Abstract
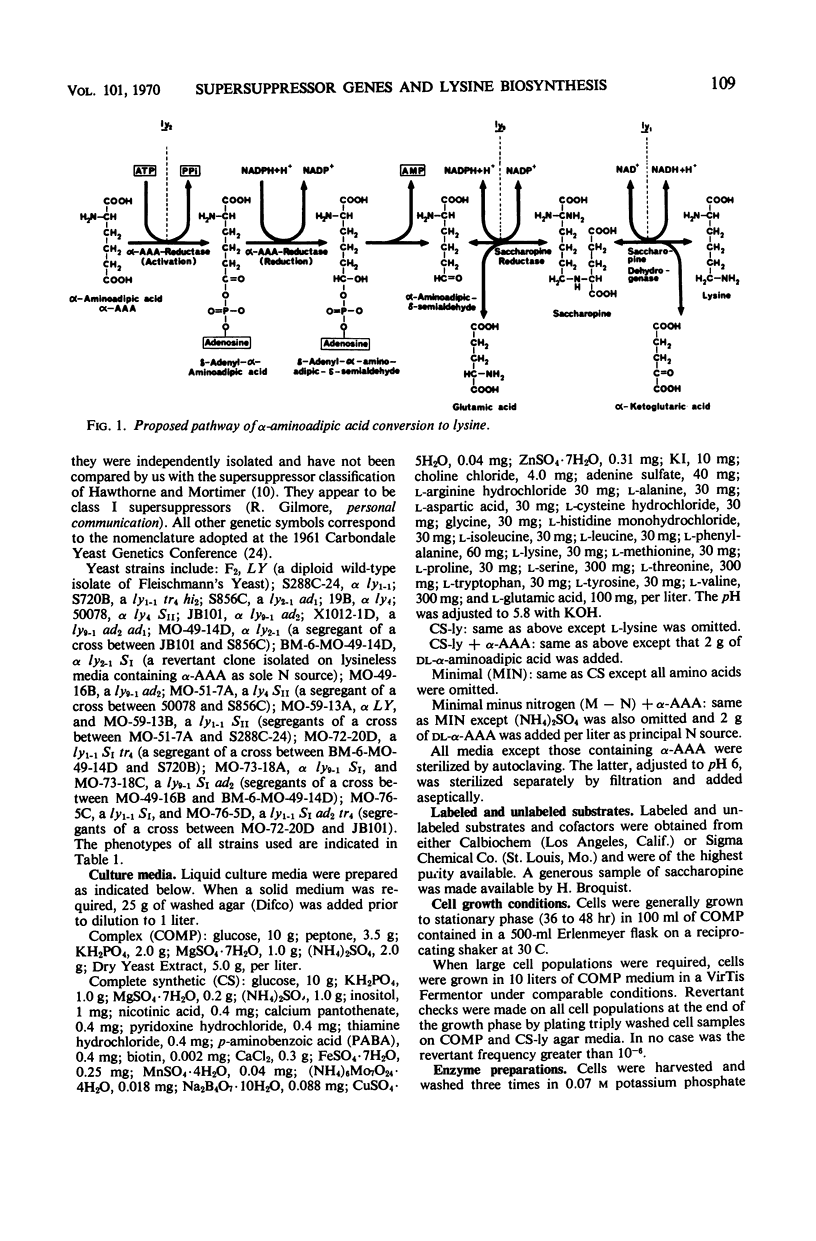
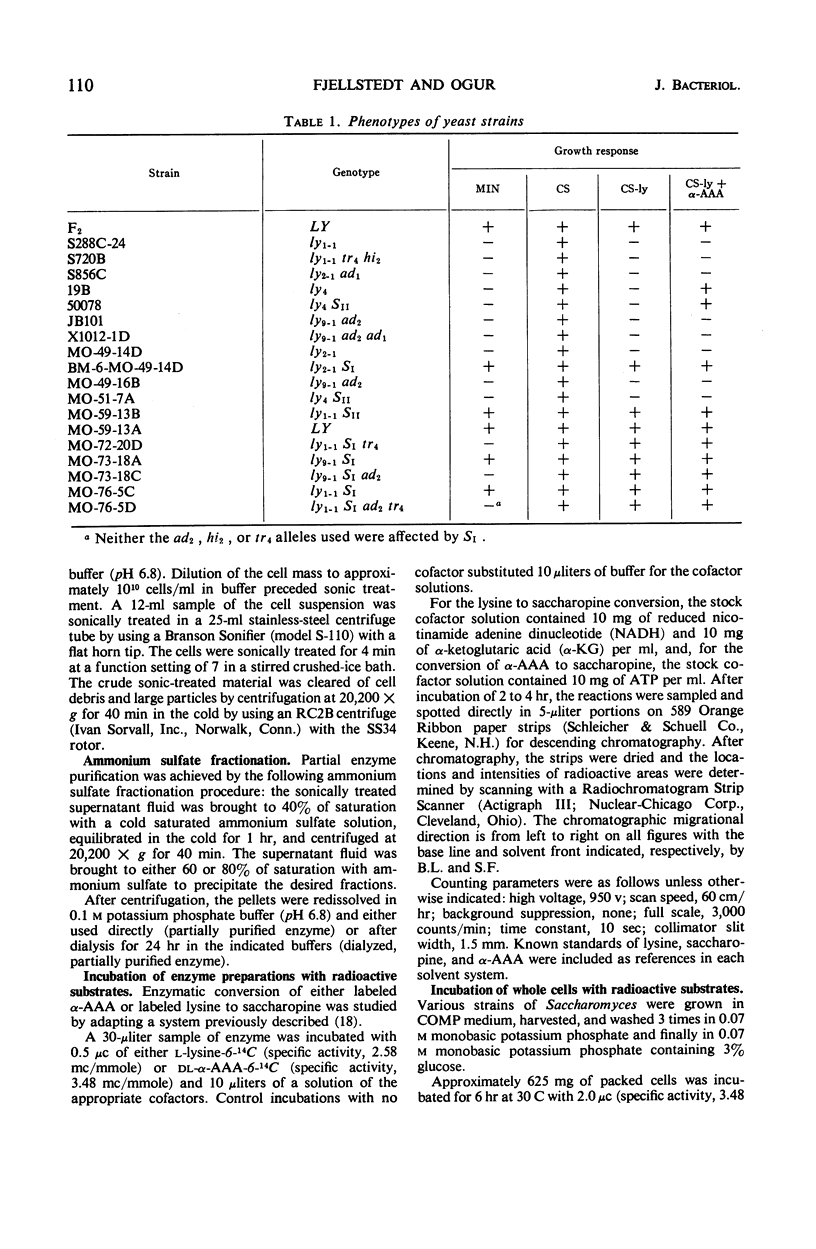
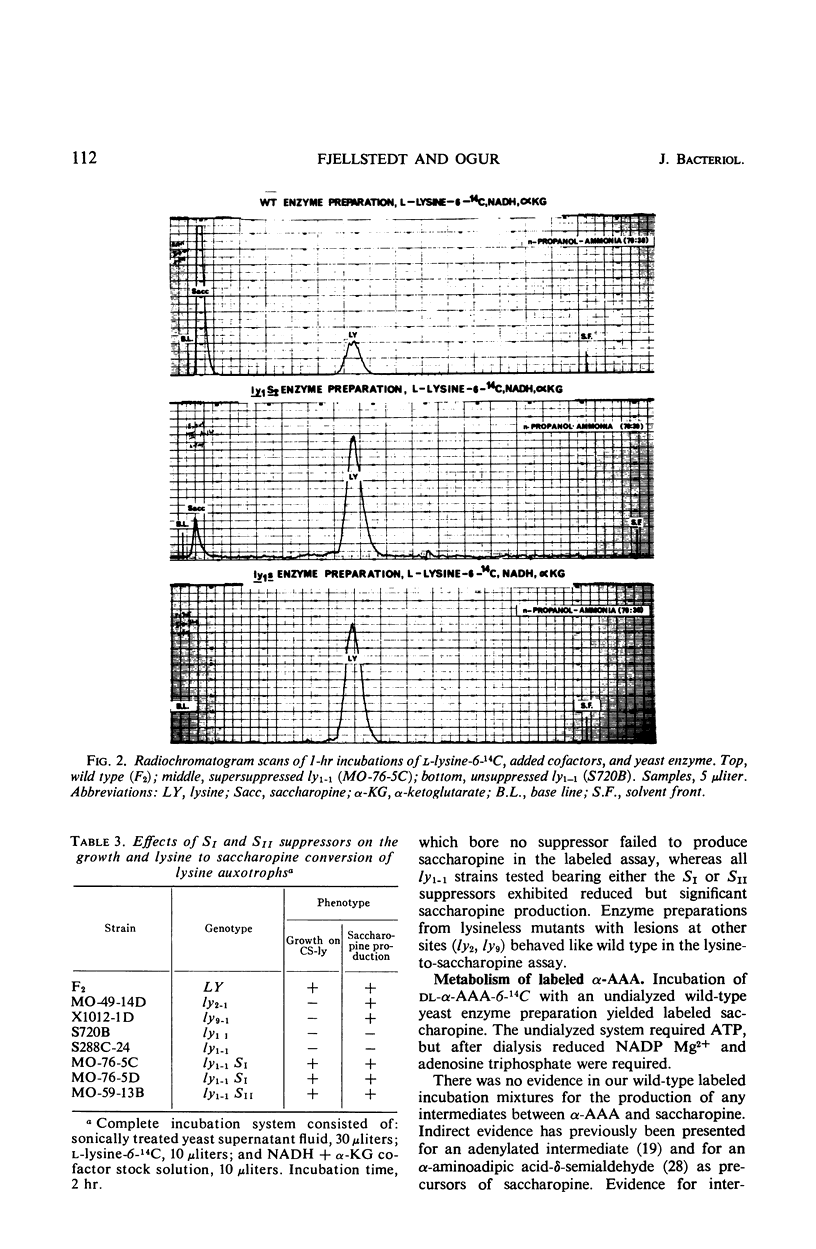
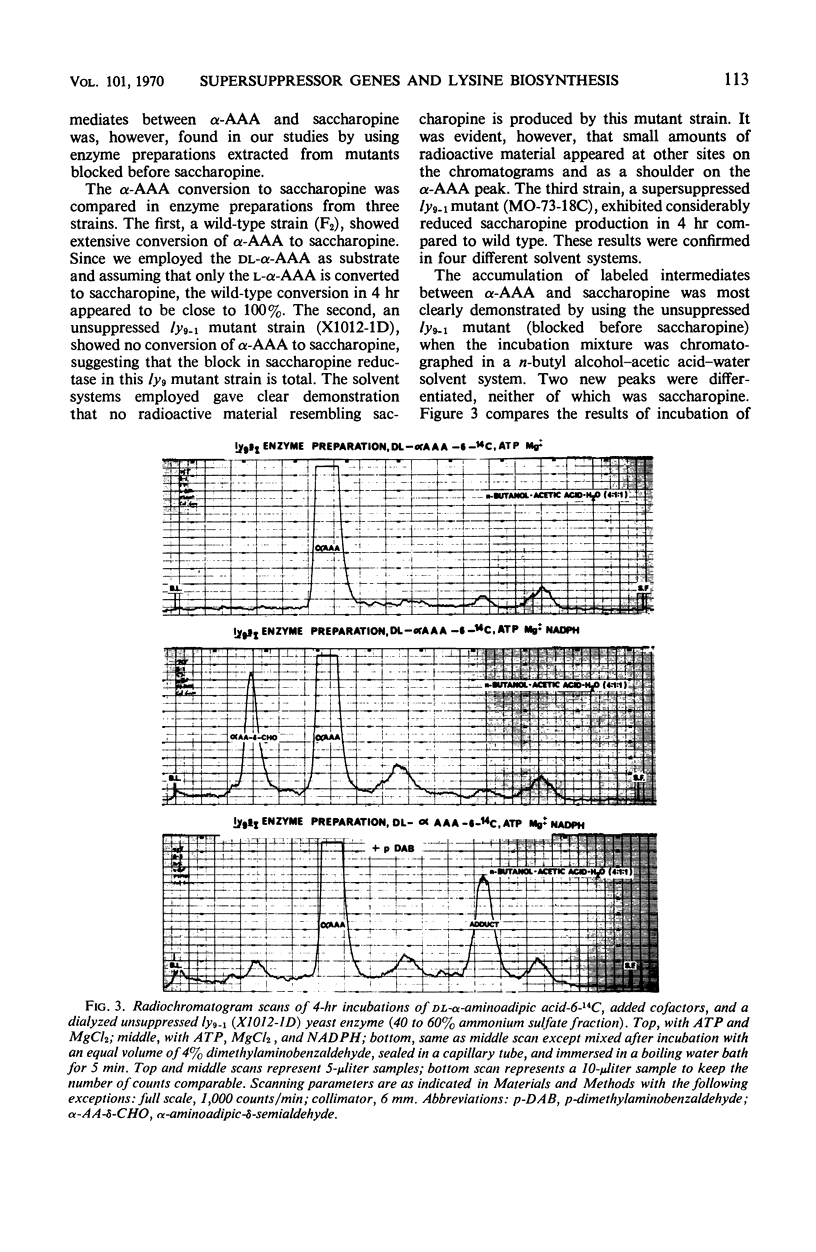
Yeast supersuppressor genes capable of masking the effects of several lysine mutant genes (ly1-1, ly9-1, ly2-1) were studied with respect to their effects on the respective enzymes (saccharopine dehydrogenase, saccharopine reductase, and α-amino-adipic acid reductase). In all strains tested, the supersuppressors functioned by allowing enzyme synthesis not found in the unsuppressed mutant. Studies by optical methods of saccharopine dehydrogenase and saccharopine reductase extracted from suppressed ly1-1 and ly9-1 cells, respectively, revealed that the Km values for these enzymes were significantly greater than those found in wild type. Saccharopine dehydrogenase from suppressed ly9-1 cells was found to have Km values similar to wild type. These findings are consistent with the inference that a supersuppressor may act by enabling nonsense codons to be read, producing altered enzyme protein. Recent findings that lysine degradation in mammals may involve saccharopine and that the human diseases, hyperlysinemia and saccharopinuria, may be due to metabolic blocks in this route of lysine degradation suggest the ly1-1 and ly9-1 yeast mutants as models for the human condition and its possible euphenic treatment.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENZER S., CHAMPE S. P. A change from nonsense to sense in the genetic code. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1114–1121. doi: 10.1073/pnas.48.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist P. L. Degenerate transfer RNAs from brewer's yeast. Cold Spring Harb Symp Quant Biol. 1966;31:435–447. doi: 10.1101/sqb.1966.031.01.056. [DOI] [PubMed] [Google Scholar]
- Carson N. A., Scally B. G., Neill D. W., Carré L. J. Saccharopinuria: a new inborn error of lysine metabolism. Nature. 1968 May 18;218(5142):679–679. doi: 10.1038/218679a0. [DOI] [PubMed] [Google Scholar]
- Gilmore R. A., Mortimer R. K. Super-suppressor mutations in Saccharomyces cerevisiae. J Mol Biol. 1966 Sep;20(2):307–311. doi: 10.1016/0022-2836(66)90066-0. [DOI] [PubMed] [Google Scholar]
- Gilmore R. A., Stewart J. W., Sherman F. Amino acid replacements resulting from super-suppression of a nonsense mutant of yeast. Biochim Biophys Acta. 1968 Jun 18;161(1):270–272. doi: 10.1016/0005-2787(68)90322-5. [DOI] [PubMed] [Google Scholar]
- Gorini L., Beckwith J. R. Suppression. Annu Rev Microbiol. 1966;20:401–422. doi: 10.1146/annurev.mi.20.100166.002153. [DOI] [PubMed] [Google Scholar]
- Grove J., Henderson L. M. The metabolism of D- and L-lysine in the intact rat, perfused liver and liver mitochondria. Biochim Biophys Acta. 1968 Aug 6;165(1):113–120. doi: 10.1016/0304-4165(68)90195-5. [DOI] [PubMed] [Google Scholar]
- HAWTHORNE D. C., MORTIMER R. K. Super-suppressors in yeast. Genetics. 1963 Apr;48:617–620. doi: 10.1093/genetics/48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashino K., Tsukada K., Lieberman I. Saccharopine, a product of lysine breakdown by mammalian liver. Biochem Biophys Res Commun. 1965 Jul 26;20(3):285–290. doi: 10.1016/0006-291x(65)90361-x. [DOI] [PubMed] [Google Scholar]
- Hutzler J., Dancis J. Conversion of lysine to saccharopine by human tissues. Biochim Biophys Acta. 1968 Apr 16;158(1):62–69. doi: 10.1016/0304-4165(68)90072-x. [DOI] [PubMed] [Google Scholar]
- JONES E. E., BROQUIST H. P. SACCHAROPINE, AN INTERMEDIATE OF THE AMINOADIPIC ACID PATHWAY OF LYSINE BIOSYNTHESIS. II. STUDIES IN SACCHAROMYCES CEREVISEAE. J Biol Chem. 1965 Jun;240:2531–2536. [PubMed] [Google Scholar]
- Jones E. E., Broquist H. P. Saccharopine, an intermediate of the aminoadipic acid pathway of lysine biosynthesis. 3. Aminoadipic semialdehyde-glutamate reductase. J Biol Chem. 1966 Jul 25;241(14):3430–3434. [PubMed] [Google Scholar]
- LEDERBERG J. Molecular biology, eugenics and euphenics. Nature. 1963 May 4;198:428–429. doi: 10.1038/198428a0. [DOI] [PubMed] [Google Scholar]
- Magni G. E., Puglisi P. P. Mutagenesis of super-suppressors in yeast. Cold Spring Harb Symp Quant Biol. 1966;31:699–704. doi: 10.1101/sqb.1966.031.01.089. [DOI] [PubMed] [Google Scholar]
- Manney T. R. Evidence for chain termination by super-suppressible mutants in yeast. Genetics. 1968 Dec;60(4):719–733. doi: 10.1093/genetics/60.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogur M. Outline for an experimental attack on the treatment of certain varieties of genetic disease. Perspect Biol Med. 1969 Spring;12(3):471–474. doi: 10.1353/pbm.1969.0060. [DOI] [PubMed] [Google Scholar]
- Piediscalzi N., Fjellstedt T., Ogur M. A glutamic-alpha-ketoadipic transaminase in Saccharomyces. Biochem Biophys Res Commun. 1968 Aug 13;32(3):380–384. doi: 10.1016/0006-291x(68)90671-2. [DOI] [PubMed] [Google Scholar]
- SAGISAKA S., SHIMURA K. A method for the quanitative determination of dehydropiperidine carboxylic acid, a reduction product of alpha-aminoadipic acid by yeast enzyme. J Biochem. 1962 Jan;51:27–31. doi: 10.1093/oxfordjournals.jbchem.a127496. [DOI] [PubMed] [Google Scholar]
- SAGISAKA S., SHIMURA K. Studies in lysine biosynthesis. IV. Mechanism of activation and reduction of alpha-aminoadipic acid. J Biochem. 1962 Sep;52:155–161. doi: 10.1093/oxfordjournals.jbchem.a127590. [DOI] [PubMed] [Google Scholar]
- Saunders P. P., Broquist H. P. Saccharopine, an intermediate of the aminoadipic acid pathway of lysine biosynthesis. IV. Saccharopine dehydrogenase. J Biol Chem. 1966 Jul 25;241(14):3435–3440. [PubMed] [Google Scholar]
- WOODY N. C. HYPERLYSINEMIA. Am J Dis Child. 1964 Nov;108:543–553. doi: 10.1001/archpedi.1964.02090010545015. [DOI] [PubMed] [Google Scholar]
- Woody N. C., Hutzler J., Dancis J. Further studies of hyperlysinemia. Am J Dis Child. 1966 Dec;112(6):577–580. doi: 10.1001/archpedi.1966.02090150121014. [DOI] [PubMed] [Google Scholar]
- YURA T., VOGEL H. J. An omega-hydroxy-alpha-amino acid dehydrogenase of Neurospora crassa; partial purification and some properties. J Biol Chem. 1959 Feb;234(2):339–342. [PubMed] [Google Scholar]


