Abstract
Background
Efforts to characterize genetic vulnerability to schizophrenia are increasingly focused on the identification of endophenotypes - neurobiological abnormalities that are evident in individuals at risk. Behavioral studies have demonstrated olfactory impairments in odor detection and identification in unaffected 1st-degree relatives of schizophrenia patients, suggesting that abnormalities in this simple sensory system may serve as candidate endophenotypes. It is unclear, however, whether these behavioral abnormalities reflect basic olfactory sensory processing deficits or nonspecific disruptions of attention and cognition.
Method
Unirhinal chemosensory olfactory evoked potentials were acquired from 14 unaffected 1st-degree relatives of schizophrenia patients and 20 healthy individuals with equivalent age and gender distributions, using 3 different concentrations of hydrogen sulfide. Subjects were also assessed behaviorally for ability to detect and identify odors.
Results
Family members exhibited left nostril olfactory detection impairments and bilateral olfactory identification abnormalities. They had reduced evoked potential response amplitudes for the initial N1 component in the left nostril. The subsequent P2 evoked potential response was reduced bilaterally. The pattern and magnitude of family member deficits were comparable to those previously observed for schizophrenia patients.
Conclusion
1st-degree relatives of schizophrenia patients exhibit specific neurophysiological impairments in early olfactory sensory processing. The presence of these neurophysiological abnormalities in both schizophrenia patients and their unaffected 1st-degree relatives suggests that these represent genetically mediated vulnerability markers or endophenotypes of the illness.
Keywords: schizophrenia, olfaction, endophenotype, evoked potentials, genetic vulnerability
1. INTRODUCTION
The complexity and heterogeneity of schizophrenia symptomatology have made it difficult to unravel the pathophysiology and etiology of the disorder. Family studies offer evidence for a significant genetic contribution to the illness (Kendler and Diehl, 1993). However, genetic vulnerability to schizophrenia need not result in overt clinical symptoms. Consequently, the nature of the genetic diathesis remains obscure and it is difficult to assess the extent of an individual’s inherited vulnerability. In an effort to reveal underlying susceptibility genes, schizophrenia research has turned to the endophenotype strategy (Turetsky et al., 2007). Endophenotypes are characteristics that reflect the actions of genes predisposing an individual to a disorder, even in the absence of diagnosable pathology. As relatively simple, well-defined and quantifiable biobehavioral characteristics, endophenotypes are presumably determined by fewer genes than the more complex phenotype of schizophrenia. Ideally, these markers could serve as dissected components of the complex schizophrenia phenotype, thereby reducing the complexity of genetic analyses.
In this context, olfactory neurocircuitry has emerged as a valuable pathway to assess the neuropathology of schizophrenia (Martzke et al., 1997; Moberg et al., 1999). Multiple studies have demonstrated that individuals with schizophrenia exhibit behavioral olfactory abnormalities, including deficits in odor detection threshold sensitivity and odor identification (Moberg et al., 1999). Such psychophysical findings, while intriguing, are inherently nonspecific and may be confounded with deficits that exist in other cognitive domains such as language and memory (Dulay et al., 2007). We have, however, reported the presence of both structural and neurophysiological abnormalities of the olfactory system. Patients with schizophrenia exhibited quantitative volume decrements in both the olfactory bulbs (Turetsky et al., 2000) and the olfactory cortex (Turetsky et al., 2003c), and physiological event-related potential (ERP) dysfunction following the presentation of olfactory chemosensory stimuli (Turetsky et al., 2003b). These findings indicate that there are fundamental abnormalities, in schizophrenia, in the neural circuitry underlying olfaction. This is consistent with recent in-vitro data suggesting abnormalities of olfactory receptor neurons in post-mortem (Arnold et al., 2001) and biopsy (Feron et al., 1999; McCurdy et al., 2006) material.
Extrapolating from the animal literature, it has been suggested that olfaction may be the sensory modality that is most highly genetically programmed (Barinaga, 2001). It is therefore reasonable to ask whether the deficits observed in patients might reflect, at least in part, a genetic predisposition consistent with an endophenotypic marker of vulnerability to the disorder. To this end, several studies have now determined that the 1st-degree relatives of schizophrenia probands exhibit psychophysical olfactory deficits that are similar to, though perhaps milder than, those of patients (Kopala et al., 1998, 2001; Roalf et al., 2006; Uqur et al., 2005). While such behavioral studies must be interpreted cautiously, the finding that unaffected family members are impaired in their ability to detect and/or identify odors suggests that olfactory deficits may indeed denote a genetically-mediated abnormality. We previously reported that 1st-degree relatives of patients have reduced olfactory bulb volumes compared to unrelated healthy comparison subjects (Turetsky et al., 2003a). This provided the first preliminary evidence that the neural substrate underlying olfaction is abnormal in unaffected family members.
The current study was designed to extend this line of inquiry by assessing the functional integrity of the olfactory neural circuitry in unaffected 1st-degree relatives of schizophrenia patients. Using a sophisticated olfactometer, we presented odorants separately to each nostril with precise control of stimulus concentration, onset and duration. This enabled us to record olfactory event-related potentials (OERPs) comparable to those elicited in other primary sensory modalities (Kobal and Hummel, 1988), with temporal resolution adequate to isolate the early primary sensory cortical response. By embedding the odorants seamlessly into a continuous air stream with precise control of temperature and humidity, we avoided the confounds of sniffing and trigeminal activation. Previous application of this methodology to schizophrenia patients confirmed the presence of a primary physiological impairment in the olfactory cortex underlying their observed behavioral deficits (Turetsky et al., 2003b). If, as we hypothesize, healthy family members exhibit similar physiological deficits, it would provide strong support for the idea that dysfunction at the level of the olfactory sensory cortex represents an endophenotypic marker of genetic vulnerability to schizophrenia.
2. METHODS
2.1 Subjects
The sample included 14 independent unaffected 1st-degree relatives of schizophrenia patients (8 siblings, 6 parents) and 20 healthy comparison subjects. The relatives were recruited from the outpatient psychiatric facilities at the Hospital of the University of Pennsylvania and through community outreach at community mental health centers and family support programs. Comparison subjects were recruited through advertisements in community newspapers and neighborhood bulletin boards. Ten family members and 7 comparison subjects were part of a larger sample for whom behavioral olfactory performance was previously reported (Roalf et al., 2006)
All subjects, including the related schizophrenia probands, received a semi-structured psychiatric interview (DIGS, Diagnostic Interview for Genetic Studies) and the Family Interview for Genetic Studies (FIGS). In order for a family member to be included in this study, the related patient required a consensus DSM-IV diagnosis of schizophrenia. Family members were excluded for any Axis I psychotic disorder or prodromal symptoms of a psychotic illness, but were not excluded for a previous non-psychotic Axis I disorder (e.g., major depression without psychotic features) if it resolved more than one year ago and was not associated with any current pharmacotherapy. Nor were they excluded on the basis of an Axis II diagnosis. Healthy comparison subjects were excluded for any history of an Axis I diagnosis (other than remitted substance abuse), Axis II Cluster A (schizotypal, schizoid, or paranoid) personality disorder, or family history of an Axis I psychotic disorder. Across groups, subjects were excluded for any history of a neurological disorder, including head trauma with loss of consciousness, any lifetime history of substance dependence, history of substance abuse within the preceding six months, or any medical condition that might affect cerebral functioning. Subjects were also excluded for any obvious cranio-facial trauma or abnormality, including septal deviation, and for any acute respiratory condition, cold, or allergy. Written informed consent was obtained after the procedures had been fully explained, in accordance with guidelines established by the University of Pennsylvania Institutional Review Board and with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Demographic and clinical characteristics of the sample are presented in Table 1. There were no significant differences in age [t(32) = 1.11, p = 0.27] or gender composition [X2(1)=1.56, p=0.21] across the two groups. There were more active smokers among the family members [X2(1)=7.22, p=0.01], as well as a significant difference in the mean number of cigarette packs smoked per day [t(14.3)=2.16, p=0.049 based on separate variance estimates]. There was also a significant increase greater history of past substance abuse among family members [X2(1)=6.48, p=0.011].
Table 1.
Demographic and Clinical Characteristics
| Family Members | Comparison Subjects | |
|---|---|---|
| Male/Female | 10/4 | 10/10 |
| Age (mean ± sd) | 38.8±17.6 | 32.7±14.5 |
| Age Range | 17 – 70 | 18 – 73 |
| Smokers (#) | 6** | 1 |
| Packs/day smoked (mean ± sd) | 0.40±0.62* | 0.04±0.17 |
| History of Major Depression (#) | 2 | 0 |
| History of Substance Abuse (#) | 4* | 0 |
| Schizotypal Personality Disorder (#) | 1 | 0 |
Group Difference: p<0.05,
p<0.01
2.2 Experimental Procedures
2.2.1 Olfactory Stimulation
Odor stimuli were presented to subjects via a dynamic multi-odorant air dilution olfactometer (OM4/B, Heinrich Burghart GmbH, Wedel, Germany). This computer-controlled apparatus allows for precisely timed pulses of odorants to be embedded in a constantly flowing air stream with specified temperature and humidity (36.5 C; 80% relative humidity) without transient pressure artifacts. A continuous airstream is delivered to one nasal chamber via a Teflon™ tube inserted approximately 1 cm into the naris. This airstream is then replaced by one of several odorized airstreams, using a non-electrical vacuum-switching device which allows for switching airstreams without pressure, thermal or auditory artifacts. This guarantees that the subject has no additional cues which could trigger other sensory systems or provide extraneous information about the timing of stimulus presentation.
Three different concentrations of hydrogen sulfide (H2S) (13.75 ppm, 8.25 ppm, 2.75 ppm, referred to as strong, medium and weak) were randomly presented, with a variable inter-stimulus interval of 20±5 seconds. Hydrogen sulfide was selected because, unlike some other odorants, it is a relatively pure olfactory nerve stimulant that does not simultaneously stimulate the trigeminal nerve through its somatosensory properties. The duration of each stimulus was 200ms with rise time less than 20ms. A total of 72 stimuli (24/condition) were presented to each nostril in two separate blocks. The order of presentation to the left and right nostrils was counterbalanced across subjects. Subjects were asked to sit quietly in a relaxed awake state and to breathe through the mouth. They practiced the proper breathing technique prior to data acquisition, and they were visually monitored throughout the study to ensure that the Teflon™ tube remained in place.
2.2.2 OERP Recording and Data Processing
Electrodes were applied to the scalp using a 32-channel Quickcap (Neuroscan, El Paso, TX). These included an expanded 10–20 system montage, with 31 scalp electrodes referenced to linked mastoids, and a pair of bipolar electrodes placed superior and lateral to the left eye to monitor eye movements. Electrical potentials were amplified with a Neuroscan Synamps 32-channel amplifier (gain: 1000; range: 5.5 mvolts; resolution: 0.084 μvolts; bandpass filter settings: 0.10–50.0 Hz).
Continuous EEG data were digitally sampled at 250 Hz and written to disk for offline post-processing. Continuous EEG data files were visually scanned for gross artifact and digitally filtered with a 0.50–20.0 Hz zero phase-shift bandpass filter (24 dB/octave). An eye-movement correction algorithm (Semlitsch et al., 1986) was applied to reduce EOG contamination. Data were then segmented into epochs extending from 500 ms pre-stimulus to 2500 ms post-stimulus and average waveforms were computed separately for each concentration of H2S.
Figure 1 illustrates the basic morphology of the olfactory ERP waveforms, with their characteristic N1 trough and P2 peak. Consistent with our previous analysis for schizophrenia patients (Turetsky et al., 2003b) the multi-channel data were reduced to underlying component waveforms using singular value decomposition (SVD). In contrast to typical peak detection methods that compare measures at a specified channel, SVD does not assume that the scalp topography of a given component is uniform across subjects and conditions. It also takes advantage of the correlation of evoked activity across electrodes to more effectively separate signal from noise (Cardenas et al., 1995). Separate component amplitudes and latencies were extracted for the N1 trough (100–600ms) and P2 peak (550–1400 ms), within the specified intervals, for each subject and condition.
Figure 1.
Grand average olfactory evoked potential waveforms for 1st-degree relatives and control subjects in response to strong and weak concentrations of hydrogen sulfide. Evoked potential morphology exhibits the characteristic N1 trough followed by the P2 peak.
2.2.3 Olfactory Psychophysical Assessment
All subjects also underwent a standardized psychophysical assessment of their ability to detect and to identify odors. Separate tests of the right and left nostril were conducted, with the other nostril being occluded by tape. The order of nostril testing was counterbalanced across subjects. A single staircase, forced-choice odor detection task was used to estimate basal detection sensitivity to phenyl ethyl alcohol (PEA), a compound with relatively low trigeminal stimulation properties (Doty et al., 1978). The subjects were required to smell successive pairs of odorants differing in concentration and to identify, which vial of odorant “smells stronger”. This test uses the geometric mean of the last four staircase reversal points of a total of seven as an estimate of odor detection threshold sensitivity. The ability to recognize and identify odors was assessed with the University of Pennsylvania Smell Identification Test (UPSIT) (Doty et al., 1984). This is a standardized, reliable (test-retest r=0.95) forty-item forced-choice test of olfactory identification (Doty et al., 1995). The stimuli are embedded in “scratch and sniff” microcapsules fixed and positioned on strips at the bottom of each page, with four response alternatives for each item located above the odorant strip. The subjects were asked to smell each scratched microencapsulated strip, and then pick one of four response alternatives that best fit the odor. Two booklets of the test were administered to the left nostril and two to the right, with booklet pairs and nostril presentation order systematically counterbalanced among subjects.
2.3 Statistical Analysis
Statistical analyses were conducted using the General Linear Model (GLM) as implemented in STATISTICA™ (StatSoft® Inc., Tulsa Oklahoma). Multivariate analyses of covariance (MANCOVA) was performed with group membership and gender as between-subjects factors, nostril and H2S concentration as within-subject factors, and age as a covariate. For the electrophysiological data, N1 and P2 latencies and amplitudes were examined separately. For the psychophysical measures (threshold detection sensitivity and odor identification), nostril was the only within-subjects factor. To control for the effects of smoking, all analyses included smoking, as measured by packs/day, as an additional continuous predictor variable. Since our a priori hypothesis was that unaffected family members of schizophrenia patients would exhibit olfactory deficits relative to healthy comparison subjects, statistical significance was assessed using a 1-tailed probability threshold of p<0.05 for family members < control subjects. Significant multivariate effects were dissected by appropriate post-hoc univariate contrasts.
Relationships between OERP and behavioral measures were examined using the general linear model (GLM) with diagnosis and OERP measures as predictors. Threshold and identification scores were dependent variables in two separate analyses, with nostril tested as a within-subjects factor for each dependent measure. To restrict the number of independent variables in the model, we included only those OERP measures that exhibited robust group differences in the initial MANCOVA analyses.
3. RESULTS
3.1 Psychophysical Measures
There was a significant group difference in the ability to detect odors at low threshold concentrations [F(1,28)=3.01, p<0.05] and a significant group X nostril interaction [F(1,28)=2.63, p<0.05]. Although family members had poorer mean odor detection threshold sensitivities for both the right and left nostrils, only the left nostril difference was statistically significant [Left nostril: F(1,28)=6.22, p<0.01; Right nostril: [F(1,28)=0.41, p=0.53]. For odor identification, there was a similar main effect of group [F(1,28)=12.95, p<.001] and a group X nostril interaction [F(1,28)=4.07, p<0.05]. In this case, family members exhibited significant odor identification deficits for both the left [F(1,28)=4.27, p<0.05] and the right [F(1,28)=15.23, p<0.001] nostrils, but the magnitude of the decrement was substantially greater on the right side. There were no significant main effects or interactions on either odor detection threshold sensitivity or odor identification. Mean scores for both measures are presented in Table 2.
Table 2.
Olfactory Psychophysical Measures (mean±sd)
| Family Members | Control Subjects | |
|---|---|---|
| Olfactory Threshold Sensitivity (-log concentration) | ||
| Left Nostril | 5.43±.1.49* | 6.28±.1.20 |
| Right Nostril | 5.70±.1.58 | 5.81±.1.38 |
| Olfactory Identification (# correct out of 20) | ||
| Left Nostril | 17.04±.2.76* | 17.75±.1.55 |
| Right Nostril | 16.79±.4.17*** | 18.00±.1.78 |
Group difference: p<0.05,
p<0.001
3.2 Olfactory Event-Related Potentials Results
N1 Component
Latency
There were no group main effects or interaction effects for N1 component latency.
Amplitude
There was a significant three-way interaction of group X odor concentration X nostril on N1 amplitude [F(2,56)=3.85, p<.05]. As illustrated in Figure 2, family members had an isolated decrement in the amplitude of the N1 when weak concentrations of the odorant were presented to the left nostril [F(1,28)=3.27, p<0.05]. There were no significant group differences for any other combination of odor concentration and nostril. There were no main or interaction effects of smoking.
Figure 2.
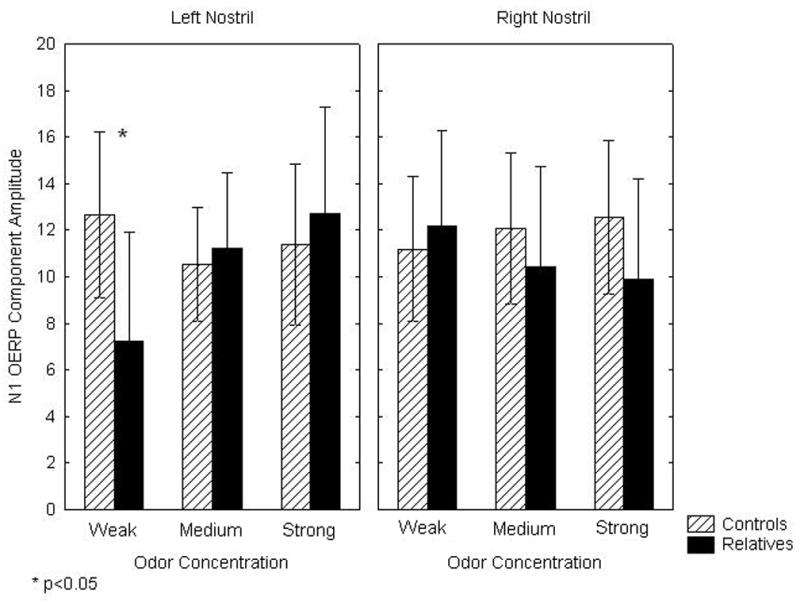
Mean (± 95% confidence interval) N1 olfactory evoked potential component amplitudes for 1st-degree relatives and control subjects in response to weak, medium and strong concentrations of hydrogen sulfide.
P2 Component
Latency
There were no group main effects or interaction effects for P2 component latency.
Amplitude
There was a significant interaction of group X odor concentration on P2 amplitude [F(2,56)=3.82, p<.05]. As illustrated in Figure 3, mean amplitude of the control subject response increased in a step-wise manner with increasing odor strength. The family member response failed to show this progressive increase with increasing odor concentration. Instead, there was a fall-off in response amplitude following the strong stimulus. This resulted in a significant family member amplitude decrement, relative to controls, for the strong odor concentration [F(1,28)=4.92, p<0.05], but not for medium [F(1,28)=1.92, p=0.18] or weak [F(1,28)=0.02, p=0.88] concentration stimuli. This group difference was observed for both the left [F(1,28)=2.91, p<0.05] and right [F(1,28)=4.55, p<0.05] nostrils, though it was nominally larger on the right side. Overall effect size for the bilateral P2 deficit (Cohen’s d) was 1.04, indicating a large effect. Smoking status had no effect on this observed group difference.
Figure 3.
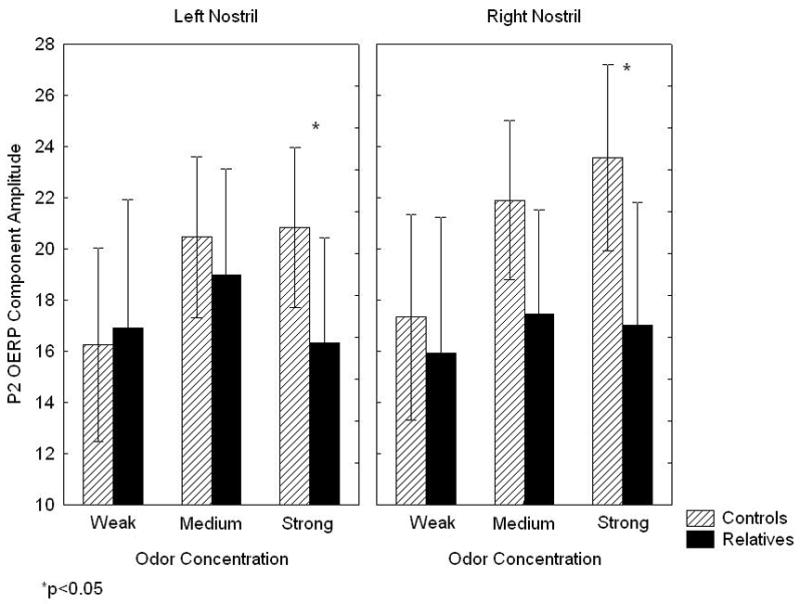
Mean (± 95% confidence interval) P2 olfactory evoked potential component amplitudes for 1st-degree relatives and control subjects in response to weak, medium and strong concentrations of hydrogen sulfide.
3.3 Relationship Between OERP and Psychophysical Measures
The MANCOVA findings above indicated that three OERP responses discriminated between family members and controls: N1 amplitude following a weak odorant presented to the left nostril and P2 amplitude following a strong odorant presented to either the left or right nostril. These three OERP measures were therefore included, with group, as independent variables, with detection threshold sensitivity and UPSIT scores as dependent measures in separate analyses. Initial tests for parallelism (i.e., homogeneity of slopes across the two groups) found no significant differences in the relationship between independent and dependent variables in patients and controls. We therefore excluded group X OERP interaction terms from the model. Since the right and left nostril P2 amplitude were significantly correlated (r=0.63, p<0.001), we also assessed potential instability due to multicollinearity. The variance inflation factor, or VIF, is a diagnostic measure of multicollinearity among independent variables. In this case, the maximum VIF was 1.86 for right nostril P2 amplitude, indicating a stable regression model.
Collectively, the model accounted for 8% and 19% of the variance, respectively, in right and left nostril detection threshold sensitivity. It accounted for 16% and 33% of the variance in right and left nostril odor identification performance. There was a specific significant relationship between the left nostril N1 response to weak odor stimuli and left nostril odor identification performance, with greater N1 amplitude predicting better identification performance [F(1,29)=8.69, p<0.01]. There was also a marginally significant relationship between this same left nostril N1 response and left nostril odor detection threshold sensitivity [F(1,29)=3.02, p<0.05 1-tailed], with greater N1 amplitude predicting greater detection sensitivity. There were no significant associations between the olfactory behavioral measures and either P2 amplitude measures or group.
4. DISCUSSION
This study demonstrates the presence of both behavioral and OERP abnormalities of the olfactory system in unaffected family members of schizophrenia patients. Behaviorally, family members had bilateral impairments in odor identification and selective left nostril impairment in odor detection threshold sensitivity. Our previous study of unirhinal olfactory deficits in unaffected 1st-degree relatives of schizophrenia patients (Roalf et al., 2006), which utilized a larger sample, reported comparable birhinal odor identification deficits, but no abnormalities in odor detection thresholds. Notably, it was the comparison subjects from that study who had worse odor detection thresholds than the comparison subjects in the current sample, rather than a difference among the family members. This highlights one of the problems associated with these behavioral tests, i.e., their test-retest variability and their sensitivity to cognitive or state-dependent factors other than olfaction. Tests of odor detection threshold sensitivity, in particular, seem to be somewhat less reliable measures of olfactory function than tests of odor identification or discrimination (Doty et al., 1995), and this likely explains the difference in behavioral findings across the two studies.
However, the presence of OERP abnormalities implies that olfactory behavioral deficits are not simply a reflection of more diffuse cognitive impairments, such as disturbed attention, associated with schizophrenia. Rather, they indicate that there are specific neurophysiological sensory impairments underlying the observed behavioral deficits. The presence of such neurophysiological abnormalities in both schizophrenia patients (Turetsky et al., 2003b) and unaffected 1st-degree relatives of patients also suggests that these neurophysiological impairments represent genetically mediated vulnerability markers or endophenotypes.
It is important to emphasize that these OERP abnormalities were elicited by repetitive stimulation of the first cranial nerve in the complete absence of any attentional or cognitive processing demands. Although odor concentration varied, none of the stimuli were qualitatively distinctive or task salient, and subjects were not cued in any way prior to stimulus presentation. Also, the long inter-stimulus interval and jitter would have made it very difficult to maintain vigilance and accurately anticipate stimulus onset. While we cannot entirely rule out the possibility that healthy participants allocated more attentional resources to olfactory stimulus processing than family members, it is also not clear that this would have had any effect on the OERP measures (Geisler and Murphy, 2000; Krauel et al., 1998; Pause et al., 1997). It is therefore very unlikely that the group differences observed in this experiment can be explained by such modality non-specific factors. Rather, this would seem to represent a specific disruption of olfactory afferent sensory processing.
Potential confounds that may have contributed to the disruption of olfactory processing in unaffected family members include the effects of other co-morbid psychiatric conditions and smoking. Family members smoked more heavily than comparison subjects. In our data analysis, we observed no statistically significant effects of smoking on any of our olfactory behavioral or ERP measures, so the abnormalities we observed cannot be attributed to this. However, we cannot rule out the possibility that the magnitudes of the deficits may have been increased by the group difference in smoking. Family members were also more likely to have a prior history of substance abuse. Unaffected family members of schizophrenia patients are known to have a greater prevalence of non-psychotic behavioral and psychiatric disturbances than is found in the general population (Walsh and Gill, 1993). These conditions are likely to represent, at least in part, alternate phenotypic expressions of the underlying genetic vulnerability of these individuals (Kendler et al., 1996). Therefore, to exclude all unaffected family members who have a history of other psychiatric disorders is to exclude those individuals who are most likely to manifest the endophenotypic traits of interest. The compromise position that we adopted was to exclude family members in which other psychiatric or substance use disorders were currently or recently active, but not to exclude those with a more remote history of these disorders. Although the presence of active substance use has been associated with olfactory impairment (Rupp et al., 2003), there is no reason to suspect that a remote past history of substance abuse without dependence would impair olfactory abilities. The same applies to a past history of non-psychotic mood disturbance (Lombion-Pouthier et al., 2006). It should also be emphasized that only a minority of the unaffected family members in this study actually had histories of either mood disturbance or substance abuse.
How then might these deficits be interpreted? The N1 is the earliest measurable OERP component. It is thought to reflect olfactory cortical activity directly related to sensory processing of the physical attributes of the chemosensory afferent input (Pause and Krauel, 2000; Rombaux et al., 2006), and source localization models suggest that it is generated in the medial temporal lobe areas around and within the primary olfactory cortex (Kettenmann et al., 1997). The P2 probably reflects subsequent complex, but still obligate, stimulus processing in secondary olfactory cortical areas. However, the functional significance of this component has not been established and there is no clear consensus as to whether this is an entirely exogenous component (Cui and Evans, 1997; Durand-Lagarde and Kobal, 1991; Kobal and Hummel, 1991; Lorig, 2000; Pause and Krauel, 2000; Rombaux et al., 2006). The fact that N1 latency was normal suggests that basic signal transmission from peripheral receptor neurons through the olfactory bulb to the primary sensory cortex was not impeded in family members, despite the fact that abnormalities may exist in more peripheral olfactory structures (Turetsky et al., 2003a).
However, the fact that the family members had reduced N1 amplitudes to weak odorants presented to the left nostril suggests that their left hemisphere olfactory cortical response to afferent inputs is deficient. This is similar to what we previously observed for schizophrenia patients (Turetsky et al., 2003b), but in that case N1 amplitude was significantly reduced across both nostrils and all three odor concentrations. This implies that the underlying physiological impairment is less severe in family members, since it is only seen when the system is required to respond to a low-level input. It also suggests that a disruption at the level of the left olfactory cortex may be a relative specific marker of genetic vulnerability independent of overt illness. It is notable, in this context, that this N1 amplitude measure also significantly predicted odor identification and odor detection performance, since olfactory behavioral measures have been shown to predict conversion to schizophrenia amongst high-risk adolescents (Brewer et al., 2003).
One necessary caveat to this interpretation of the N1 amplitude decrement is the extent to which left nostril deficits can be equated with deficits in the left olfactory cortex. Although the vast majority of olfactory afferents project from the nasal epithelium to the ipsilateral olfactory bulb and, from there, to the ipsilateral cortex, a small number of axons project from the olfactory bulb to the contralateral cortex via the anterior commissure (Lohman and Mentink, 1969). Also, although subjects were instructed to breathe through their mouths and were monitored for compliance, it is possible that there was a degree of contralateral olfactory receptor neuron stimulation from retro-nasal transmission of odorant to the contralateral nostril. It is likely that any contralateral activation through these two mechanisms would be relatively small compared to the ipsilateral activity, and the general equation of nostril with hemisphere is valid. Nevertheless, the conclusion that family members have a left olfactory cortex abnormality requires confirmation with either a three-dimensional imaging modality (such as fMRI) or a more sensitive source localization method (such as magnetoencephalography).
Family members also exhibited an abnormal ceiling effect for P2 amplitude. Among the control subjects, P2 amplitude increased in a step-wise manner with increased odor concentrations. Family members, however, failed to show this incremental response. Instead, their response to the strong odor was smaller than their response to the medium concentration stimulus. It is as if the elements of the olfactory system denoted by the P2 ERP are operating at a lower “maximum capacity” that can not respond to a further increase in odor strength. This is very similar to the pattern of P2 abnormalities that we observed in schizophrenia patients (Turetsky et al., 2003b), and the magnitude of the effect (1.04) is also comparable to the effect size for patients (1.14).
An important unanswered question is the relative diagnostic specificity of these OERP abnormalities to schizophrenia patients and their relatives. As we have already noted, behavioral olfactory deficits are not unique to schizophrenia (Moberg et al., 1997; Doty et al., 1988). However, to the extent that these electrophysiological measures are independent of higher cognitive processes, they are likely to be more specific indicators of selective olfactory deficits. It is possible, therefore, that neuropsychiatric disorders that are indistinguishable on psychophysical measures will exhibit different profiles of OERP abnormalities, both among affected individuals and among those at genetic risk of illness. While these findings must be considered preliminary, they are consistent with the conclusion that neurophysiological disturbances in the olfactory system may be sensitive endophenotypic markers of genetic vulnerability to schizophrenia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold SE, Han LY, Moberg PJ, Turetsky BI, Gur RE, Trojanowski JQ, Hahn CG. Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry. 2001;58:829–835. doi: 10.1001/archpsyc.58.9.829. [DOI] [PubMed] [Google Scholar]
- Barinaga M. Olfaction. Smell’s course is predetermined. Science. 2001;294:1269–1271. doi: 10.1126/science.294.5545.1269. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, McGorry PD, Francey SM, Phillips LJ, Yung AR, Anderson V, Copolov DL, Singh B, Velakoulis D, Pantelis C. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160:1790–4. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Yingling CD, Jewett D, Fein G. A multichannel, model-free method for estimation of event-related potential amplitudes and its comparison with dipole source localization. J Med Eng Technol. 1995;19:88–98. doi: 10.3109/03091909509030282. [DOI] [PubMed] [Google Scholar]
- Cui L, Evans WJ. Olfactory event-related potentials to amyl acetate in congenital anosmia. Electroenceph Clin Neurophysiol. 1997;102:303–6. doi: 10.1016/s0013-4694(96)96109-x. [DOI] [PubMed] [Google Scholar]
- Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav. 1978;20:175–85. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]
- Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237–44. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- Doty RL, McKeown DA, Lee WW, Shaman P. A study of the test-retest reliability of ten olfactory tests. Chem Senses. 1995;20:645–56. doi: 10.1093/chemse/20.6.645. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Dulay MF, Gesteland RC, Shear PK, Ritchey PN, Frank RA. Assessment of the influence of cognition and cognitive processing speed on three tests of olfaction. J Clin Exp Neuropsychol. 2007;25:1–11. doi: 10.1080/13803390701415892. [DOI] [PubMed] [Google Scholar]
- Durand-Lagarde M, Kobal G. P300: a new technique for recording the cognitive component in olfactory evoked potentials. Chem Senses. 1991;16:379. [Google Scholar]
- Feron F, Perry C, Hirning MH, McGrath J, Mackay-Sim A. Altered adhesion, proliferation and death in neural cultures from adults with schizophrenia. Schizophr Res. 1999;40:211–218. doi: 10.1016/s0920-9964(99)00055-9. [DOI] [PubMed] [Google Scholar]
- Geisler MW, Murphy C. Event-related brain potentials to attended and ignored olfactory and trigeminal stimuli. Int J Psychophysiol. 2000;37:309–15. doi: 10.1016/s0167-8760(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Diehl SR. The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull. 1993;19:261–285. doi: 10.1093/schbul/19.2.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski-Shuman L, Walsh D. The Risk for Psychiatric Illness in Siblings of Schizophrenics: The Impact of Psychotic and Non-Psychotic Affective Illness and Alcoholism in Parents. Acta Psychiatr Scand. 1996;94:49–55. doi: 10.1111/j.1600-0447.1996.tb09824.x. [DOI] [PubMed] [Google Scholar]
- Kettenmann B, Hummel C, Stefan H, Kobal G. Multiple olfactory activity in the human neocortex identified by magnetic source imaging. Chem Senses. 1997;22:493–502. doi: 10.1093/chemse/22.5.493. [DOI] [PubMed] [Google Scholar]
- Kobal G, Hummel C. Cerebral chemosensory evoked potentials elicited by chemical stimulation of the human olfactory and respiratory nasal mucosa. Electroenceph Clin Neurophysiol. 1988;71:241–250. doi: 10.1016/0168-5597(88)90023-8. [DOI] [PubMed] [Google Scholar]
- Kobal G, Hummel T. Olfactory evoked potentials in humans. In: Getchell TV, Doty RL, Bartoshuk LM, Snow JB, editors. Smell and Taste in Health and Disease. Raven Press Inc; New York: 1991. pp. 255–275. [Google Scholar]
- Kopala LC, Good KP, Morrison K, Bassett AS, Alda M, Honer WG. Impaired olfactory identification in relatives of patients with familial schizophrenia. Am J Psychiatry. 2001;158:1286–1290. doi: 10.1176/appi.ajp.158.8.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopala LC, Good KP, Torrey EF, Honer WG. Olfactory function in monozygotic twins discordant for schizophrenia. Am J Psychiatry. 1998;155:134–136. doi: 10.1176/ajp.155.1.134. [DOI] [PubMed] [Google Scholar]
- Krauel K, Pause BM, Sojka B, Schott P, Ferstl R. Attentional modulation of central odor processing. Chem Senses. 1998;23:423–32. doi: 10.1093/chemse/23.4.423. [DOI] [PubMed] [Google Scholar]
- Lohman AHM, Mentink GM. The lateral olfactory tract, the anterior commissure and the cells of the olfactory bulb. Brain Res. 1969;12:396–413. doi: 10.1016/0006-8993(69)90008-0. [DOI] [PubMed] [Google Scholar]
- Lombion-Pouthier S, Vandel P, Nezelof S, Haffen E, Millot JL. Odor perception in patients with mood disorders. J Affect Disord. 2006;90:187–191. doi: 10.1016/j.jad.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Lorig TS. The application of electroencephalographic techniques to the study of human olfaction: a review and tutorial. Int J Psychophysiol. 2000;36:91–104. doi: 10.1016/s0167-8760(99)00104-x. [DOI] [PubMed] [Google Scholar]
- Maffei C, Fossati A, Agostoni I, Barraco A, Bagnato M, Deborah D, Namia C, Novella L, Petrachi M. Interrater reliability and internal consistency of the structured clinical interview for DSM-IV axis II personality disorders (SCID-II), version 2.0. J Personal Disord. 1997;11:279–284. doi: 10.1521/pedi.1997.11.3.279. [DOI] [PubMed] [Google Scholar]
- Martzke JS, Kopala LC, Good KP. Olfactory dysfunction in neuropsychiatric disorders: review and methodological considerations. Biol Psychiatry. 1997;42:721–732. doi: 10.1016/s0006-3223(96)00442-8. [DOI] [PubMed] [Google Scholar]
- McCurdy RD, Féron F, Perry C, Chant DC, McLean D, Matigian N, Hayward NK, McGrath JJ, Mackay-Sim A. Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophr Res. 2006;82:163–173. doi: 10.1016/j.schres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology. 1999;21:325–340. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Doty RL, Mahr RN, Mesholam RI, Arnold SE, Turetsky BI, Gur RE. Olfactory identification in elderly schizophrenia and Alzheimer’s disease. Neurobiol Aging. 1997;18:163–7. doi: 10.1016/s0197-4580(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Pause BM, Krauel K. Chemosensory event-related potentials (CSERP) as a key to the psychology of odors. Int J Psychophysiol. 2000;36:105–22. doi: 10.1016/s0167-8760(99)00105-1. [DOI] [PubMed] [Google Scholar]
- Pause BM, Sojka B, Ferstl R. Central processing of odor concentration is a temporal phenomenon as revealed by chemosensory event-related potentials (CSERP) Chem Senses. 1997;22:9–26. doi: 10.1093/chemse/22.1.9. [DOI] [PubMed] [Google Scholar]
- Roalf DR, Turetsky BI, Owzar K, Balderston CC, Johnson SC, Brensinger CM, Gur RE, Siegel SJ, Moberg PJ. Unirhinal olfactory function in schizophrenia patients and first-degree relatives. J Neuropsychiatry Clin Neurosci. 2006;18:389–96. doi: 10.1176/jnp.2006.18.3.389. [DOI] [PubMed] [Google Scholar]
- Rombaux P, Mouraux A, Bertrand B, Guerit JM, Hummel T. Assessment of olfactory and trigeminal function using chemosensory event-related potentials. Neurophysiol Clin. 2006;36:53–62. doi: 10.1016/j.neucli.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Kurz M, Kemmler G, Mair D, Hausmann A, Hinterhuber H, Fleischhacker WW. Reduced olfactory sensitivity, discrimination, and identification in patients with alcohol dependence. Alcohol Clin Exp Res. 2003;27:432–439. doi: 10.1097/01.ALC.0000057945.57330.2C. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: The viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Arnold SA, Doty RL, Gur RE. Low olfactory bulb volume in first-degree relatives of patients with schizophrenia. Am J Psychiatry. 2003a;160:703–708. doi: 10.1176/appi.ajp.160.4.703. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Owzar K, Johnson SA, Doty RL, Gur RE. Physiological impairment of olfactory stimulus processing in schizophrenia. Biol Psychiatry. 2003b;53:403–411. doi: 10.1016/s0006-3223(02)01865-6. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Roalf DR, Arnold SA, Gur RE. Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch Gen Psychiatry. 2003c;60:1193–1200. doi: 10.1001/archpsyc.60.12.1193. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE. Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry. 2000;157:828–830. doi: 10.1176/appi.ajp.157.5.828. [DOI] [PubMed] [Google Scholar]
- Ugur T, Weisbrod M, Franzek E, Pfuller U, Sauer H. Olfactory impairment in monozygotic twins discordant for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2005;255:94–8. doi: 10.1007/s00406-004-0536-8. [DOI] [PubMed] [Google Scholar]
- Walsh C, Gill M. Psychiatric Morbidity in the Relatives of Schizophrenic Probands. Br J Psychiatry. 1993;163:695. doi: 10.1192/bjp.163.5.695b. [DOI] [PubMed] [Google Scholar]



