Abstract
The common technique of growing cells in two-dimensions (2-D) is gradually being replaced by culturing cells on matrices with more appropriate composition and stiffness, or by encapsulation of cells in three-dimensions (3-D). The universal acceptance of the new 3-D paradigm has been constrained by the absence of a commercially available, biocompatible material that offers ease of use, experimental flexibility, and a seamless transition from in vitro to in vivo applications. The challenge – the puzzle that needs a solution – is to replicate the complexity of the native extracellular matrix (ECM) environment with the minimum number of components necessary to allow cells to rebuild and replicate a given tissue. For use in drug discovery, toxicology, cell banking, and ultimately in reparative medicine, the ideal matrix would therefore need to be highly reproducible, manufacturable, approvable, and affordable. Herein we describe the development of a set of modular components that can be assembled into biomimetic materials that meet these requirements. These semi-synthetic ECMs, or sECMs, are based on hyaluronan derivatives that form covalently crosslinked, biodegradable hydrogels suitable for 3-D culture of primary and stem cells in vitro, and for tissue formation in vivo. The sECMs can be engineered to provide appropriate biological cues needed to recapitulate the complexity of a given ECM environment. Specific applications for different sECM compositions include stem cell expansion with control of differentiation, scar-free wound healing, growth factor delivery, cell delivery for osteochondral defect and liver repair, and development of vascularized tumor xenografts for personalized chemotherapy.
Keywords: semi-synthetic extracellular matrices, modular macromonomers, biomimetic materials
1. Deconstructing the Extracellular Matrix (ECM)
Natural cellular environments encompass both issues of scaffolding and proper cellular signaling. The ECM consists of an intricate mesh of glycosaminoglycans, structural and matricellular proteins that orchestrate complex signaling events and also confer structural support for various cellular processes. Depending on the cell/organ type, the native ECM has variable composition and compliance. Moreover, both spatially and temporally, cells are constantly remodeling and adapting their microenvironment to their developmental needs by synthesizing de novo ECM components and degrading preexisting ones.
While cell isolation and passage are by now routine processes, the scientific community is still searching for an optimal ECM equivalent that would accurately provide normal cell physiology, morphology, behavior, and gene expression under in vitro culturing conditions. The dramatic differences in signaling events and cellular genotype and phenotype for cells cultured in two-dimensional (2D) versus three-dimensional (3D) conditions underscore the limitations of tissue culture plastic (TCP) as a substratum [1, 2]. However, the caveat is finding a proper replacement for very convenient and easy-to-use TCP [3]. To address these unmet needs, a variety of natural, synthetic and semi-synthetic ECM analogs have now become available [3, 4].
Natural ECM sources provide the required natural biological molecules, the natural presentation of ligands, and the ability to be remodeled by cell-induced enzyme activity. However, murine sarcoma-derived materials such as Matrigel [5] raise issues such as limited availability, batch-to-batch variability, pathogen transmission, immunogenicity, technical challenges in handling, and experimental inertness (inability to experimentally vary composition and compliance) [3, 4]. Synthetic analogs, while addressing all the problems posed by their natural equivalents, most often fail to recapitulate essential biological features such as biodegradability or biological recognition [6, 7]. The solution to this impasse seems to be offered by semi-synthetic ECM analogs that encompass the positive features of both natural and synthetic matrices. These materials use natural components (i.e., glycosaminoglycans, collagens, silk, fibronectin) that are chemically processed or altered. The design features for these semi-synthetic biomaterials can be compared with puzzle pieces (identical to those found in native ECMs) that have chemically-designed “tabs” and “notches” required to reconstitute the “big picture” (the cellular microenvironment).
Following the same rationale, we chose to re-engineer the complexity of the ECM by starting with the simplicity of well-defined, manufacturable chemical entities. We therefore deconstructed the ECM into component protein and glycosaminoglycan components, synthesized semi-synthetic, modular macromonomers, and reassembled them into biomimetic hydrogels to be evaluated as engineered synthetic ECMs for 3-D cell culture, tissue engineering, and reparative medicine.
2. Solutions for the puzzle
Hyaluronan, also known as hyaluronic acid (HA), is one of the major components of native ECMs. HA is essential in water homeostasis of tissues, joint lubrication [8], stabilization of cartilage matrix [8, 9], cell motility [10, 11], morphogenesis and embryogenesis [12], and modulation of inflammation [13]. Unmodified HA has been used in numerous drug delivery and surgical applications [14], in viscosurgery, viscosupplementation and wound healing [15]. In many applications, however, the beneficial properties of HA were overshadowed by its rapid in vivo degradation and poor biomechanical properties. HA is an unbranched, polyanionic polymer composed of alternating units of glucuronic acid and N-acetylglucosamine [15]. The numerous primary hydroxyl and carboxyl groups offer attractive targets for chemical modification [16, 17], and a variety of both monolithic and living derivatives of HA have been prepared for use as medical devices and as scaffolds for cell culture and cell therapy [18].
Thus, we selected HA as the fundamental building block, the central piece to implement the vision of a modular, clinically versatile and readily-manufactured sECM. We will use the puzzle metaphor to illustrate the “mix and match” approach to assembling many different solutions to the puzzles presented by biology. A small number of puzzle pieces allows us to retain the simplicity, ease of sourcing, ease of manufacture, and straightforward regulatory pathway essential for a product that would be produced commercially for clinical use. Additional puzzle pieces can be introduced to recreate a particular biomimetic microenvironment, ultimately leading to a solution to the puzzle [3, 19].
One of our first synthetic routes used carbodiimide-mediated hydrazide chemistry to obtain a thiol-modified version of hyaluronan, known as HA-DTPH [20]. The HA-DTPH product was readily cross-linkable via disulfide bonds, and yielded biocompatible hydrogels but did not support cell attachment and proliferation. To better control the cross-linking process, cytocompatible cross-linkers were employed, e.g. polyethylene glycol diacrylate (PEGDA) [21], PEG divinyl sulfone [22], or other bivalent PEG electrophiles [23].
To obtain materials on which cells could readily attach and spread, the addition of “living” macromonomers with free thiol groups was required. Thus, we incorporated thiol-modified gelatin (Gtn-DTPH) [24, 25], RGD peptides [26] and cysteine-containing domains of recombinant human fibronectin [27] into the hydrogel formulations. Alternatively, the HA-DTPH was crosslinked into a decellularized matrix that provided attachment factors and a physical scaffold [28]. Or, simple addition of collagen provided a non-contracting 3-D hydrogel suitable for culture of human fibroblasts [29]. Such two-component hydrogels were tested with good results in three-dimensional (3-D) cell culture and tissue engineering applications [21].
Most tissue engineering applications require neovascularization and controlled growth factor release, and thus thiolated heparin (HP-DTPH) and chondroitin sulfate (CS-DTPH) macromonomers were developed. Each of these new modules added new biomimetic features and improved the therapeutic performance of the initial biomaterial. Thus, HP-DTPH containing hydrogels acted as heparan sulfate proteoglycan mimetics, providing spatiotemporal control of the release growth factors over a time course of weeks to months. Basic fibroblast growth factor (bFGF) was released in vitro with a half-live of one month from an HA-DTPH/HP-DTPH blend[30]; when released from a CS-DTPH/HP-DTPH blend, bFGF showed dose-dependent acceleration of re-epithelialization and improved formation of vascularized dermal tissue in the repair of large acute wounds in diabetic mice [31]. Dual delivery of vascular endothelial growth factor (VEGF) with bFGF [32] and VEGF with angiopoetin-1 (Ang-1) lead to enhanced neovascularization in vivo. CS-DTPH incorporation resulted in composites supporting fibroblast growth and offering a CS proteoglycan-like hydrogel for bone and cartilage repair [25].
To further stabilize HA-DTPH against hyaluronidase degradation and to increase cross-linking sites, a second-generation of HA derivatives was developed [3]. These biopolymers were synthesized starting from a carboxymethylated alternative of HA, to yield a thiol-enriched HA-DTPH version, known as CMHA-S or Carbylan-S. This newly-optimized puzzle piece became the basis for assembling modular puzzles in a large variety of combinations (Figure 1).
Figure 1.
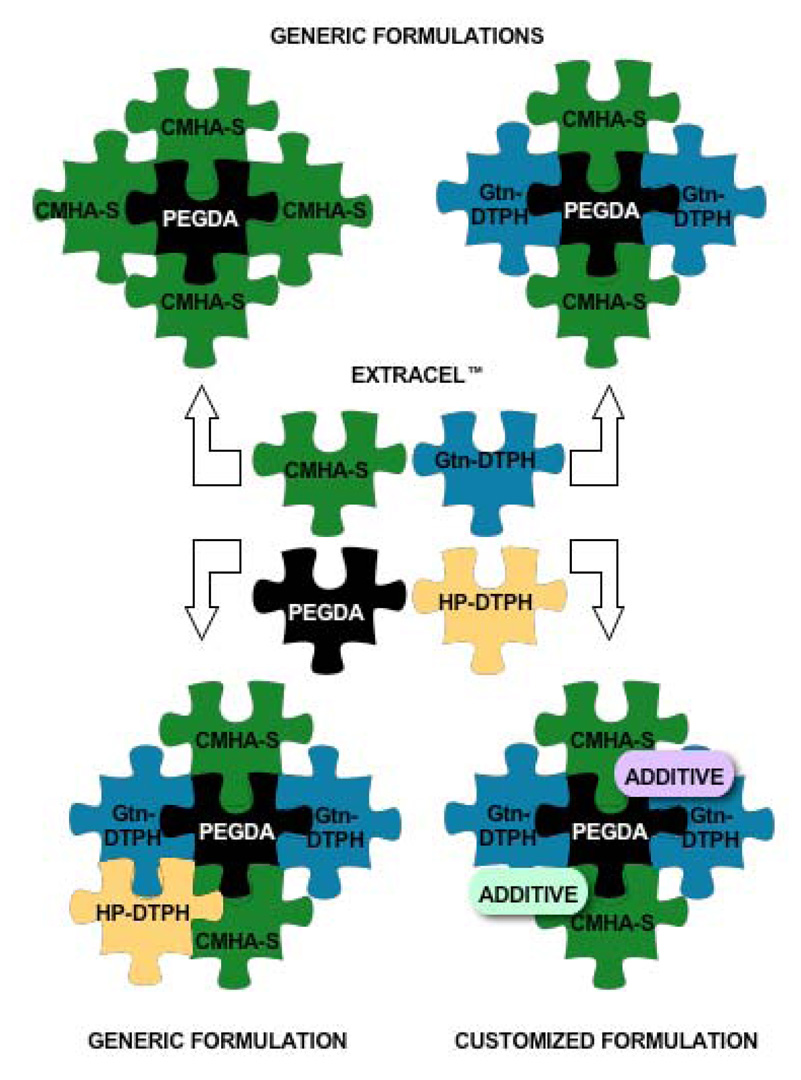
Mixing and matching modules to solve the biological puzzle.
As found for HA-DTPH, cross-linked CMHA-S biomaterials did not sustain cell attachment and spreading. However, these hydrogels promoted scar-free wound healing and prevented adhesion formations and were successfully employed in vivo for vocal fold repair [33], reduced post-operative intra-abdominal adhesions [34], reduced airway stenosis [35] and reduced pericardial adhesions [36], improved healing following endoscopic sinus surgery [37], and scar-free healing following middle ear injury [38].
As with HA-DTPH, addition of Gtn-DTPH to CMHA-S and crosslinking with PEGDA resulted in biomaterials that were cytoadherent had broad general utility for 3-D cell culture and cell delivery [3]. It was this sECM formulation that became available as Extracel (Glycosan BioSystems, Inc., Salt Lake City). It has been shown to be effective in vocal fold repair [39, 40], osteochondral defect repair via autologous stem cell delivery [41], femoral defect repair in conjunction with delivery of human demineralized bone matrix [42], tympanic membrane repair [43], in vivo liver repair with xenografted hepatocytes [19], and establishment of orthotopic human tumor xenografts in mice [44]. For many 3D cell culture and tissue engineering applications a generic 1:1 (w/w) CMHA-S to Gtn-DTPH composition appeared to be suitable. However, this was not always optimal; for example, an CMHA-S:Gtn-DTPH ratio of 95:5 (w/w) was optimal for tissue repair in a biopsied rabbit vocal fold [39, 40], indicating that an HA-rich milieu was important in this elastic and HA-rich tissue. In 3-D cell cultures, the use of Extracel™ as the cellular microenvironment yielded tissue-like constructs with a variety of cell types including human dermal fibroblasts, human breast epithelial cells, human adipose-derived stem cells L929 and 3T3 cell lines, and many others [45].
Nevertheless, in an in vitro study involving T31 human tracheal scar fibroblasts, the common hydrogel formulation lead to rounded, aberrant fibroblast morphology [4]. Our hypothesis was that the generic Extracel™ formulation was not providing sufficient attachment sites for the cell type being tested. Because of the puzzle-like, modular structure of the hydrogel, we decided to examine an easy-to-make, high-gelatin version of Extracel™ and use Matrigel™ as a benchmark for the 3D culture microenvironment in this study. Matrigel is natural, murine sarcoma-derived product, enriched in laminin, type IV, collagen, entactin and growth factors. It has been employed and tested in numerous 3-D cell culturing applications, for invasion assays, for tumor xenografts, and has become established as a valuable biological ECM replacement [5]. Nonetheless, Matrigel™ is not without drawbacks, the most serious of which include (i) difficulty of use, (ii) lack of experimental control of composition, (iii) batch-to-batch variability, and (iv) lack of utility in translational research for cell therapy [4]. The principal attributes of the two biomaterials tested in this study are summarized in Table 1.
Table 1.
Summary of key properties of Matrigel™ and Extracel™.
| ECM analog | ||
|---|---|---|
| Properties | Matrigel™ | Extracel™ |
| Source | Murine tumor | Semi-synthetic |
| Composition | Laminin, collagen, entactin, growth factors | Chemically-modified hyaluronan and chemically-modified gelatin Polyethylene glycol diacrylate (crosslinker) |
| Batch-to-batch consistency | No | Yes |
| Protocol features | Handled with pre-chilled equipment Gels rapidly at room temperature | Prepared at ambient physiological conditions Controllable gelation time |
| Growth factors present | Yes | No |
| Immunogenicity | Yes | No |
To reiterate, the generic formulation of Extracel™ consists of a 1:1 volume ratio of CMHA-S to Gtn-DTPH, crosslinked with PEGDA in a 4:1 volume ratio. The customized version that we intended to test contained a 1:4 (w/w) of CMHA-S to Gtn-DTPH. The morphology and viability of 3-D cultured fibroblasts after 6 days in culture was first examined (Figure 2A). The end point of this assay was arbitrarily set as the time by which cells had completely adapted to the new microenvironment. In both Matrigel™ and customized Extracel™, the live (green) cells adopted an extended, spindle-shaped morphology and the overall viable cell population was estimated to be approximately 95%. Based on the visual analysis of calcein AM/ ethidium homodimer-1 (EthD-1) stained cells, it appeared that cells proliferated faster in Extracel™ that those encapsulated in Matrigel™. This was further verified by quantifying the proliferation rates of 3-D cultured fibroblasts in an MTS colorimetric assay (Figure 2B). The variance for Matrigel™ was higher that that of Extracel™, because Matrigel™ gelled quite rapidly at room temperature, leading to pipetting inconsistencies. Compared to Matrigel™, Extracel™ sustained almost a two-fold higher increase in cell growth (p < 0.005).
Figure 2.
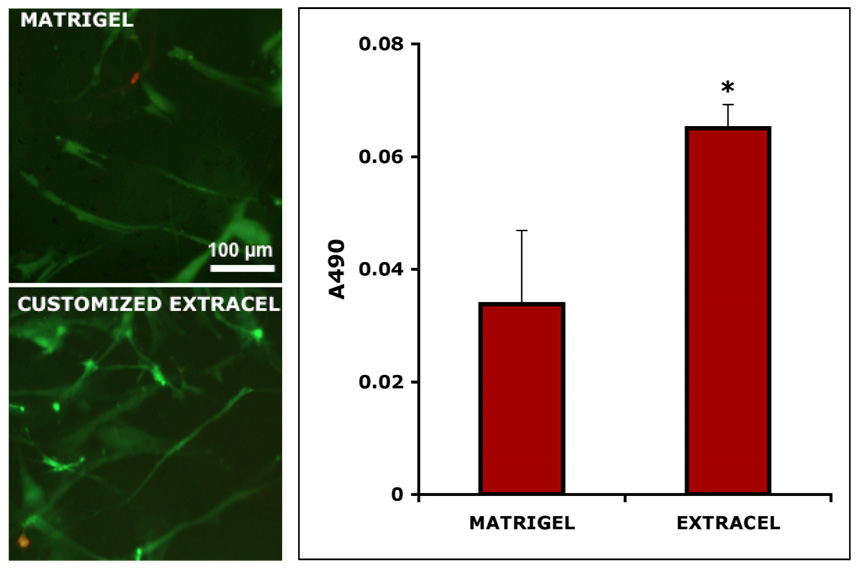
Customized Extracel™. Panel A: live/dead staining of Matrigel™ (left) and customized Extracel™ (right) encapsulated T31 fibroblasts. Panel B: proliferation rates (MTS assay) of 3-D cultured T31 fibroblasts.
Previously, our “puzzle pieces” had nucleophilic “tabs” in the form of thiol groups that could react with other thiolated “notches” to form disulfide bonds. Alternatively, the thiol “tabs” could react with electrophilic “notches” such as those in PEGDA to form crosslinked materials that were less stiff, more viscoelastic, more readily remodeled, and with better diffusivity for nutrient flow. As an alternative, we also developed thiol-reactive, electrophililic derivatives of HA that could react with the nucleophilic CMHA-S to yield “crosslinker-free” hydrogels [46]. To this end, reactive bromo- and iodoacetate functionalities were introduced at some of the primary hydroxyl groups of the N-acetylglucosamine residues to yield hyaluronan-haloacetate (HAHA) biopolymers. Hyaluronan-bromoacetate (HABA) was prepared directly from HA and bromoacetic anhydride and used to prepare hyaluronan-iodoacetate (HAIA) as well. The HAHA derivatives had an “inverted” reactivity that confers reactivity towards with polyvalent macromolecular nucleophiles. In vitro, both HABA and HAIA showed dose-dependent but mild cytotoxic effects on T31 fibroblasts, as expected for reactive electrophiles. However, cytocompatible hydrogels were obtained simply by reacting HABA or HAIA with macromolecular polynucleophiles (Figure 3).
Figure 3.
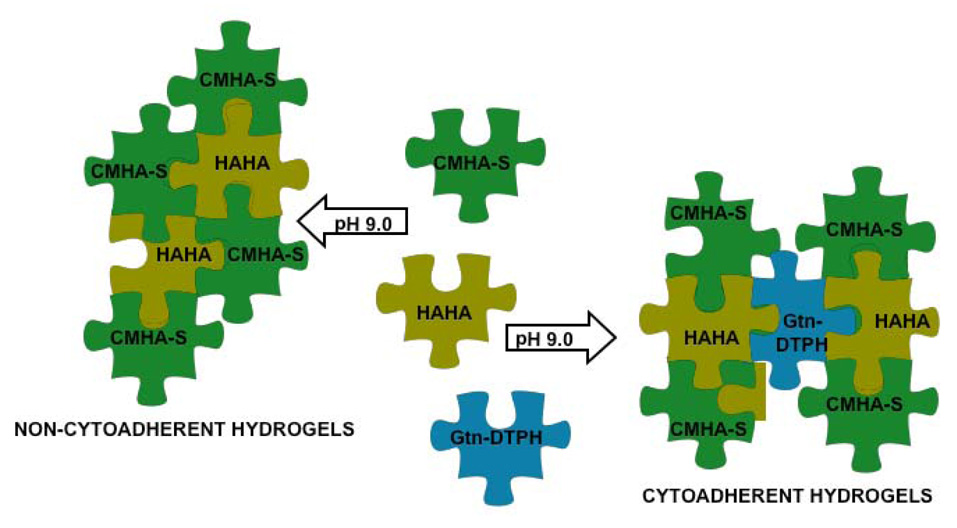
HAHA hydrogels.
When mixed with CMHA-S only, HAHA containing hydrogels were non-cytoadhesive, preventing cell attachment and proliferation. However, when mixed with CMHA-S plus Gtn-DTPH, a cytoadherent hydrogel was obtained that showed cell attachment and proliferation characteristics similar to Extracel. Thus, combining electrophilic and nucleophilic HA derivatives could provide novel hydrogels that are intriguing candidates for adhesion prevention and suitable as non-immunogenic, non-inflammatory, and non-cytoadhesive coating for medical devices such as stents and surgical implants. The crosslinker-free HAHA/CMHA-S hydrogels tend to be less rapidly degraded, both in the presence and absence of hyaluronidase, than the corresponding PEGDA-crosslinked CMHA-S hydrogel [46].
3. From the lab to the clinic: Why the modular approach is essential
A growing diversity of ECM analogs and 3-D scaffolds have reached the marketplace. Each ECM equivalent or scaffold offers unique properties that renders it appropriate for a specific research application or drug evaluation need. The common denominator however, is that none of the equivalents are suitable for all experimental needs. In contrast, the modular sECMs bring two unique and attractive features into play: versatility and translatability. A “one formulation fits all” approach cannot be viable for tissue engineering, drug efficacy evaluation or drug safety testing for all tissues. Ultimately, different products will likely be selected for a particular cell type and experimental design, based on the desired composition and intended end use of the cellularized construct.
The sECM concept deconstructs the complex ECM into simple, manufacturable component parts that can be reassembled in many ways. These modular sECMs are designed with the end-user in mind. First, they offer complete experimental flexibility, in that the user can adjust the composition, the stiffness, and the rate of degradation independently. The simplest crosslinked HA derivative allows separate incorporation of any additional factors in a combinatorial fashion to establish the minimum construct required to recapitulate full biological performance. Second, the materials can be fashioned as hydrogels or 3-D porous sponges for different uses in cell and tissue culture. In the hydrogel form, cells may be incorporated prior to crosslinking and gelation, allowing facile in vivo injection for cell delivery. Third, the cells can be readily recovered from the cellularized constructs after expansion, differentiation, and matrix remodeling has occurred. Fourth, the modules are separately synthesized, quality-controlled, and stored to give long-shelf life, batch-to-batch consistent materials that keep experimental variability to an absolute minimum. Fifth, the modular materials are readily recombined and handled at room temperature or physiological temperature at neutral pH. Sixth, the simplicity of handling and reproducibility allows incorporation into high-content and high-throughput cell based assays in academic and pharmaceutical industry environments. Finally, and most importantly, the modular sECMs offer seamless translation from in vitro to in vivo work, and from an animal-based experiment to an approvable cell therapy approach in the clinic.
Acknowledgments
This research was supported by the State of Utah Centers of Excellence Program, by NIH Grant 2 R01 DC04336 (to S. L. Thibeault and G.D. Prestwich), and by NSF FIBR Award EF0526854. We thank Dr. J. A. Scott, Glycosan BioSystems, Inc., for providing the Extracel™ components used for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bissell MJ, Kenny PA, Radisky DC. Cold Spring Harb. Symp Quant. Biol. 2005;70:343–356. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissell MJ, Rizki A, Mian IS. Curr. Opin. Cell Biol. 2003;15:753–762. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prestwich GD. J. Cell Biochem. 2007;101:1370–1383. doi: 10.1002/jcb.21386. [DOI] [PubMed] [Google Scholar]
- 4.Serban MA, Liu Y, Prestwich G. Acta Biomater. 2007 doi: 10.1016/j.actbio.2007.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 6.Cima LG. J. Cell Biochem. 1994;56:155–161. doi: 10.1002/jcb.240560206. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg M, Langer R, Jia X. J. Biomater. Sci. Polym. Ed. 2007;18:241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser JR, Laurent TC, Laurent UB. J. Intern. Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 9.Dowthwaite GP, Edwards JC, Pitsillides AA. J. Histochem. Cytochem. 1998;46:641–651. doi: 10.1177/002215549804600509. [DOI] [PubMed] [Google Scholar]
- 10.Collis L, Hall C, Lange L, Ziebell M, Prestwich R, Turley EA. FEBS Lett. 1998;440:444–449. doi: 10.1016/s0014-5793(98)01505-1. [DOI] [PubMed] [Google Scholar]
- 11.Hardwick C, Hoare K, Owens R, Hohn HP, Hook M, Moore D, Cripps V, Austen L, Nance DM, Turley EA. J. Cell Biol. 1992;117:1343–1350. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toole BP. J. Intern. Med. 1997;242:35–40. doi: 10.1046/j.1365-2796.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- 13.Gerdin B, Hallgren R. J. Intern. Med. 1997;242:49–55. doi: 10.1046/j.1365-2796.1997.00173.x. [DOI] [PubMed] [Google Scholar]
- 14.Saettone MF, Monti D, Torracca MT, Chetoni P. J. Ocul. Pharmacol. 1994;10:83–92. doi: 10.1089/jop.1994.10.83. [DOI] [PubMed] [Google Scholar]
- 15.Davidson JM, Nanney LB, Broadley KN, Whitsett JS, Aquino AM, Beccaro M, Rastrelli A. Clin. Mater. 1991;8:171–177. doi: 10.1016/0267-6605(91)90027-d. [DOI] [PubMed] [Google Scholar]
- 16.Allison D, Grande-Allen K. Biomaterials. 2006;12:2131–2140. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 17.Shu XZ, Prestwich GD. In: Chemistry and Biology of Hyaluronan, Vol. Garg HG, Hales CA, editors. Amsterdam: Elsevier Press; 2004. pp. 475–504. [Google Scholar]
- 18.Prestwich GD, Kuo J-w. Curr. Pharm. Biotech. 2007 in press. [Google Scholar]
- 19.Prestwich GD. Acc. Chem. Res. 2007 doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 20.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Biomacromolecules. 2002;3:1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 21.Shu XZ, Liu Y, Palumbo FS, Luo Y, Prestwich GD. Biomaterials. 2004;25:1339–1348. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh K, Shu XZ, Mou R, Lombardi J, Prestwich GD, Rafailovich MH, Clark RAF. Biomacromolecules. 2005;6:2857–2865. doi: 10.1021/bm050361c. [DOI] [PubMed] [Google Scholar]
- 23.Vanderhooft JL, Mann BK, Prestwich GD. Biomacromolecules. 2007;8:2883–2889. doi: 10.1021/bm0703564. [DOI] [PubMed] [Google Scholar]
- 24.Shu XZ, Liu Y, Palumbo F, Prestwich GD. Biomaterials. 2003;24:3825–3834. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 25.Shu XZ, Ahmad S, Liu Y, Prestwich GD. J. Biomed. Mater. Res. 2006;A 79:902–912. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 26.Shu XZ, Ghosh K, Liu Y, Palumbo FS, Luo Y, Clark RA, Prestwich GD. J. Biomed. Mater. Res. 2004;A 68:365–375. doi: 10.1002/jbm.a.20002. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh K, Ren XD, Shu XZ, Prestwich GD, Clark RA. Tissue Eng. 2006;12:601–613. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- 28.Flynn L, Prestwich GD, Semple JL, Woodhouse KA. Biomaterials. 2007;28:3834–3842. doi: 10.1016/j.biomaterials.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Mehra TD, Ghosh K, Shu XZ, Prestwich GD, Clark RAF. J. Invest. Dermatol. 2006;126:2202–2209. doi: 10.1038/sj.jid.5700380. [DOI] [PubMed] [Google Scholar]
- 30.Cai S, Liu Y, Zheng Shu X, Prestwich GD. Biomaterials. 2005;26:6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Cai S, Shu XZ, Shelby J, Prestwich GD. Wound Rep. Reg. 2007;15:245–251. doi: 10.1111/j.1524-475X.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- 32.Pike DB, Cai S, Pomraning KR, Firpo MA, Fisher RJ, Shu XZ, Prestwich GD, Peattie RA. Biomaterials. 2006;27:5242–5251. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Hansen JK, Thibeault SL, Walsh JF, Shu XZ, Prestwich GD. Ann. Otol. Rhinol. Laryngol. 2005;114:662–670. doi: 10.1177/000348940511400902. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Shu XZ, Prestwich GD. Fertil. Steril. 2007;87:940–948. doi: 10.1016/j.fertnstert.2006.07.1532. [DOI] [PubMed] [Google Scholar]
- 35.Sondrup C, Liu Y, Shu XZ, Prestwich GD, Smith ME. Otolaryngol. Head Neck Surg. 2006;135:28–35. doi: 10.1016/j.otohns.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Connors RC, Muir JJ, Liu Y, Reiss GR, Kouretas PC, Whitten MG, Sorenson TK, Prestwich GD, Bull DA. J. Surg. Res. 2007;140:237–242. doi: 10.1016/j.jss.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Orlandi RR, Shu XZ, McGill L, Petersen E, Prestwich GD. Laryngoscope. 2007;117:1288–1295. doi: 10.1097/MLG.0b013e318058a083. [DOI] [PubMed] [Google Scholar]
- 38.Park AH, Jackson A, Hunter L, McGill L, Simonsen SE, Alder SC, Shu XZ, Prestwich GD. Otol. Neurotol. 2006;27:1170–1175. doi: 10.1097/01.mao.0000227893.50162.9e. [DOI] [PubMed] [Google Scholar]
- 39.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Tissue Eng. 2006;12:2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 40.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Tissue Eng. 2006;12:3201–3207. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Shu XZ, Prestwich GD. Tissue Eng. 2006;12:3405–3416. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Ahmad S, Shu XZ, Sanders RK, Kopesec SA, Prestwich GD. J. Orthop. Res. 2006;24:1454–1462. doi: 10.1002/jor.20148. [DOI] [PubMed] [Google Scholar]
- 43.Park AH, Hughes CW, Jackson A, Hunter L, McGill L, Simonsen SE, Alder SC, Shu XZ, Prestwich GD. Otolaryngo.l Head Neck Surg. 2006;135:877–883. doi: 10.1016/j.otohns.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Shu XZ, Prestwich GD. Tissue Eng. 2007;13:1091–1101. doi: 10.1089/ten.2006.0297. [DOI] [PubMed] [Google Scholar]
- 45.Prestwich GD, Shu XZ, Liu Y, Cai S, Walsh JF, Hughes CW, Ahmad S, Kirker KR, Yu B, Orlandi RR, Park AH, Thibeault SL, Duflo S, Smith ME. Adv. Exp. Med. Biol. 2006;585:125–133. doi: 10.1007/978-0-387-34133-0_9. [DOI] [PubMed] [Google Scholar]
- 46.Serban MA, Prestwich GD. Biomacromolecules. 2007;8:2821–2828. doi: 10.1021/bm700595s. [DOI] [PubMed] [Google Scholar]


