Abstract
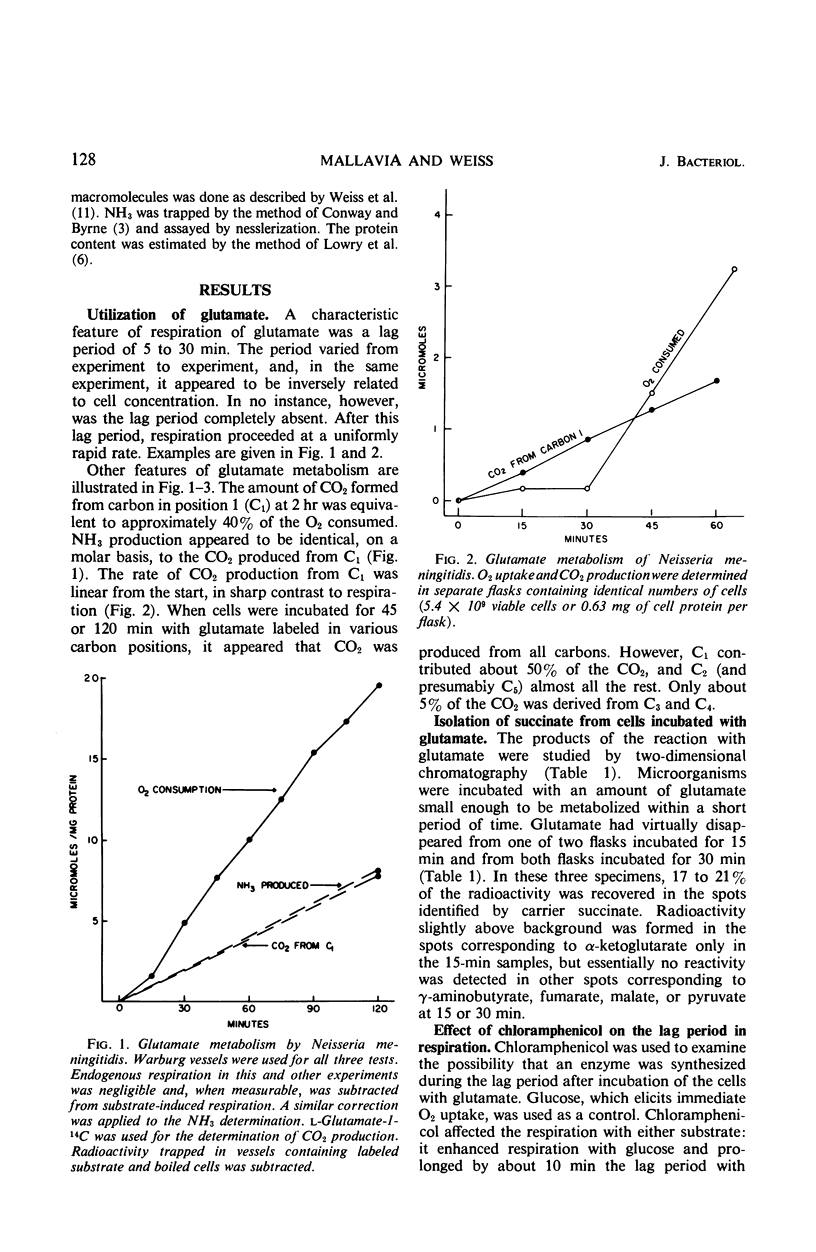
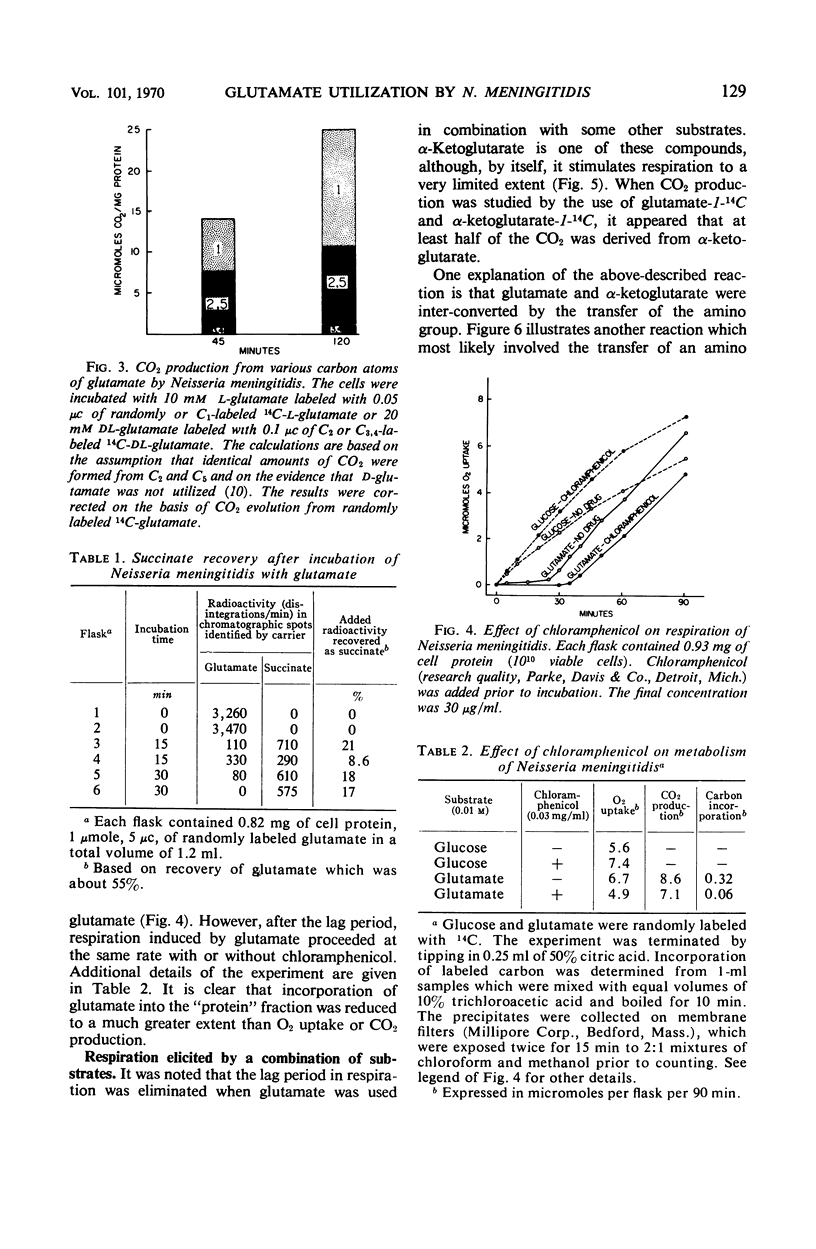
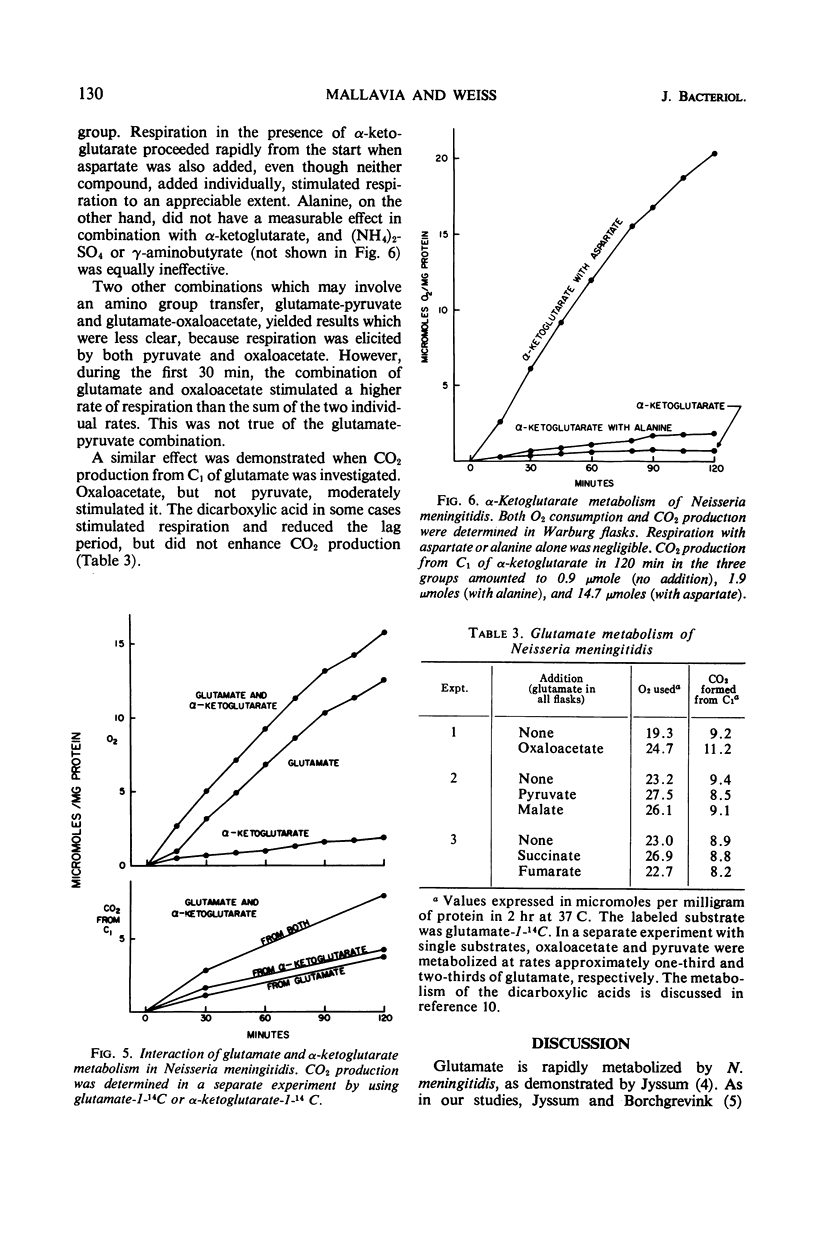
Glutamate was catabolized at a rapid rate by Neisseria meningitidis, group B. Surprisingly, there was a lag of 5 to 30 min in respiration, but not in CO2 production from C1, and an appreciable amount of succinate accumulated. The eventual rapid rate of respiration was not prevented by the addition of chloramphenicol. The lag period was eliminated by combinations of substrates that favored the activity of a glutamate-oxaloacetate transaminase. It is suggested that with glutamate as the sole substrate, the reaction terminated at succinate, required only moderate O2 uptake, and did not result in the transport of succinate to enzymatic sites. The lag period represented the time required for the accumulation of succinate and its transport to enzymatic sites by energy provided by the metabolism of the remaining glutamate. When the transaminase was operative, on the other hand, successive products of the reaction were immediately placed in contact with enzymatic sites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conway E. J., Byrne A. An absorption apparatus for the micro-determination of certain volatile substances: The micro-determination of ammonia. Biochem J. 1933;27(2):419–429. [PMC free article] [PubMed] [Google Scholar]
- JYSSUM K. Assimilation of nitrogen in meningococci grown with the ammonium ion as sole nitrogen source. Acta Pathol Microbiol Scand. 1959;46:320–332. [PubMed] [Google Scholar]
- JYSSUM K., BORCHGREVINK B. The adaptive oxidation of L-glutamic acid in meningococci. Acta Pathol Microbiol Scand. 1960;48:361–366. doi: 10.1111/j.1699-0463.1960.tb04779.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Myers W. F., Huang K. Y. Separation of intermediates of the citric acid cycle and related compounds by thin-layer chromatography. Anal Biochem. 1966 Nov;17(2):210–213. doi: 10.1016/0003-2697(66)90199-0. [DOI] [PubMed] [Google Scholar]
- TONHAZY N. E., PELCZAR M. J., Jr Oxidation of amino acids and compounds associated with the tricarboxylic acid cycle by Neisseria gonorrhoeae. J Bacteriol. 1953 Apr;65(4):368–377. doi: 10.1128/jb.65.4.368-377.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS E., NEPTUNE E. M., Jr, DAVIES J. A. LIPID METABOLISM OF THE RICKETTSIALIKE MICROORGANISM WOLBACHIA PERSICA. III. COMPARISON WITH OTHER METABOLIC ACTIVITIES. J Infect Dis. 1964 Feb;114:50–54. doi: 10.1093/infdis/114.1.50. [DOI] [PubMed] [Google Scholar]
- Weiss E. Catabolic Activities of Neisseria meningitidis: Utilization of Succinate. J Bacteriol. 1970 Jan;101(1):133–137. doi: 10.1128/jb.101.1.133-137.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Transaminase activity and other enzymatic reactions involving pyruvate and glutamate in Chlamydia (psittacosis-trachoma group). J Bacteriol. 1967 Jan;93(1):177–184. doi: 10.1128/jb.93.1.177-184.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



