Abstract
Studies of the electrophysiological response to acetylcholine (ACh) in mammalian outer hair cells (OHCs) are hindered by the presence of a large potassium current, IK,n, most likely mediated by channels containing the KCNQ4 subunit. Since IK,n can be blocked by linopirdine, cholinergic effects might be better revealed in the presence of this compound. The aim of the present work was to study the effects of linopirdine on the ACh-evoked responses through α9α10-containing native and recombinant nicotinic cholinergic receptors. Responses to ACh were blocked by linopirdine in both OHCs and inner hair cells (IHCs) of rats at postnatal days 21–27 (OHCs) and 9–11 (IHCs). In addition, linopirdine blocked responses of recombinant α9α10 nicotinic cholinergic receptors (nAChRs) in a concentration-dependent manner with an IC50 of 5.2 μM. Block by linopirdine was readily reversible, voltage independent, and surmountable at high concentrations of ACh, thus suggestive of a competitive type of interaction with the receptor. The present results contribute to the pharmacological characterization of α9α10-containing nicotinic receptors and indicate that linopirdine should be used with caution when analyzing the cholinergic sensitivity of cochlear hair cells.
Keywords: nicotinic receptors, linopirdine, ion channels, acetylcholine, K+ channels
INTRODUCTION
Cochlear outer hair cells (OHCs) are responsible for the active mechanical amplification process that leads to the fine tuning and high sensitivity of the mammalian inner ear (Dallos 1992; Dallos and Evans 1995). This active amplification is the consequence of the ability of OHCs to alter their cell length in response to changes in membrane potential, a process referred to as electromotility (Brownell et al. 1985). Cochlear amplification is controlled by efferent olivocochlear fibers that originate in the brain stem and synapse directly at the base of OHCs (Guinan 1996). Acetylcholine (ACh) is the principal neurotransmitter released by medial olivocochlear efferent axons (Eybalin 1993), and existing pharmacological and electrophysiological data suggest a central role for an atypical, nicotinic ACh receptor (nAChR) located at the synapse between efferent fibers and vertebrate OHCs (Fuchs 1996). Current data support a model in which inhibition can be attributed to a transient, ACh-gated depolarization followed by activation of small-conductance, calcium-activated potassium channels (SK2) and subsequent hair cell hyperpolarization (Art et al. 1984; Housley and Ashmore 1991; Fuchs and Murrow 1992; Evans 1996; Blanchet et al. 1996).
The cholinergic hair cell receptor displays an unusual pharmacological profile which does not resemble the one described for muscle or neuronal nAChRs (Housley and Ashmore 1991; Fuchs and Murrow 1992; Kakehata et al. 1993; Erostegui et al. 1994; Blanchet et al. 1996; Chen et al. 1996; Dulon and Lenoir 1996; Evans 1996; McNiven et al. 1996), thus suggesting the presence of a new receptor subtype (Fuchs 1996). The more recently cloned α9 and α10 gene subunits are distant members of the nAChR family and, when expressed in Xenopus laevis oocytes, form a heteromeric receptor–channel complex that displays a pharmacological profile distinctly different from that of other cholinergic receptors, with sensitivity to glycinergic, gabaergic, serotonergic, as well as nicotinic and muscarinic antagonists (Elgoyhen et al. 1994, 2001). However, the properties of the recombinant α9α10 receptor are strikingly similar to those described for the cholinergic receptor that mediates synaptic transmission between efferent fibers and cochlear OHCs. Moreover, the α9 and α10 gene subunits exhibit a unique and restricted expression pattern that includes the cochlear and vestibular hair cells (Elgoyhen et al. 1994, 2001; Morley et al. 1998; Hiel et al. 2000). This has led to the proposal that efferent modulation of cochlear and vestibular hair cell function occurs, at least in part, via heteromeric nAChRs assembled from both α9 and α10 subunits (Elgoyhen et al. 2001).
Studies of the electrophysiological response to ACh in mammalian OHCs are hindered by the presence of a potassium current, IK,n, most likely mediated by channels containing the KCNQ4 subunit (Housley and Ashmore 1992; Marcotti and Kros 1999). IK,n provides a large potassium conductance that determines the OHCs membrane potential and membrane time constant. IK,n is also present in adult inner hair cells (IHCs). It is developmentally regulated and contributes only to mouse IHCs’ resting membrane potential from P19 onward (Marcotti et al. 2003; Oliver et al. 2003). IK,n can be blocked by linopirdine with an IC50 near 1 μM (Marcotti and Kros 1999); therefore, cholinergic effects might be better studied in the presence of this compound. Indeed, recordings from OHCs of electrophysiological cholinergic responses in acute cochlear explants are performed in the presence of 10–100 μM linopirdine (Lioudyno and Fuchs, unpublished observations; Oliver et al. 2000, 2001). However, the α9α10-containing hair cell nicotinic cholinergic receptors exhibit unusually nonselective antagonist pharmacology, raising the possibility that this IK,n blocker might also interfere with these receptors. Indeed, in the present work we show that linopirdine reduced the response to ACh of both native OHCs and IHCs, as well as of recombinant α9α10 receptors expressed in Xenopus laevis oocytes. Thus, linopirdine should be used with caution when studying responses to ACh in mammalian hair cells.
METHODS
Electrophysiological recordings from hair cells
Apical turns of the organ of Corti were excised from Sprague–Dawley rats at postnatal ages P9–11 for IHCs and P21–27 for OHCs and used within 3 h. Day of birth was considered postnatal day 0, P0. Cochlear preparations were mounted under an Axioskope microscope (Zeiss, Oberkochem, Germany) and viewed with differential interference contrast (DIC) using a 63× water immersion objective and a camera with contrast enhancement (Hamamatsu C2400-07, Hamamatsu City, Japan). Methods to record from IHCs and OHCs were essentially as described previously (Glowatzki and Fuchs 2000; Oliver et al. 2000).
Briefly, IHCs were identified visually with the 63× objective and, during recordings, by the size of their capacitance (7–12 pF), by their characteristic voltage-dependent Na+ and K+ currents, and at older ages by a fast-activating K+ conductance (Kros et al. 1998). Some cells were removed to access IHCs, but mostly the pipette moved through the tissue under positive pressure. The extracellular solution was as follows (in mM): 155 NaCl, 5.8 KCl, 1.3 CaCl2, 0.9 MgCl2, 0.7 NaH2PO4, 5.6 D-glucose, and 10 HEPES buffer, pH 7.4. The pipette solution contained (in mM): 150 KCl, 3.5 MgCl2, 0.1 CaCl2, 5 HEPES buffer, 2.5 Na2ATP, pH 7.2. In order to isolate the nAChR current from the currents through the SK channels, 10 mM of the fast calcium chelator l,2-bis(2-aminophenoxy)ethaneN,N,N′,N′-tetraacetic acid (BAPTA) was added to the pipette solution. In addition, 1 nM apamin, a specific SK channel blocker, was added to the extracellular solution. Glass pipettes, 1.2 mm i.d., had resistances of 7–10 MΩ . Experiments on IHCs were done at a holding voltage of −90 mV and all working solutions (containing ACh and/or linopirdine) were made up in a saline solution containing low Ca2+ (0.5 mM) and no Mg2+ so as to optimize the experimental conditions for measuring currents flowing through the α9α10 receptors (Weisstaub et al. 2002).
Outer hair cells in the first and second rows of the apical turn were visually chosen for whole-cell recording. In these experiments the extracellular solution was as follows in (mM): 150 NaCl, 4 KCl, 1.3 CaCl2, 0.9 MgCl2, 0.7 NaH2PO4, 5.6 glucose, 10 HEPES, 1 ascorbate, pH 7.4. The pipette solution was (mM): 115 KCl, 20 K2SO4, 3.5 MgCl2, 0.1 CaCl2, 5 EGTA, 5 HEPES, 2.5 Na2ATP, 6 glucose, pH 7.2. Recordings from OHCs were done at a holding voltage of −30 mV.
Solutions containing ACh with or without linopirdine were applied by a gravity-fed multichannel glass pipette (~150 μM tip diameter), positioned about 300 μM from the recorded cell. Currents in both IHCs and OHCs were recorded in the whole-cell patch-clamp mode with an Axopatch 200B amplifier, low-pass filtered at 2–10 kHz, and digitized at 5–20 kHz with a Digidata 1200 board (Axon Instruments Corp., Union City, CA). Recordings were made at room temperature (22–25°C). Holding potentials were not corrected for liquid junction potentials or for the voltage drop across the uncompensated series resistance.
Expression of recombinant α9α10 receptors in Xenopus laevis oocytes
For expression studies, α9 and α10 rat nAChR subunits were subcloned into a modified pGEMHE vector (Liman et al. 1992). Capped cRNAs were in vitro transcribed from linearized plasmid DNA templates using the mMessage mMachine T7 Transcription Kit (Ambion Corporation, Austin, TX). The maintenance of Xenopus laevis as well as the preparation and cRNA injection of stage V and VI oocytes has been described in detail elsewhere (Weisstaub et al. 2002). Typically, oocytes were injected with 50 nl of RNase-free water containing 0.01–1.0 ng of cRNAs (at a 1:1 molar ratio) and maintained in Barth’s solution at 17°C.
Electrophysiological recordings were performed 2–6 days after cRNA injection under two-electrode voltage clamp with a Geneclamp 500 amplifier (Axon Instruments Corp.). Both voltage and current electrodes were filled with 3 M KCl and had resistances of ~1–2 MΩ. Data acquisition was performed using a Digidata 1200 and pClamp 7.0 software (Axon Instruments Corp.). During electrophysiological recordings, oocytes were continuously superfused (~10 ml/min) with normal frog saline composed of (mM): 115 NaCl, 2.5 KCl, 1.8 CaCl2, and 10 HEPES buffer, pH 7.2. Unless otherwise indicated, the membrane potential was clamped to −70 mV. Drugs were applied in the perfusion solution of the oocyte chamber. To minimize activation of the endogenous Ca2+-sensitive chloride current (Elgoyhen et al. 2001), all experiments were performed in oocytes incubated with the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethaneN,N,N′,N′-tetraacetic acid–acetoxymethyl ester (BAPTA–AM, 100 μM) for 3–4 h prior to electrophysiological recordings.
Concentration-response curves were normalized to the maximal agonist response in each oocyte. For the linopirdine inhibition curves, responses were referred to as a percentage of the response to ACh presented alone. The mean and standard error of the mean of peak current responses are represented. Agonist concentration–response curves were iteratively fitted with the equation I/Imax=An/(An+ECn50), where I is the peak inward current evoked by agonist at concentration A, Imax is the current evoked by the concentration of agonist eliciting a maximal response, EC50 is the concentration of agonist inducing half-maximal current response, and n is the Hill coefficient. An equation of the same form was used to analyze the concentration dependence of antagonist-induced blockage. The parameters derived were the concentration of antagonist producing a 50% block of the control response to ACh (IC50) and the associated interaction coefficient (n). Current-voltage (I–V) relationships were obtained by applying 2-s voltage ramps from −120 to +50 mV, 10 s after the peak response to 10 μM ACh from a holding potential (Vhold) of −70 mV. Leakage correction was performed by digital subtraction of the I–V curve obtained by the same voltage ramp protocol prior to the application of ACh. Generation of voltage protocols and data acquisition were performed using a Digidata 1200 and the pClamp 6.1 or 7.0 software. Statistical significance was evaluated by the Student’s t-test (two-tailed, unpaired samples). A p < 0.05 was considered significant.
ACh-evoked current amplitudes in OHCs, IHCs, and α9α10-expressing oocytes were always measured at the peak of the response.
Materials
ACh chloride and linopirdine were bought from Sigma Chemical Co. (St. Louis, MO). ACh was dissolved in distilled water as 100 mM stocks and stored at −20°C. Linopirdine was dissolved in dimethyl sulfoxide (DMSO) as a 100 mM stock and diluted in saline. Final concentration of DMSO in the recording solution was always equal or less than 0.1%. This concentration of DMSO did not have any effect on either the holding current or the ACh-evoked currents in OHCs, IHCs, or α9α10-expressing oocytes (data not illustrated). BAPTA–AM (Molecular Probes, Eugene, OR) was stored at −20°C as aliquots of a 100 mM solution in DMSO, thawed, and diluted 1000-fold into saline solution shortly before incubation of the oocytes.
All experimental protocols were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 80-23) revised 1978.
RESULTS
Effect of linopirdine on rat cochlear hair cells
Figure 1 shows a representative response to 100 μM ACh recorded in OHCs from acutely excised organs of Corti of 3–4-week-old rats. At a holding potential of −30 mV, currents were outward since efflux of K+ through calcium-activated SK2 channels predominates over influx through the α9α10-containing nAChRs (Art et al. 1984; Housley and Ashmore 1991; Fuchs and Murrow 1992; Evans 1996). Application of linopirdine in the absence of ACh reduced the holding current by 16.8 ± 1.3 pA (data not illustrated). At 100 μM, the coapplication of linopirdine blocked responses to 100 μM ACh by 34.5 ± 3.3% (n = 3 cells; values were corrected for the effect of linopirdine on the holding current in each cell). This blocking effect increased to ~50% when OHCs were superfused with linopirdine for 5 min prior to the coapplication of linopirdine plus ACh (53.3 ± 7.6%, n = 3 cells). This effect of linopirdine was reversed after washing the preparation with extracellular solution for 1 min.
Figure 1.
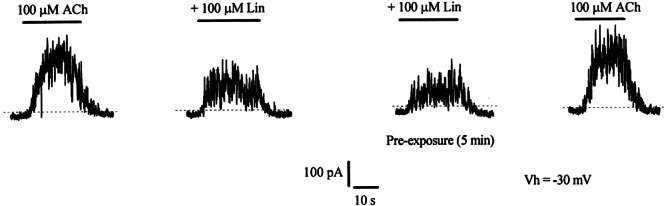
Effect of linopirdine on ACh-evoked responses in outer hair cells. Cells (P21–27) were voltage-clamped at −30 mV. Shown are representative traces (n = 3) of the responses to 100 μM ACh either alone or in the presence of 100 μM linopirdine. Holding current was −17.5 pA. Each dashed line indicates the zero-current level. Linopirdine was coapplied with ACh or, where indicated, preincubated for 5 min prior to the application of the agonist. During the preincubation with linopirdine, the holding current changed to −34.5 pA.
ACh-evoked responses in neonatal inner as well as outer hair cells result from the activation of α9α10-containing nAChRs plus the subsequent gating of SK channels by Ca2+ entry through the nAChR (Glowatzki and Fuchs 2000; Oliver et al. 2000). Therefore, in order to evaluate the effects of linopirdine on the nAChR in isolation, the following recordings were taken from IHCs with 10 mM BAPTA in the pipette solution and 1 nM apamin in the extracellular solution (see Methods) to prevent activation of SK channels. Isolated nAChR responses were studied in neonatal (P9-P11) IHCs to take advantage of their lower resting potassium conductance and thus obtain a higher signal to noise ratio than that of the OHCs. Figure 2A shows representative traces of responses of IHCs to 30 μM ACh, and block of these responses in the presence of different concentrations of linopirdine. Block by linopirdine was concentration dependent as shown on the representative traces and inhibition curves of Figures 2A and B. The concentration of linopirdine required to block 50% of the response to 30 μM ACh (IC50) was 3.1 ± 0.2 μM, with a Hill coefficient of 1.5 ± 0.3 (n = 3–6). In addition, block by linopirdine was reversed by superfusion of the preparation for 40 s with control bath solution (not shown).
Figure 2.
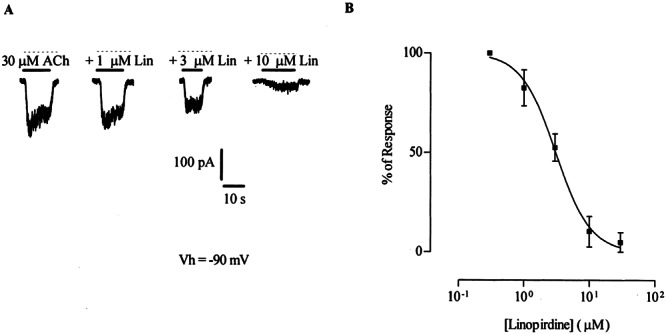
Effect of linopirdine on ACh-evoked responses in inner hair cells (P9–11) voltage-clamped at −90 mV. A. Representative traces (n = 3–6) of the isolated nAChR responses to 30 μM ACh either alone or in the presence of 1, 3, or 10 μM linopirdine. The holding current was −152 pA; the dashed line indicates the zero-current level. B. Inhibition curve resulting from the application of 30 μM ACh with increasing concentrations of linopirdine. The antagonist was preincubated for 3 min prior to application of the agonist. Peak current values are expressed as the percentage of the peak control current evoked by ACh. The mean and SEM of 3–6 experiments per point are shown and fitted according to Methods; IC50 = 3.1 ± 0.2 μM.
Effect of linopirdine on recombinant α9α10 nicotinic receptors expressed in Xenopus laevis oocytes
it is currently accepted that olivocochlear efferent inhibition of OHCs and transient efferent innervation to IHCs is subserved, at least in part, by a nAChR composed from both α9 and α10 nicotinic subunits (Glowatzki and Fuchs 2000; Oliver et al. 2000; Elgoyhen et al. 2001; Lustig et al. 2001). We therefore studied the effects of linopirdine on recombinant α9α10 nicotinic receptors expressed in Xenopus laevis oocytes. This system offers the possibility of a more extended study of the mechanism underlying the blocking effect of linopirdine.
As shown in Figure 3A, when coapplied with the agonist, 100 μM linopirdine reduced the response of α9α10-injected oocytes to 10 μM ACh by 83 ± 2.1% (n = 3). This effect was readily reversible; 99.2 ± 8.8% of the initial response to ACh was recovered after washing the oocytes with frog saline solution for 3 min. The effect of linopirdine occurred rapidly. This became apparent when applying linopirdine in the middle of a prolonged exposure to 10 μM ACh (Fig. 3B). The onset and decay of block by various concentrations of linopirdine occurred with the same timing as the effect of ACh itself. Presumably the perfusion system was rate-limiting in both cases.
Figure 3.
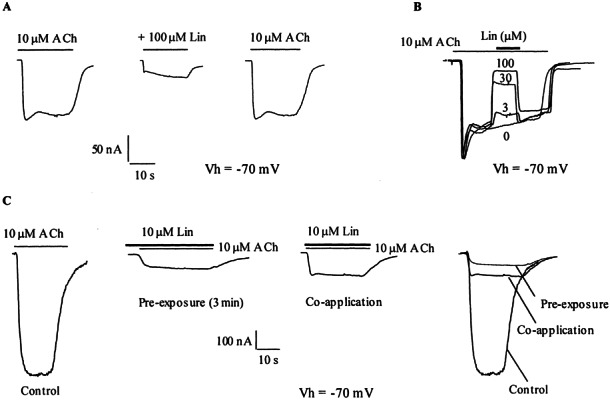
Effect of linopirdine on ACh-evoked responses in α9α10-injected oocytes. A. Responses to 10 μM ACh either alone, during coapplication with 100 μM linopirdine, or after washing with control bath solution for 3 min. B. Application of different concentrations of linopirdine during responses to 10 μM ACh show a fast onset and decay of the blockage. C. Responses to 10 μM ACh either alone or in the presence of 10 μM linopirdine. Linopirdine was coapplied with ACh or, where indicated, preincubated for 3 min prior to application of the agonist. Shown are representative traces of n = 3 in all cases. Oocytes were voltage-clamped at −70 mV.
Pre-exposure of the oocytes for 3 min with 10 μM linopirdine before the application of 10 μM ACh only slightly enhanced the percentage of block from 80 ± 2% to 88 ± 2% (n = 3, p < 0.05, Fig. 3C). Consequently, the following experiments were performed by coapplying linopirdine with ACh.
Block by linopirdine was concentration dependent as shown on the representative traces and inhibition curves of Figure 4. The concentration of linopirdine required to block 50% of the response to 10 μM ACh (IC50) was 5.2 ± 0.2 μM, with a Hill coefficient of 0.6 ± 0.04 (n = 3). This IC50 value is similar to that obtained in IHCs (Fig. 2), To further characterize the mechanism underlying the blocking action of linopirdine on the α9α10 receptor, the effect of this drug was analyzed at increasing concentrations of ACh (Vhold = −70 mV). As shown in Figure 5A, 10 μM linopirdine produced a significant shift of the concentration–response curves to ACh to the right without a reduction of the maximum agonist response. The EC50 value for ACh increased from the control value of 15.4 ± 0.8 μM to 382.5 ± 6.7 μM (n = 6) in the presence of linopirdine. This result is compatible with a competitive mechanism of block. Moreover, as shown in the representative I–V curves of Figure 5B, block by linopirdine of ACh-evoked currents was voltage independent, i.e., currents were diminished equally both at negative as well as at positive membrane potentials (Ilino/Icontrol = 41.7 ± 5.2% and 44.7 ± 6.3% at −90 mV and +40 mV, respectively, n = 3).
Figure 4.
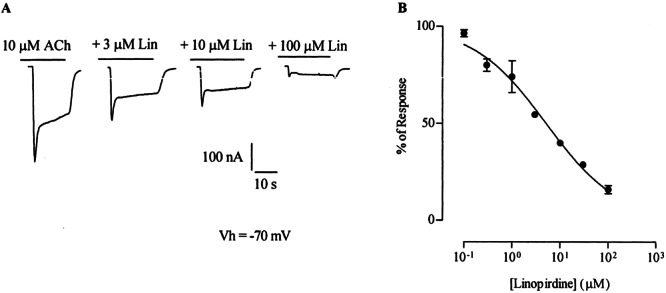
Block by linopirdine of ACh-evoked responses in α9α10-injected oocytes was concentration dependent. A. Representative traces to ACh either alone or in the presence of different concentrations of linopirdine. B. Inhibition curve resulting from the coapplication of 10 μM ACh with increasing concentrations of linopirdine. Peak current values are expressed as the percentage of the peak control current evoked by ACh. The mean and SEM of three experiments are shown and fitted according to Methods; IC50 = 5.2 ± 0.2 μM.
Figure 5.
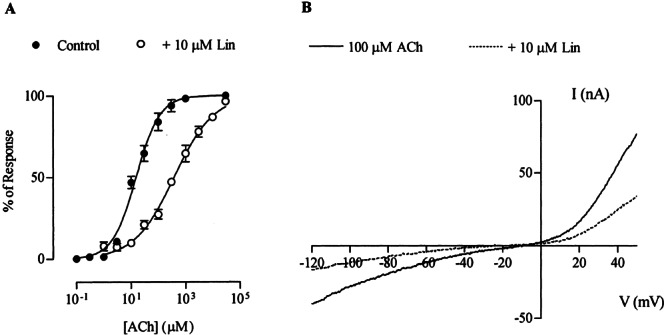
Mechanism of block by linopirdine of ACh-evoked responses. A. Concentration–response curves to ACh either alone or in the presence of 10 μM linopirdine. Peak current values were normalized and referred to the maximal peak response to ACh. The mean and SEM of six experiments per group are shown. Data were fitted according to Methods; EC50 = 15.4 ± 0.8 μM in control conditions and 382.5 ± 6.7 μM in the presence of linopirdine (n = 6). B. Leak-corrected, representative I–V curves obtained upon application of 2-s voltage ramps (−120 to +50 mV) 10 s after the peak response to 10 μM ACh from a holding potential (Vhold) of −70 mV, either alone or in the presence of 10 μM linopirdine (n = 3 for each curve).
DISCUSSION
The present study shows that linopirdine, a classic blocker of IK,n potassium currents of mammalian cochlear hair cells (Housley and Ashmore 1992; Marcotti and Kros 1999), and of IM potassium currents of peripheral sympathetic and central nervous system neurons (Costa and Brown 1997; Lamas et al. 1997), also behaves as an antagonist of native and recombinant α9α10-containing hair cell nAChRs. The interaction of linopirdine with these nAChRs appears as efficient as that with the IK,n potassium current, since the IC50 of linopirdine for the blockage of α9α10-containing nAChRs (5 μM recombinant and 3 μM native IHC) is within the same order of magnitude (micromolar) as that reported for native IK,n and IM currents (Costa and Brown 1997; Lamas et al. 1997; Marcotti and Kros 1999). Block of recombinant α9α10 nAChRs by linopirdine is also within the same range of concentrations as those required to block recombinant channels formed by the cloned KCNQ2 and KCNQ3 subunits (Wang et al. 1998). In addition, linopirdine exerts a more potent block of recombinant α9α10 nAChRs than that reported for recombinant channels composed of the KCNQ4 subunit, 30% block at a concentration of 200 μM (Kubisch et al. 1999). Thus, at the concentrations used in preparations of the organ of Corti to study the ACh-evoked responses, 10–100 μM (Lioudyno and Fuchs, unpublished; Oliver et al. 2000, 2001), linopirdine will interfere with this response.
Since linopirdine is an established potassium channel blocker, one might speculate that linopirdine is acting on the associated SK2 potassium channel to produce its antagonistic effect on the hair cells response to ACh. While direct effects on SK channels were not examined in the organ of Corti, linopirdine does not block apamin-sensitive Ca2+-activated K+ currents of rat superior cervical ganglion neurons (Lamas et al. 1997). Moreover, it is clear from experiments performed on the isolated currents through the native nAChR in IHCs and on recombinant receptors in Xenopus oocytes that linopirdine can interact with the α9α10 nAChRs themselves. However, the possibility that linopirdine also affects SK channels cannot be disregarded.
The observation that linopirdine antagonized α9α10 nAChRs was not totally unexpected. It has previously been reported that, in addition to blocking M-type K+ currents of rat superior sympathetic ganglion cells, linopirdine also blocks transmitter-gated channels of those cells, i.e., nAChRs and GABAA receptors with an IC50 of 8 and 26 μM, respectively (Lamas et al. 1997). Furthermore, it also has been proposed that linopirdine influences several processes that are unique to ACh-containing neurons (Vickroy 1993). Thus, linopirdine facilitates both potassium- and calcium-stimulated [3H]ACh release from hippocampal cholinergic synaptosomes and causes a lasting calcium-dependent stimulation of synaptosomal [3H]choline uptake and [3H]ACh formation (Vickroy 1993). Linopirdine increases K+-evoked release of ACh in brain slices (Nickolson et al. 1990), blocks acetylcholinesterase activity in homogenates from rat cerebral cortex (Nickolson et al. 1990), and enhances cholinergic synaptic transmission at the rodent neuromuscular junction through a presynaptic facilitatory effect (Tsai et al. 1992). While the present work examined only the effects of exogenous ACh, a potential effect of linopirdine on efferent synaptic release of ACh in this organ of Corti preparation should be considered in future studies.
The expression of recombinant α9α10 nAChRs in Xenopus laevis oocytes allowed a more extensive study of the mechanism of interaction of linopirdine with these receptors, thus complementing the experiments performed in cochlear hair cells. Block by linopirdine of the IM K+ current is due to direct channel block (Costa and Brown 1997; Lamas et al. 1997). However, linopirdine does not appear to act as a pore blocker of the α9α10 nAChR. Support for this conclusion is provided by the finding that linopirdine blocking effect was voltage independent. It is further supported by the observation that block by linopirdine was overcome by raising the concentration of ACh, thus suggesting that this compound acts as an antagonist by competing for the ACh binding site.
The striking similarities found so far between the pharmacology of recombinant nAChRs assembled from α9 and α10 subunits (Elgoyhen et al. 1994, 2001) and that of native nAChRs present in isolated OHCs from different species (Housley and Ashmore 1991; Fuchs and Murrow 1992; Kakehata et al. 1993; Erostegui et al. 1994; Blanchet et al. 1996; Chen et al. 1996; Dulon and Lenoir 1996; Evans 1996; McNiven et al. 1996) suggest that the native cholinergic hair cell receptor is assembled from both α9 and α10 subunits. Moreover, this notion is further reinforced by in situ hybridization studies carried out in rat cochlear OHCs and IHCs at the same postnatal ages as those employed in the present study (Elgoyhen et al. 2001; Morley and Simmons 2002). α9α10-containing recombinant and native nAChRs exhibit unusually nonselective antagonist pharmacology (Elgoyhen et al. 2001). The present work demonstrates that the IK,n blocker linopirdine antagonizes hair cell nAChRs with the same potency as reported for classical muscarinic (atropine) and nicotinic (nicotine) compounds (Elgoyhen et al. 2001), and it contributes to the pharmacological characterization of this receptor subtype.
Acknowledgments
This work was supported by an International Research Scholar Grant from the Howard Hughes Medical Institute, a grant from Agencio Nacional de Promociones Científicas y Técnicas Argentina, a John Simon Guggenheim Memorial Foundation Fellowship, and Laboratorios Temis-Lostaló, Argentina, to ABE; NIH Research Grant No. R03TW006247 to PAF and ABE from the Fogarty International Center and the National Institute on Deafness and Other Communication Disorders (NIDCD); and NIDCD DC01508 to PAF.
References
- 1.Art J, Fettiplace R, Fuchs P. Synaptic hyperpolarization and inhibition of turtle cochlear hair cells. J. Physiol. (Lond) 1984;365:525–550. doi: 10.1113/jphysiol.1984.sp015481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchet C, Erostegui C, Sugasawa M, Dulon D. Acetylcholine-induced potassium current of guinea pig outer hair cells: its dependence on a calcium influx through nicotinic-like receptors. J. Neurosci. 1996;16:2574–2584. doi: 10.1523/JNEUROSCI.16-08-02574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownell W, Bader C, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, LeBlanc C, Bobbin R. Differences in cholinergic responses from outer hair cells of rat and guinea pig. Hear. Res. 1996;98:9–17. doi: 10.1016/0378-5955(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 5.Costa A, Brown B. Inhibition of M-current in cultured rat superior cervical ganglia by linopirdine: mechanism of action studies. Neuropharmacol. 1997;36:1747–1753. doi: 10.1016/S0028-3908(97)00155-X. [DOI] [PubMed] [Google Scholar]
- 6.Dallos P. The active cochlea. J. Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dallos P, Evans BN. High-frequency motility of outer hair cells and the cochlear amplifier. Science. 1995;267:2006–2009. doi: 10.1126/science.7701325. [DOI] [PubMed] [Google Scholar]
- 8.Dulon D, Lenoir M. Cholinergic responses in developing outer hair cells of the rat cochlea. Eur. J. Neurosci. 1996;8:1945–1952. doi: 10.1111/j.1460-9568.1996.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 9.Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 10.Elgoyhen AB, Vetter D, Katz E, Rothlin C, Heinemann S, Boulter J. Alpha 10: A determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc. Natl. Acad. Sci. USA. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erostegui C, Norris CH, Bobbin RP. In vitro characterization of a cholinergic receptor on outer hair cells. Hear. Res. 1994;74:135–147. doi: 10.1016/0378-5955(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 12.Evans M. Acetylcholine activates two currents in guinea-pig outer hair cells. J. Physiol (Lond.) 1996;491:563–578. doi: 10.1113/jphysiol.1996.sp021240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eybalin M. Neurotransmitters and neuromodulators of the mammalian cochlea. Physiol. Rev. 1993;73:309–373. doi: 10.1152/physrev.1993.73.2.309. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs P. Synaptic transmission at vertebrate hair cells. Curr. Opin. Neurobiol. 1996;6:514–519. doi: 10.1016/s0959-4388(96)80058-4. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs PA, Murrow BW. A novel cholinergic receptor mediates inhibition of chick cochlear hair cells. Proc. R. Soc. Lond. B Biol. Sci. 1992;248:35–40. doi: 10.1098/rspb.1992.0039. [DOI] [PubMed] [Google Scholar]
- 16.Glowatzki E, Fuchs P. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science. 2000;288:2366–2368. doi: 10.1126/science.288.5475.2366. [DOI] [PubMed] [Google Scholar]
- 17.Guinan JJ. Physiology of olivocochlear efferents. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer-Verlag; 1996. pp. 435–502. [Google Scholar]
- 18.Hiel H, Luebke AE, Fuchs PA. Cloning and expression of the 9 nicotinic acetylcholine receptor subunit in cochlear hair cells of the chick. Brain Res. 2000;858:215–225. doi: 10.1016/S0006-8993(00)01947-8. [DOI] [PubMed] [Google Scholar]
- 19.Housley GD, Ashmore JF. Direct measurement of the action of acetylcholine on isolated outer hair cells of the guinea pig cochlea. Proc. R. Soc. Lond. B Biol. Sci. 1991;244:161–167. doi: 10.1098/rspb.1991.0065. [DOI] [PubMed] [Google Scholar]
- 20.Housley GD, Ashmore JF. Ionic currents of outer hair cells isolated from the guinea-pig cochlea. J. Physiol (Lond) 1992;448:73–98. doi: 10.1113/jphysiol.1992.sp019030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakehata S, Nakagawa T, Takasaka T, Akaike N. Cellular mechanism of acetylcholine-induced response in dissociated outer hair cells of guinea-pig cochlea. J. Physiol. (Lond) 1993;463:227–244. doi: 10.1113/jphysiol.1993.sp019592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kros CJ, Ruppersberg JP, Rusch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- 23.Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch TJ. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96:437–446. doi: 10.1016/S0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- 24.Lamas JA, Selyanko AA, Brown DA. Effects of a cognition-enhancer, linopirdine (DuP 996), on M-type potassium currents (IK(M)) and some other voltage- and ligand-gated membrane currents in rat sympathetic neurons. Eur. J. Neurosci. 1997;9:605–616. doi: 10.1111/j.1460-9568.1997.tb01637.x. [DOI] [PubMed] [Google Scholar]
- 25.Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-A. [DOI] [PubMed] [Google Scholar]
- 26.Lustig LR, Peng H, Hiel H, Yamamoto T, Fuchs P. Molecular cloning and mapping of the human nicotinic acetylcholine receptor α10 (CHRNA10) Genomics. 2001;73:272–283. doi: 10.1006/geno.2000.6503. [DOI] [PubMed] [Google Scholar]
- 27.Marcotti W, Kros CJ. Developmental expression of the potassium current IK, n contributes to maturation of mouse outer hair cells. J. Physiol (Lond) 1999;520((Pt 3):653–660. doi: 10.1111/j.1469-7793.1999.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J. Physiol (Lond) 2003;548:383–400. doi: 10.1113/jphysiol.2002.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNiven AI, Yuhas WA, Fuchs PA. Ionic dependence and agonist preference of an acetylcholine receptor in hair cells. Audit Neurosci. 1996;2:63–77. [Google Scholar]
- 30.Morley BJ, Simmons DD. Developmental mRNA expression of the alpha10 nicotinic acetylcholine receptor subunit in the rat cochlea. Brain Res. Dev. Brain Res. 2002;139:87–96. doi: 10.1016/S0165-3806(02)00514-X. [DOI] [PubMed] [Google Scholar]
- 31.Morley B, Li H, Heil H, Drescher D, Elgoyhen AB. Identification of the subunits of the nicotinic cholinergic receptors in the rat cochlea using RT-PCR and in situ hybridization. Mol. Brain Res. 1998;53:78–87. doi: 10.1016/S0169-328X(97)00272-6. [DOI] [PubMed] [Google Scholar]
- 32.Nickolson V, Tam S, Myers M, Cook L. Dup 996 (3,3-bis(pyrindinylmethyl)-1-phenyl-2-one) enhances the stimulus-induced release of acetylcholine from rat brain in vitro and in vivo. Drug Dev. Res. 1990;19:285–300. [Google Scholar]
- 33.Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron. 2000;26:595–601. doi: 10.1016/S0896-6273(00)81197-6. [DOI] [PubMed] [Google Scholar]
- 34.Oliver D, Ludwig J, Reisinger E, Zoellner W, Ruppersberg JP, Fakler B. Memantine inhibits efferent cholinergic transmission in the cochlea by blocking nicotinic acetylcholine receptors of outer hair cells. Mol. Pharmacol. 2001;60:183–189. doi: 10.1124/mol.60.1.183. [DOI] [PubMed] [Google Scholar]
- 35.Oliver D, Knipper M, Derst C, Fakler B. Resting potential and submembrane calcium concentration of inner hair cells in the isolated mouse cochlea set by KCNQ-type potassium channels. J. Neurosci. 2003;23:2141–2149. doi: 10.1523/JNEUROSCI.23-06-02141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai M, Su J, Chen M, Fan S, Cheng C. The effect of 3,3-dipyridyl-methyl-1-phenyl-2-indolinone on the neuromuscular transmission in the rodent skeletal muscles. Neuropharmacol. 1992;31:89–94. doi: 10.1016/0028-3908(92)90166-M. [DOI] [PubMed] [Google Scholar]
- 37.Vickroy T. Presynaptic cholinergic actions by the putative cognitive enhancing agent DuP 996. J. Pharmacol. Exp. Ther. 1993;264:910–917. [PubMed] [Google Scholar]
- 38.Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 39.Weisstaub N, Vetter D, Elgoyhen A, Katz E. The alpha9/alpha10 nicotinic acetylcholine receptor is permeable to and is modulated by divalent cations. Hear. Res. 2002;167:122–135. doi: 10.1016/S0378-5955(02)00380-5. [DOI] [PubMed] [Google Scholar]


