also decrease BDNF mRNA (Smith et al., 1995). Conversely, physical exercise is associated with decreased depression and increased BDNF mRNA (Russo-Neustadt et al., 1999; Cotman and Berchtold, 2002). Existing treatments for depression are thought to act primarily by increasing endogenous monoaminergic (i.e. serotonergic and nor-adrenergic) synaptic transmission, and recent studies have shown that effective antidepressants increase BDNF mRNA (Dias et al., 2003) and protein (Chen et al., 2001; Altar et al., 2003). Exogenous delivery of BDNF promotes the function and sprouting of serotonergic neurons in adult rat brains (Mamounas et al., 1995), and BDNF-deficient mice are also deficient in serotonergic innervation (Lyons et al., 1999). Thus, new pharmacologic strategies are focused on the potential antidepressant role of BDNF.
It has also been hypothesized that BDNF may be involved in bipolar disorder (Tsai, 2004). Interestingly, lithium, a major drug for the treatment of bipolar disorder, increases BDNF and trkB activation in cerebral cortical neurons (Hashimoto et al., 2002). BDNF is an attractive candidate gene for susceptibility to bipolar disorder, and some (Neves-Pereira et al., 2002; Sklar et al., 2002) but not other (Hong et al., 2003; Nakata et al., 2003) studies suggest linkage between BDNF polymorphisms and disease susceptibility (Green and Craddock, 2003). How alterations in BDNF activity may relate to fluctuating bouts of mania and depression in bipolar disorder is still a matter of speculation.
SUMMARY
Since the purification of BDNF in 1982, a great deal of evidence has mounted for its central roles in brain development, physiology, and pathology. Aside from its importance in neural development and cell survival, BDNF appears essential to molecular mechanisms of synaptic plasticity. Basic activity-related changes in the central nervous system are thought to depend on BDNF modification of synaptic transmission, especially in the hippocampus and neocortex. Pathologic levels of BDNF-dependent synaptic plasticity may contribute to conditions such as epilepsy and chronic pain sensitization, whereas application of the trophic properties of BDNF may lead to novel therapeutic options in neurodegenerative diseases and perhaps even in neuropsychiatric disorders.
References
- Acheson A, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- Alderson RF, et al. Truncated TrkB mediates the endocytosis and release of BDNF and neurotrophin-4/5 by rat astrocytes and schwann cells in vitro. Brain Res. 2000;871:210–222. doi: 10.1016/s0006-8993(00)02428-8. [DOI] [PubMed] [Google Scholar]
- Alonso M, et al. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12:551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- Altar CA. Neurotrophins and depression. Trends Pharmacol Sci. 1999;20:59–61. doi: 10.1016/s0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci. 1998;21:431–437. doi: 10.1016/s0166-2236(98)01273-9. [DOI] [PubMed] [Google Scholar]
- Altar CA, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Altar CA, et al. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54:703–709. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabe-Heider F, Miller FD. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J Neurosci. 2003;23:5149–5160. doi: 10.1523/JNEUROSCI.23-12-05149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, et al. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffo S, et al. Selective binding and internalisation by truncated receptors restrict the availability of BDNF during development. Developmemt. 1995;121:2461–2470. doi: 10.1242/dev.121.8.2461. [DOI] [PubMed] [Google Scholar]
- Binder DK, Routbort MJ, McNamara JO. Immunohistochemical evidence of seizure-induced activation of trk receptors in the mossy fiber pathway of adult rat hippocampus. J Neurosci. 1999a;19:4616–4626. doi: 10.1523/JNEUROSCI.19-11-04616.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, et al. Selective inhibition of kindling development by intraventricular administration of TrkB receptor body. J Neurosci. 1999b;19:1424–1436. doi: 10.1523/JNEUROSCI.19-04-01424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, et al. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 2001;24:47–53. doi: 10.1016/s0166-2236(00)01682-9. [DOI] [PubMed] [Google Scholar]
- Black IB. Trophic regulation of synaptic plasticity. J Neurobiol. 1999;41:108–118. [PubMed] [Google Scholar]
- Bramham CR, et al. Unilateral LTP triggers bilateral increases in hippocampal neurotrophin and trk receptor mRNA expression in behaving rats: evidence for interhemispheric communication. J Camp Neurol. 1996;368:371–382. doi: 10.1002/(SICI)1096-9861(19960506)368:3<371::AID-CNE4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bregola G, et al. Different patterns of induction of fibroblast growth factor-2 and brain-derived neurotrophic factor messenger RNAs during kindling epileptogenesis, and development of a herpes simplex vector for fibroblast growth factor-2 gene transfer in vivo. Epilepsia. 2000;41(Suppl 6):S122–S126. doi: 10.1111/j.1528-1157.2000.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Burbach GJ, et al. Induction of brain-derived neurotrophic factor in plaque-associated glial cells of aged APP23 transgenic mice. J Neurosci. 2004;24:2421–2430. doi: 10.1523/JNEUROSCI.5599-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelli RJ, Hohn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, et al. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, et al. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Castrén E, et al. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E, et al. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Castrén E, Thoenen H, Lindholm D. Brain-derived neurotrophic factor messenger RNA is expressed in the septum, hypothalamus and in adrenergic brain stem nuclei of adult rat brain and is increased by osmotic stimulation in the paraventricular nucleus. Neuroscience. 1995;64:71–80. doi: 10.1016/0306-4522(94)00386-j. [DOI] [PubMed] [Google Scholar]
- Castrén E, et al. Regulation of brain-derived neurotrophic factor mRNA levels in hippocampus by neuronal activity. Prog Brain Res. 1998;117:57–64. doi: 10.1016/s0079-6123(08)64007-8. [DOI] [PubMed] [Google Scholar]
- Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- Chao MV, Bothwell M. Neurotrophins: to cleave or not to cleave. Neuron. 2002;33:9–12. doi: 10.1016/s0896-6273(01)00573-6. [DOI] [PubMed] [Google Scholar]
- Chen B, et al. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chen WG, et al. Depression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Chmielnicki E, et al. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Climent E. Astrocytes in culture express the full-length Trk-B receptor and respond to brain derived neurotrophic factor by changing intracellular calcium levels: effect of ethanol exposure in rats. Neurosci Lett. 2000;288:53–56. doi: 10.1016/s0304-3940(00)01207-6. [DOI] [PubMed] [Google Scholar]
- Conner JM, et al. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Croll SD, et al. Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience. 1999;93:1491–1506. doi: 10.1016/s0306-4522(99)00296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechant G, Barde YA. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5:1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- Dias BG, et al. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45:553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA, Patterson SL. Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J Neurosci. 1999;19:8009–8026. doi: 10.1523/JNEUROSCI.19-18-08009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eide FF, et al. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, et al. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Esposito D, et al. The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J Biol Chem. 2001;276:32687–32695. doi: 10.1074/jbc.M011674200. [DOI] [PubMed] [Google Scholar]
- Farhadi HF, et al. Neurotrophin-3 sorts to the constitutive secretory pathway of hippocampal neurons and is diverted to the regulated secretory pathway by coexpression with brain-derived neurotrophic factor. J Neurosci. 2000;20:4059–4068. doi: 10.1523/JNEUROSCI.20-11-04059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JP, et al. Detection of brain-derived neurotrophic factor in a vesicular fraction of brain synaptosomes. J Biol Chem. 1997;272:8837–8840. doi: 10.1074/jbc.272.14.8837. [DOI] [PubMed] [Google Scholar]
- Fawcett JP, et al. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci. 1998;18:2808–2821. doi: 10.1523/JNEUROSCI.18-08-02808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JP, et al. Evidence that brain-derived neurotrophic factor from presynaptic nerve terminals regulates the phenotype of calbindin-containing neurons in the lateral septum. J Neurosci. 2000;20:274–282. doi: 10.1523/JNEUROSCI.20-01-00274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, et al. BDNF and full-length and truncated TrkB expression in Alzheimer disease. Implications in therapeutic strategies. J Neuropathol Exp Neurol. 1999;58:729–739. doi: 10.1097/00005072-199907000-00007. [DOI] [PubMed] [Google Scholar]
- Figurov A, et al. Regulation of synaptic responses lo high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Frade JM, Rodriguez-Tebar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- Frisen J, et al. Characterization of glial trkB receptors: differential response to injury in the central and peripheral nervous systems. Proc Natl Acad Sci USA. 1993;90:4971–4975. doi: 10.1073/pnas.90.11.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer RH, Kaplan DR, Kromer LF. Truncated trkB receptors on nonneuronal cells inhibit BDNF-induced neurite outgrowth in vitro. Exp Neurol. 1997;148:616–627. doi: 10.1006/exnr.1997.6699. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, et al. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21:4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall CM, Isackson PJ. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989;245:758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- Gall C, et al. Seizures and the regulation of neurotrophic factor and neuropeptide gene expression in brain. Epilepsy Res Suppl. 1991;4:225–245. [PubMed] [Google Scholar]
- Gall CM, et al. Seizures and the regulation of neurotrophic factor expression: associations with structural plasticity in epilepsy. Adv Neurol. 1997;72:9–24. [PubMed] [Google Scholar]
- Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol. 2002;12:268–274. doi: 10.1016/s0959-4388(02)00326-4. [DOI] [PubMed] [Google Scholar]
- Goggi J, et al. The control of [125I]BDNF release from striatal rat brain slices. Brain Res. 2003;967:201–209. doi: 10.1016/s0006-8993(03)02225-x. [DOI] [PubMed] [Google Scholar]
- Green E, Craddock N. Brain-derived neurotrophic factor as a potential risk locus for bipolar disorder: evidence, limitations, and implications. Curr Psychiatry Rep. 2003;5:469–476. doi: 10.1007/s11920-003-0086-1. [DOI] [PubMed] [Google Scholar]
- Griesbeck O, et al. Are there differences between the secretion characteristics of NGF and BDNF? Implications for the modulatory role of neurotrophins in activity-dependent neuronal plasticity. Microsc Res Tech. 1999;45:262–275. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<262::AID-JEMT10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Gustafsson E, Lindvall O, Kokaia Z. Intraventricular infusion of TrkB-Fc fusion protein promotes ischemia-induced neurogenesis in adult rat dentate gyrus. Stroke. 2003;34:2710–2715. doi: 10.1161/01.STR.0000096025.35225.36. [DOI] [PubMed] [Google Scholar]
- Haapasalo A, et al. Truncated trkB.T1 is dominant negative inhibitor of trkB. TK +-mediated cell survival. Biochem Biophys Res Commun. 2001;280:1352–1358. doi: 10.1006/bbrc.2001.4296. [DOI] [PubMed] [Google Scholar]
- Haapasalo A, et al. Regulation of TRKB surface expression by brain-derived neurotrophic factor and truncated TRKB isoforms. J Biol Chem. 2002;277:43160–43167. doi: 10.1074/jbc.M205202200. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hallbook F, Ibanez CF, Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron. 1991;6:845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, et al. Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology. 2002;43:1173–1179. doi: 10.1016/s0028-3908(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Hong CJ, et al. Association study of a brain-derived neurotrophic-factor genetic polymorphism and mood disorders, age of onset and suicidal behavior. Neuropsychobiology. 2003;48:186–189. doi: 10.1159/000074636. [DOI] [PubMed] [Google Scholar]
- Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Howells DW, et al. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol. 2000;166:127–135. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip NY, et al. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci USA. 1992;89:3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isackson PJ, et al. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, et al. Tool-use learning induces BDNF expression in a selective portion of monkey anterior parietal cortex. Brain Res Mol Brain Res. 2002;102:110–112. doi: 10.1016/s0169-328x(02)00201-2. [DOI] [PubMed] [Google Scholar]
- Ito H, et al. Neurotrophins facilitate neuronal differentiation of cultured neural stem cells via induction of mRNA expression of basic helix-loop-helix transcription factors Mash 1 and Math 1. J Neurosci Res. 2003;71:648–658. doi: 10.1002/jnr.10532. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Patterson PH. The role of cytokines and growth factors in seizures and their sequelae. Prog Neurobiol. 2001;63:125–149. doi: 10.1016/s0301-0082(00)00022-8. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, et al. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafitz KW, et al. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kang H, et al. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, et al. Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. FASEB J. 2002;16:1328–1330. doi: 10.1096/fj.02-0143fje. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr BJ, et al. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19:5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K, et al. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- Kokaia M, et al. Suppressed epileptogenesis in BDNF mutant mice. Exp Neurol. 1995;133:215–224. doi: 10.1006/exnr.1995.1024. [DOI] [PubMed] [Google Scholar]
- Korte M, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, et al. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahteinen S, et al. Decreased BDNF signalling in transgenic mice reduces epileptogenesis. Eur J Neurosci. 2002;15:721–734. doi: 10.1046/j.1460-9568.2002.01897.x. [DOI] [PubMed] [Google Scholar]
- Larsson E, et al. Suppression of insult-induced neurogenesis in adult rat brain by brain-derived neurotrophic factor. Exp Neurol. 2002;177:1–8. doi: 10.1006/exnr.2002.7992. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, et al. Differential effects of protein synthesis inhibition on the activity-dependent expression of BDNF transcripts: evidence for immediate-early gene responses from specific promoters. J Neurosci. 1996;16:7428–7436. doi: 10.1523/JNEUROSCI.16-23-07428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, et al. The uniqueness of being a neurotrophin receptor. Curr Opin Neurobiol. 2001a;11:281–286. doi: 10.1016/s0959-4388(00)00209-9. [DOI] [PubMed] [Google Scholar]
- Lee R, et al. Regulation of cell survival by secreted proneurotrophins. Science. 2001b;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Hamburger V. Selective growth- stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116:321–361. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- Lindholm D, et al. Activity-dependent and hormonal regulation of neurotrophin mRNA levels in the brain—implications for neuronal plasticity. J Neurobiol. 1994;25:1362–1372. doi: 10.1002/neu.480251105. [DOI] [PubMed] [Google Scholar]
- Lindvall O, et al. Neurotrophins and brain insults. Trends Neurosci. 1994;17:490–496. doi: 10.1016/0166-2236(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Luikart BW, et al. In vivo role of truncated trkb receptors during sensory ganglion neurogenesis. Neuroscience. 2003;117:847–858. doi: 10.1016/s0306-4522(02)00719-4. [DOI] [PubMed] [Google Scholar]
- Lyons WE, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, et al. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990;247:1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Lessmann V. A common thread for pain and memory synapses? Brain-derived neurotrophic factor and trkB receptors. Trends Pharmacol Sci. 2003;24:116–121. doi: 10.1016/S0165-6147(03)00025-7. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, et al. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty S, et al. GABAergic stimulation regulates the phenotype of hippocampal interneurons through the regulation of brain-derived neurotrophic factor. Neuron. 1996;16:565–570. doi: 10.1016/s0896-6273(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Mathern GW, et al. Granule cell mRNA levels for BDNF. NGF, and NT-3 correlate with neuron losses or supragranular mossy fiber sprouting in the chronically damaged and epileptic human hippocampus. Mol Chem Neuropathol. 1997;30:53–76. doi: 10.1007/BF02815150. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- Merlio JP, et al. Increased production of the trkB protein tyrosine kinase receptor after brain insults. Neuron. 1993;10:151–164. doi: 10.1016/0896-6273(93)90307-d. [DOI] [PubMed] [Google Scholar]
- Metsis M, et al. Differential usage of multiple brain-derived neurotrophic factor promoters in the rat brain following neuronal activation. Proc Natl Acad Sci USA. 1993;90:8802–8806. doi: 10.1073/pnas.90.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FD, Kaplan DR. On Trk for retrograde signaling. Neuron. 2001;32:767–770. doi: 10.1016/s0896-6273(01)00529-3. [DOI] [PubMed] [Google Scholar]
- Minichiello L, et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Mowla SJ, et al. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosci. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla SJ, et al. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, et al. Association study of the brain-derived neurotrophic factor (BDNF) gene with bipolar disorder. Neurosci Lett. 2003;337:17–20. doi: 10.1016/s0304-3940(02)01292-2. [DOI] [PubMed] [Google Scholar]
- Neeper SA, et al. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Neves-Pereira M, et al. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Patterson SL, et al. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Patterson SL, et al. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Pencea V, et al. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, et al. Noxious stimulation induces Trk receptor and downstream ERK phosphorylation in spinal dorsal horn. Mol Cell Neurosci. 2002;21:684–695. doi: 10.1006/mcne.2002.1205. [DOI] [PubMed] [Google Scholar]
- Phillips HS, et al. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Reibel S, et al. Brain-derived neurotrophic factor delays hippocampal kindling in the rat. Neuroscience. 2000;100:777–788. doi: 10.1016/s0306-4522(00)00351-1. [DOI] [PubMed] [Google Scholar]
- Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Rivera C, et al. BDNF-induced TrkB activation down-regulates the K + –Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell BioI. 2002;159:747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roback JD, et al. BDNF-activated signal transduction in rat cortical glial cells. Eur J Neurosci. 1995;7:849–862. doi: 10.1111/j.1460-9568.1995.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Rocamora N, et al. Upregulation of BDNF mRNA expression in the barrel cortex of adult mice after sensory stimulation. J Neurosci. 1996;16:4411–4419. doi: 10.1523/JNEUROSCI.16-14-04411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, et al. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–78. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- Roux PP, et al. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci. 1999;19:6887–6896. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21:679–682. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, et al. Transgenic mice overexpressing truncated trkB neurotrophin receptors in neurons show increased susceptibility to cortical injury after focal cerebral ischemia. Mol Cell Neurosci. 2000a;16:87–96. doi: 10.1006/mcne.2000.0863. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, et al. Transgenic mice overexpressing truncated trkB neurotrophin receptors in neurons have impaired long-term spatial memory but normal hippocampal LTP. Synapse. 2000b;38:102–104. doi: 10.1002/1098-2396(200010)38:1<102::AID-SYN11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J Neurophysiol. 1997;78:1082–1095. doi: 10.1152/jn.1997.78.2.1082. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Actions of brain-derived neurotrophic factor in slices from rats with spontaneous seizures and mossy fiber sprouting in the dentate gyrus. J Neurosci. 1999;19:5619–5631. doi: 10.1523/JNEUROSCI.19-13-05619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, et al. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp Neurol. 2002;174:201–214. doi: 10.1006/exnr.2002.7869. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, et al. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Shieh PB, Ghosh A. Molecular mechanisms underlying activity-dependent regulation of BDNF expression. J Neurobiol. 1999;41:127–134. [PubMed] [Google Scholar]
- Shieh PB, et al. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Simonato M, et al. Dendritic targeting of mRNAs for plasticity genes in experimental models of temporal lobe epilepsy. Epilepsia. 2002;43(Suppl 5):153–158. doi: 10.1046/j.1528-1157.43.s.5.32.x. [DOI] [PubMed] [Google Scholar]
- Sklar P, et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002;7:579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- Smith MA, et al. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, et al. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J Neurosci. 2004;24:2270–2276. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, et al. Patients with temporal lobe epilepsy show an increase in brain-derived neurotrophic factor protein and its correlation with neuropeptide Y. Brain Res. 1999;818:579–582. doi: 10.1016/s0006-8993(98)01355-9. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, et al. Ca2+influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Tao X, et al. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002;33:383–395. doi: 10.1016/s0896-6273(01)00561-x. [DOI] [PubMed] [Google Scholar]
- Thakker-Varia S, et al. Rab3A is required for brain-derived neurotrophic factor-induced synaptic plasticity: transcriptional analysis at the population and single-cell levels. J Neurosci. 2001;21:6782–6790. doi: 10.1523/JNEUROSCI.21-17-06782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SW, et al. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc Natl Acad Sci USA. 1999;96:7714–7718. doi: 10.1073/pnas.96.14.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk T, et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Tolwani RJ, et al. BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience. 2002;114:795–805. doi: 10.1016/s0306-4522(02)00301-9. [DOI] [PubMed] [Google Scholar]
- Tsai SJ. Is mania caused by overactivity of central brain-derived neurotrophic factor? Med Hypotheses. 2004;62:19–22. doi: 10.1016/s0306-9877(03)00297-4. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Perrett SP, Pozzo-Miller LD. The role of neurotrophins in neurotransmitter release. Neuroscientist. 2002;8:524–531. doi: 10.1177/1073858402238511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle RA, Poo MM. Brain-derived neurotrophic factor modulation of GABAergic synapses by post-synaptic regulation of chloride transport. J Neurosci. 2003;23:8722–8732. doi: 10.1523/JNEUROSCI.23-25-08722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, et al. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- Yan Q, et al. Immunocytochemical localization of trkB in the central nervous system of the rat. J Comp Neurol. 1997a;378:135–157. [PubMed] [Google Scholar]
- Yan Q, et al. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997b;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- Zaccaro MC, et al. p75 Co-receptors regulate ligand-dependent and ligand-independent Trk receptor activation, in part by altering Trk docking subdomains. J Biol Chem. 2001;276:31023–31029. doi: 10.1074/jbc.M104630200. [DOI] [PubMed] [Google Scholar]
- Zigova T, et al. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]
- Zuccato C, et al. Loss of huntington-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- Zuccato C, et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]


