Abstract
Damage to auditory hair cells in the inner ear as a consequence of aging, disease, acoustic trauma, or exposure to ototoxins underlies most cases of hearing impairment. Because the mammalian ear cannot replace damaged hair cells, loss of hearing is irreversible and progressive throughout life. One of the current goals of inner ear biology is to develop therapeutic strategies to prevent hair cell degeneration. Although important progress has been made in discovering factors that mediate hair cell death, very little is known about the molecular pathway(s) that signal survival. Here we considered the role of NF-κB, a ubiquitous transcription factor that plays a major role in the regulation of many apoptosis- and stress-related genes, in mediating hair cell survival. NF-κB was detected in a constitutively active form in the organ of Corti of 5-day-old rats. Selective inhibition of NF-κB through use of a cell-permeable inhibitory peptide in vitro caused massive degeneration of hair cells within 24 h of inhibitor application. Hair cell death occurred through an apoptotic pathway through activation of caspase-3 and may involve transcriptional down-regulation of the gadd45β gene, an anti-apoptotic NF-κB target. In view of our results, it seems likely that NF-κB may participate in normal hair cell function.
Keywords: apoptosis, inhibitor of κB, inner ear, organ of Corti, RelA
Introduction
An estimated 500 million people worldwide are affected by some form of hearing loss (http://www.who.org). In the majority of hearing-impairment cases, the cause is directly or indirectly linked to degeneration and death of cochlear sensory elements (i.e., hair cells) and their associated spiral ganglion neurons. In mammals, unlike in birds and lower vertebrates, loss of hair cells is irreversible because regeneration does not take place. A better understanding of the survival pathways and molecular events involved in protection of auditory epithelium is essential for developing therapeutic strategies to prevent hearing loss.
Studies show that reactive oxygen species (ROS) and activated c-Jun N-terminal kinase (JNK) signaling are some of the key mediators of hair cell death (Clerici et al. 1996; Huang et al. 2000; Pivola et al. 2000; Sha and Schacht 1999). However, surprisingly little is known about the factors that signal hair cell survival. Here we focus on the role of NF-κB/Rel family transcription factors, which have been described as key regulators of genes involved in many biological processes (e.g., immunity and inflammation, cell survival, apoptosis, differentiation, development, and neoplastic transformation; Karin et al. 2002).
The five mammalian NF-κB subunits [RelA (p65), NFκB1 (p50/p105), NFκB2 (p52/p100), RelB, c-Rel] each contain a Rel homology domain that allows them to dimerize and bind DNA. In most cells, NF-κB dimers are kept inactive in the cytoplasm through the interaction with the IκB complex. Nuclear translocation occurs after stimuli-induced proteosomal IκB degradation. In addition to the inducible form, constitutively active NF-κB has been identified in the nuclei of immune cells (e.g., in mature B lymphocytes, thymocytes, and adherent macrophages), in the eye photoreceptor cells, a subset of hippocampal and cortical neurons, and in many cancer cell types (Kaltschmidt et al. 1994; Korner et al. 1991; Krishnamoorthy et al. 1999; Pagliari et al. 2000; Sen and Baltimore 1986). RelA/p50 and RelA/c-Rel heterodimers have been shown to play an important role in neuronal development and survival (Bhakar et al. 2002; Kucharczak et al. 2003; Mattson et al. 2000). Because of the close developmental relationship between the brain and the ear, and also because NF-κB was found to have an important role in other sensory systems (i.e., photoreceptors of the eye), we considered the role of NF-κB in the cochlea.
Methods
Tissue culture
All animal procedures were carried out according to an approved animal research protocol (Kantonales Veterinaeramt Zurich, Switzerland). Five-day-old Sprague–Dawley (SD) rats were sacrificed, and cochlear microdissections were performed under a light microscope to isolate the organ of Corti, stria vascularis, and spiral ganglion. Following isolation, all explants were transferred to cell culture plates and maintained on 0.4 μm culture plate inserts (Millipore) in Dulbecco's Modified Eagle Medium, supplemented with 10% fetal calf serum, 25 mM HEPES, and 30 U/ml penicillin (all Invitrogen; Van de Water and Ruben 1971, 1974; Sobkowicz et al. 1993).
Inhibition of NF-κB activity
To inhibit NF-κB activity, an HPLC-purified synthetic inhibitor (NFκB inhibitor, AAVALLPAVLLALLAPVQRKRQKLMP, Santa Cruz Biotechnology Inc.) was added to the cell culture medium at the final concentration of 25 μg/ml. In the control experiments, an inactive control for the NF-κB inhibitor wasused (NFκB control, AAVALLPAVLLALLAPVQRDGQKLMP, Santa Cruz Biotechnology Inc.) at the same final concentration. The sesquiterpene lactone parthenolide (Santa Cruz Biotechnology Inc.), an NF-κB and MAP kinase inhibitor, was dissolved in DMSO and used at a final concentration of 50 mM.
Immunohistochemistry
Five-day-old SD rats were anesthetized and perfusions were performed as described previously (Nagy et al. 2005). Following deparaffinization, inner ear tissue slices were washed once in PBS and incubated in 0.5% Triton-X (Sigma) in 0.15% BSA/PBS and normal goat serum for 2 h. Incubation with the primary antibody directed against RelA (p65) was performed overnight at 4°C. In the negative controls, the primary antibody was replaced by PBS. Following overnight incubation, slices were washed three times in PBS, and detection was performed using a fluorescein-conjugated secondary antibody for 2 h at room temperature. For phalloidin staining, samples were incubated with F-actin binding compound rhodamine-conjugated phalloidin (Molecular Probes) for 2 h at room temperature. Samples were then washed three times in PBS for 5 min, and nuclei were visualized by DAPI staining for 1 min. Slices were viewed on a fluorescence microscope (Olympus IX71) and photographed using AxioCam (Zeiss) immediately following staining. NF-κB p65 antibody (C-20) and antirabbit–IgG FITC conjugate were purchased from Santa Cruz Biotechnology Inc. and used at 1:100 dilutions. Phalloidin (T7471) was diluted 1:100. A total of five animals (10 cochleae) were examined.
Protein isolation
For preparation of protein extracts, five explants were pooled. After the medium was aspirated, samples were washed in PBS, collected in hypotonic homogenization buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EGTA, 0.1 mM EDTA), and placed on a rotating wheel at 4°C for 30 min. Following incubation, 10% nonidet P-40 was added to each sample and tissue was lysed with Ultra-Turrax T8 (IKA-Werke) tissue homogenizer. Samples were centrifuged for 10 min at 13,000 rpm; the cytosolic fraction was collected and stored separately. Nuclear pellets were resuspended in 20 mM HEPES, pH 7.9, 25% glycerol, 0.25 M NaCl, 1 mM EDTA, and placed in a chilled Thermomixer (Eppendorf) for 20 min, after which centrifugation was performed for 30 min at 13,000 rpm to remove cellular debris. Total protein extraction was performed essentially as described above, but samples were placed in 50 mM Tris, pH 8.0, 170 mM NaCl, and 0.5% nonidet P-40 for lysis. All buffers were supplemented with 2.5 mM DTT, 5 μg/ml leupeptin, and 1.2 mM PMSF before use.
Immunoblotting
Antibodies against NF-κB p65 (C-20) and GADD45β (C-18) were purchased from Santa Cruz Biotechnology Inc. and used in dilutions of 1:1000. Protein concentrations were determined by Bio-Rad Protein Assay (Bio-Rad). Five micrograms of proteins was fractionated on 10% Bis–Tris Acrylamide gels (Invitrogen). Following electrophoresis, proteins were transferred to PVDF membranes using Mini-Cell Blot® (Invitrogen). Immunoreactive proteins were visualized with WesternBreeze® Chemiluminescence Kits (Invitrogen). In the negative control assays, the membranes were incubated with secondary antibodies after blocking. At least three immunoblots for each experiment were performed independently.
Caspase-3 activity assay
Protein lysates were prepared essentially as described above, but in 10 mM HEPES, pH 7.5, 2 mM EDTA, and 0.1% CHAPS, supplemented with 5 mM DTT, 1 mM PMSF, 10 μg/ml pepstatin A, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. Cell lysates were mixed 1:1 with caspase assay buffer (200 mM HEPES, pH7.5, 1 mM EDTA, 20% sucrose, 0.2% CHAPS, 0.2 mg/ml BSA, 20 mM DTT). Ac-DEVD-pNA (Alexis Biochemicals) was added to each reaction at the final concentration of 50 mM. Reactions were incubated for 3 h at 37°C. Reactions were stopped by placing tubes on ice. Caspase-3 activity was measured by spectrophotometer (Tecan) at wavelength 405 nm.
Statistical analysis
Results obtained in the caspase activity assays were analyzed by an unpaired t-test using GraphPad Prism 3.03 software (GraphPad Software Inc.). The unpaired t-test compares the means of two groups, assuming the data are sampled from Gaussian populations. Statistical significance is given in form of p value and confidence interval. A p value of >0.05 signifies that the differences observed in comparison of two samples are statistically significant, i.e., that it is unlikely that the differences observed are due to a coincidence of random sampling. Data obtained in three independent experiments were used for analysis.
Fluorescence confocal microscopy
For immunofluorescence studies, organ of Corti explants were fixed in 4% paraformaldehyde in PBS and permeabilized with 5% Triton X-100 in PBS with 10% fetal calf serum. The explants were incubated with a 1:300 dilution of Texas Red X-phalloidin (Molecular Probes) for 1 h at room temperature. Visualization was performed on a Leica inverted confocal laser scanning microscope with a red filter (excitation/emission wavelength 596/615 nm). Images were assembled by stacking 25 single digital scans (4 μm in thickness) using the Imaris® Image Processing Software (Bitplane AG).
Hair cell counts
For each control organ of Corti explant (i.e., nontreated and control for inhibitor-treated), OHCs associated with 30 IHCs were counted at one or two sites located on the basal and/or middle turns (Bodmer et al. 2002). From the control explants, it was determined that the length of 130 μm corresponds to 30 IHCs. For inhibitor-treated explants, one or two sites located on the basal and/or middle turns, 130 μm in length, were counted. The hair cells were characterized as missing if no stereocilia and cuticular plate were observed. At least eight sites were counted for each condition. No more than two sites were counted per explant.
Reverse transcriptase–PCR
RNA was prepared as described previously (Nagy et al. 2004). RT-PCR reactions were carried out using the One-step RT-PCR Kit (Qiagen). Total RNA (1.2 ng) was used in each reaction. Reactions were performed in the Eppendorf Mastercycler® under the following conditions: 40 min at 50°C, 10 min at 94°C, 35 cycles (for semiquantitative RT-PCR: 20 cycles) of PCR (1 min at 94°C, 1 min at 50°C, and 1 min at 72°C), 10 min at 94°C. The following primer sets were used: (p50) 5′-agcccccaacgcgtccaacct-3′ and 5′-tcaatcagcagggagaccct-3′; (p52) 5′-actgggcagacacgggtggtg-3′ and 5′-acattagcatggagcttggtgac-3; (RelA) 5′-cagcggggcatgcgtttccg-3′ and 5′-gatgcgctggctaatggcttg-3′; (cRel) 5′-actcacaaggtgtccttc-3′ and 5′-tgctgctgccatactggcagt-3′; (RelB) 5′-acaggcagatcgccattgtgttc-3′ and 5′-agcgttgggggcagagggatcgtag-3′; (IκBα) 5′-gagacctggccttcctcaac-3′ and 5′-caaaagtcaccaagtgctcca-3′; (xiap) 5′-ggtgataaagtgaagtgctttcactgt-3′ and 5′-cagcagttcttaccacagattc-3′; (gadd45β) 5′-cagcaggctacccagctacc-3′ and 5′-aggagggtggagagggctcg-3′; (gapdh) 5′-tcggagtcaacggatttggtcgta-3′ and 5′-atggactgtggtcatgagtccttc-3′, yielding 380-, 372-, 317-, 322-, 435-, 319-, 186-, 379-, and 520-bp size products, respectively. Primers were designed to cover rat exon sequences, except gapdh which was constructed based on human/mouse sequence; the GenBank accession numbers are as follows: XM_342346, BC085800, NM_199267, XM_223699, XM_345064, XM_343065, AB033366, L39010, and NM_017008, respectively. Negative control reactions were performed essentially as described above, but without the RT cycle preceding PCR amplification. Reactions were performed at least three times independently.
Results
Expression of NF-κB in the cochlea
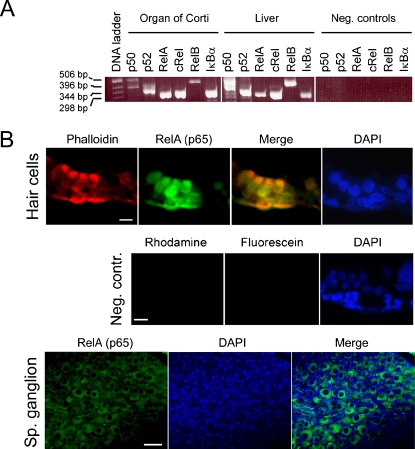
To investigate expression of NF-κB in the cochlea, we isolated RNA from the organs of Corti of p5 rats and performed RT-PCR analysis. Messenger RNA encoding for the five NF-κB subunits, as well as the inhibitor of NF-κB (IκB α-subunit), were detected (Fig. 1A). Total RNA isolated from the rat liver served as positive control; in the negative controls, the RT cycle was omitted.
Fig. 1.
NF-κB/Rel expression in the cochlea. (A) RT-PCR showing expression of NF-κB/Rel mRNA in p5 rat organs of Corti and liver (positive control). Negative control reactions were amplified without the RT cycle preceding PCR. (B) Tissue sections of rat p5 cochlea showing localization of RelA (p65) in the hair cells of the organ of Corti (upper panel) and spiral ganglion neurons (lower panel). Phalloidin-rhodamine was used to identify hair cells in the upper panel. Cell nuclei were stained with DAPI (in the lower panel, the condensed nuclei of Schwann cells are stained more intensely than the neuronal nuclei). In the negative controls (middle panel), samples were incubated in absence of RelA (p65) and phalloidin antibodies, but with antirabbit-IgG conjugated to fluorescein. Scale bars represent 15 μm.
Immunohistochemistry was performed to localize at least one of the NF-κB subunits to the various cell types found in the organ of Corti. Tissue sections of cochleae of p5 rats were fixed and stained with an antibody directed against RelA (p65), the most ubiquitously expressed NF-κB subunit, and a hair cell marker phalloidin. Nuclei were visualized with DAPI. Positive NF-κB staining could be observed in the nuclei of cochlear hair cells, as well as in Deiters's supporting cells (Fig. 1B, upper panel). In contrast to the nuclear staining detected in the organ of Corti, and consistent with a recent report on subcellular distribution of NF-κB in the spiral ganglion, RelA (p65) was found localized to the cytoplasm of spiral ganglion neurons (Fig. 1B, lower panel; Lang et al. 2005).
Nuclear localization of NF-κB in the organ of Corti, but not in the spiral ganglion and the stria vascularis
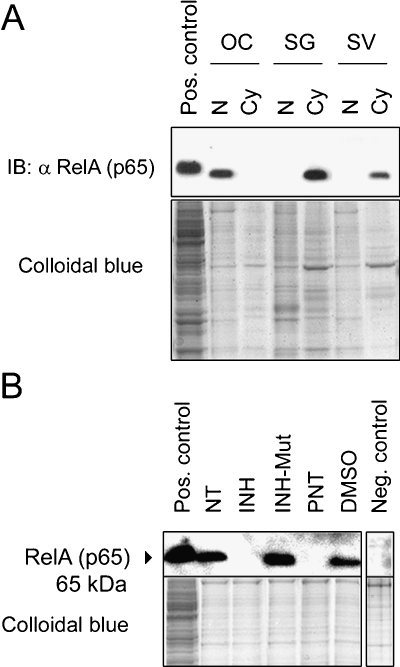
To corroborate the subcellular distribution of NF-κB in the cochlea, immunoblottings of nuclear and cytoplasmic protein lysates prepared separately from the organ of Corti, spiral ganglion, and stria vascularis were performed. Immunoblottings confirmed that RelA (p65) resides largely in the nuclear subfractions isolated from the organs of Corti, in contrast to its cytoplasmic localization in both the spiral ganglion and stria vascularis (Fig. 2A; staining of all protein was performed with colloidal blue and is shown as a loading control in the lower panel).
Fig. 2.
Subcellular distribution of NF-κB in the cochlea. (A) Immunoblotting (IB) showing nuclear vs. cytoplasmic distribution of RelA (p65) in the organ of Corti (OC), spiral ganglion (SG), and stria vascularis (SV) lysates; nuclear lysate (N), cytoplasmic lysate (Cy). (B) IB of nuclear lysates prepared from the organ of Corti after treatment with 25 μg/ml inhibitor of NF-κB (INH), 25 μg/ml control for the inhibitor of NF-κB (INH-Mut), 50 mM parthenolide (PNT), or 50 mM DMSO, all for 24 h. In the negative control, nontreated (NT) nuclear protein lysate was incubated with antirabbit-IgG secondary antibody immediately after blocking. All explants were kept in culture for an equal amount of time and collected for protein extraction at the same end time point. Positive controls show HEK293 whole cell lysate; total protein loading amount was visualized by staining with colloidal blue in the lower images.
Inhibition of NF-κB activity results in hair cell degeneration
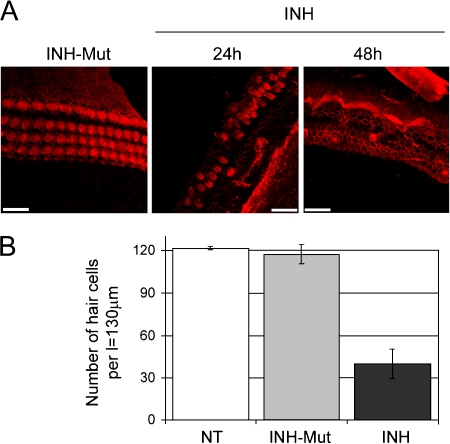
Nuclear localization of transcriptional activator RelA (p65) suggests that NF-κB may be in an activated state in the p5 organ of Corti. Thus, we were interested to test the effect of NF-κB inhibition. Inhibition of NF-κB activity can be achieved with cell-permeable synthetic peptides bearing the nuclear localization sequence (amino acids 360–369) of NFκB1. Such peptides were found to effectively block nuclear translocation of the NF-κB active complex (Lin et al. 1995). In addition, sesquiterpene lactones (e.g., parthenolide that targets IKKβ) are alkaloids capable of inhibiting NF-κB at different levels (Bork et al. 1997). Here we utilized a cell-permeable inhibitor of NF-κB (INH) and parthenolide (PNT) to block NF-κB activity in organ of Corti explants. To ensure that our observations were not a result of an effect that is not related to inhibition of NF-κB activity (e.g., cytotoxicity of the treatment itself), experiments with the inactive control for the inhibitor of NF-κB (INH-Mut) and DMSO were performed in parallel. INH-Mut is a compound analogous to INH which is unable to inhibit NF-κB dimers because of a mutation within the nuclear localization sequence of NFκB1 (changing the amino acid residues lysine–arginine to aspartic acid–glycine). DMSO treatment was performed as a control for PNT. Distribution of NF-κB after treatment was verified by immunoblotting (Fig. 2B). Immunofluorescence was performed to analyze the effect of the inhibitor on hair cell morphology. After culturing for 72 h, the organ of Corti explants treated with the inactive inhibitor (INH-Mut) stained with phalloidin–rhodamine, a high affinity probe for F-actin, showed normal morphological appearance consisting of a single row of IHCs and three rows of OHCs in the basal and middle turns of the organ of Corti (Fig. 3A). In contrast, explants treated with the inhibitor of NF-κB (INH) for the indicated time periods exhibited severe damage and loss of hair cells (Fig. 3A). The effect was quantitatively analyzed by counting the number of hair cells across 130-μm length in the basal and/or middle turns. The resulting data indicated approximately 60% of hair cell loss following 24-h treatment with the NF-κB inhibitor, when compared to nontreated and mutant inhibitor-treated controls (Fig. 3B).
Fig. 3.
NF-κB inhibitors damage hair cells. (A) Middle turns of rat p5 organs of Corti immunostained with phalloidin-rhodamine analyzed by laser scanning confocal microscopy. Representative stacked images obtained with the Multiple Image Processing tool of Imaris are shown. Explants were treated with 25 μg/ml inhibitor of NF-κB (INH) for 24 and 48 h or with 25 μg/ml control for the inhibitor of NF-κB (INH-Mut) for 24 h as indicated. Scale bars represent 20 μm. (B) Quantitative analysis of hair cell survival in organ of Corti basal and middle turns; nontreated (NT), treated with 25 μg/ml inhibitor of NF-κB (INH) or control for the inhibitor of NF-κB (INH-Mut) for 24 h. Values on the y-axis represent total number of hair cells (one row of IHCs + three rows of OHCs) counted per length (l) = 130 μm. Error bars represent standard deviation of the mean.
Caspase-3 activation in organs of Corti exposed to NF-κB inhibitors
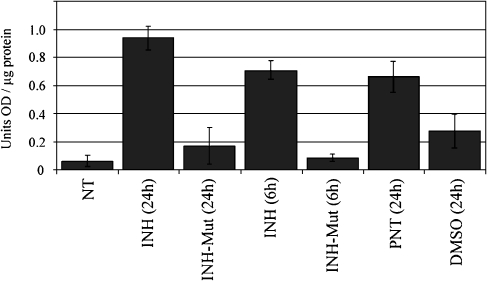
To evaluate whether hair cell death following NF-κB inhibition occurs by an apoptosis-directed pathway, we looked at caspase-3 activation in the organ of Corti. The activation of caspases (cystein–aspartic acid–proteases), arguably the most important transducers of apoptosis, is an early event in programmed cell death. Caspases initiate cellular breakdown by degrading specific structural, regulatory, and DNA-repair proteins. Activation of caspase-3 plays a key role in this process, and once it has been activated, the program for cell death is irreversibly activated. Caspase activity assays utilizing Ac-DEVD-pNA caspase-3 substrate (Alexis Biochemicals) indicated a 2- to 4.5-fold increase in caspase-3 activity in the organs of Corti following inhibition of NF-κB by various treatments (Fig. 4). Results were statistically analyzed using an unpaired t-test and indicate that the inhibitor-treated samples have significantly higher caspase-3 activity than their inactive control counterparts. Inactive controls do not differ significantly from the nontreated control, except for DMSO treatment which may by itself introduce changes to in vitro culturing conditions (Forman et al. 1999). The p values are given in Table 1.
Fig. 4.
Onset of apoptosis after NF-κB inhibition in the organ of Corti. Caspase-3 activation assay; untreated (NT), treated with 25 μg/ml of the inhibitor of NF-κB (INH), 25 μg/ml control for INH (INH-Mut), 50 mM parthenolide (PNT), or dimethyl sulfoxide (DMSO). Baseline buffer and Ac-DEVD-pNA activity (as measured in samples not containing protein lysates) were subtracted from each sample. Data bars represent mean values obtained in three independent experiments, and error bars represent standard deviation of the mean; optical density (OD).
Table 1.
Caspase assay unpaired t-test p values
| INH (24 h) | INH-Mut (24 h) | INH (6 h) | INH-Mut (6 h) | PNT (24 h) | DMSO (24 h) | |
|---|---|---|---|---|---|---|
| NT | <0.0001, Yes | 0.2441, No | 0.0001, Yes | 0.4508, No | 0.0010, Yes | 0.0404, Yes |
| INH (24 h) | 0.0010, Yes | |||||
| INH (6 h) | 0.0001, Yes | |||||
| PNT (24 h) | 0.0149, Yes |
The p values obtained in unpaired t-test are shown. Each sample was compared to the NT control (first line) and then with its negative control [e.g., INH (24 h) was compared with INH-Mut (24 h) and gave the p value of 0.0010]. A yes or no beneath the p value indicates whether means are significantly different (p < 0.05) between two compared samples.
Transcriptional down-regulation of Gadd45β following inhibition of NF-κB
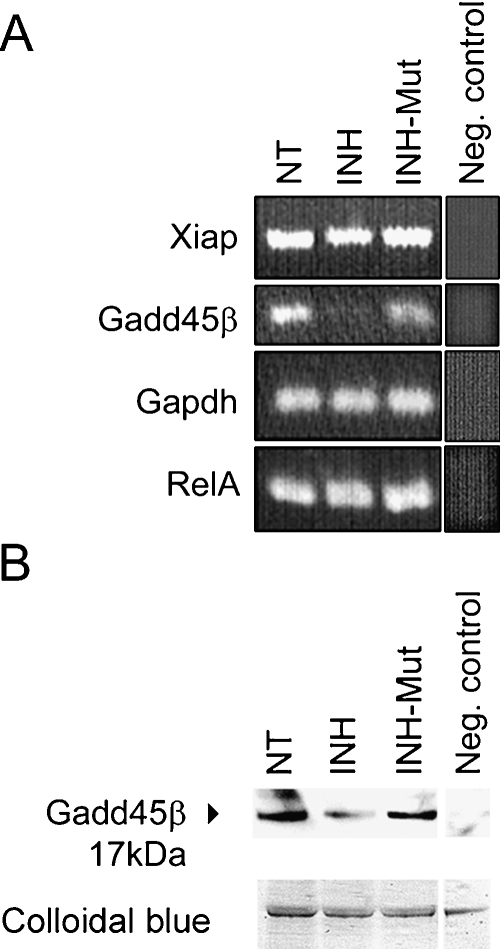
The exact mechanisms by which NF-κB contributes to cell survival are not completely understood. It is thought that NF-κB enhances transcriptional activity of genes that counter pro-apoptotic signals, although only a few of these genes have been identified so far. gadd45β and xiap have been proposed as antiapoptotic NF-κB transcriptional targets with a role in suppressing pro-apoptotic JNK signaling (De Smaele et al. 2001; Jin et al. 2002; Papa et al. 2004; Tang etal. 2001). We looked at expression levels of mRNAs encoding GADD45β and XIAP (i.e., RIAP3 in rat) in organs of Corti exposed to NF-κB inhibitors by semiquantitative RT-PCR. Although this method is not strictly quantitative, a decrease of gadd45β transcripts after treatment with the NF-κB inhibitor could be detected (Fig. 5A). In contrast, no significant change in xiap expression was observed in these experiments (Fig. 5A). RelA and gapdh mRNAs, which are ubiquitously expressed and therefore not expected to change, served as controls. The experiments were repeated three times with different RNA material and equal results.
Fig. 5.
Down-regulation of GADD45β mRNA and protein. (A) RT-PCR showing a decrease of gadd45β transcripts in the organ of Corti following inhibition of NF-κB. Shown are 24-h treatments with 25 μg/ml of indicated peptides. In the neg. control reactions, RNA isolated from nontreated samples was amplified without the RT cycle preceding PCR. (B) Representative immunoblot (IB) of total protein lysates, prepared from organs of Corti treated as in A, stained with goat anti-GADD45β antibody; lower panel shows colloidal blue staining of loaded proteins; in the neg. control, the membrane was stained with antigoat secondary antibody directly after blocking.
To determine whether the observed transcriptional down-regulation of gadd45β gene leads to decreased amounts of GADD45β protein in the organ of Corti, immunoblotting using organ of Corti total protein lysates was performed. The lysates collected from organs of Corti that have been treated with 25 μg/ml INH for 24 h revealed decreased levels of GADD45β protein, as compared to nontreated and INH-Mut-treated controls (Fig. 5B).
Discussion
We initiated this study to investigate the role of transcription factor NF-κB in the cochlea. NF-κB has been associated historically with inflammatory and immune responses, activating transcription of numerous genes involved in host defense. Very little is known about the role of NF-κB in the ear in general. Previously, NF-κB was found expressed in the murine cisplatin-treated cochlea, mostly in the stria vascularis and the spiral ligament, where it was proposed to activate transcription of inducible nitric-oxide synthase (iNOS) leading to increased production of NO and consequently to ear dysfunction (Watanabe et al. 2002). Another study reported increased NF-κB activity in spiral ganglion neurites of aged mice (Inafuku et al. 2000). NF-κB has also been tied to the inflammatory reactions of the middle ear epithelia during otitis media (Barrett et al. 2003). Here we find that NF-κB/Rel family is expressed in the cochlea, and that at least one subunit, transcriptional activator RelA (p65), is present also in the nuclei of the hair cells and supporting cells of p5 rat organ of Corti (Fig. 1). Nuclear localization of NF-κB in the organ of Corti was confirmed independently, in vivo by immunohistochemistry (Fig. 1B) and in vitro by immunoblotting of subcellular fractions (Fig. 2). For the in vitro biochemical studies, to minimize the possibility that changes in the localization and activity of RelA occur because of stress caused by the isolation procedure, explants were kept in culture for 72 h prior to further treatment.
Nuclear localization of RelA (p65) in hair cells and some supporting cells as well implies constitutive NF-κB activation but does not guarantee it per se (Fig. 1B). However, the morphology of hair cells as it was observed after treatment with the NF-κB inhibitor suggests that some form of NF-κB activity is required for hair cell survival. Disruption of orderly hair cell rows, degeneration, and loss of hair cells was observed within 24 h of treatment with NF-κB inhibitor (Fig. 3). To ensure our observations were not due to reasons other than specific inhibition of NF-κB activity, control experiments were performed with an inactive mutated form of INH (INH-Mut). To determine whether cell death occurs by an apoptotic pathway following inhibition of NF-κB, caspase-3 activity assays were performed. Two different time points were assessed for the specific NF-κB inhibitor (INH). Comparison with a less specific NF-κB inhibitor parthenolide (PNT), which is also known to inhibit MAPK signaling, is provided. Assays showed 2- to 4.5-fold increase in caspase-3 activity in the organ of Corti after treatment with inhibitors, as compared to their inactive controls (Fig. 4). The data obtained in these experiments strongly suggest that damage to hair cells occurs as a consequence of NF-κB inhibition and indicate that transcriptional activity of NF-κB is required for survival of neonatal cochlear hair cells in vitro even in resting conditions.
Because all our studies were performed with material isolated from p5 animals, we must leave open the possibility that expression patterns of NF-κB family may differ at distinct developmental stages. P5 animals were chosen for this study primarily because at this early neonatal stage, the otic capsule is not incorporated in dense bone, making isolation of material for biochemical studies plausible and relatively quick. It is worth noting that the rat cochlea is still immature in development 5 days after birth. While this article was in preparation, Jiang et al. (2005) reported on the protective role of the NF-κB pathway in kanamycin-induced hair cell death in adult mice. Another very recent study conducted in the gerbil cochlea proposed a neuroprotective role of NF-κB through enhancing the survival of type II spiral ganglion neurons exposed to ouabain (i.e., G-strophanthidin), a poisonous plant alkaloid that blocks the sodium–potassium ATPase (Lang et al. 2005).
In our experimental setting, inhibition of NF-κB activity resulted in rapid activation and execution of the apoptotic program, whereas hair cells seemed to suffer most. The findings are reminiscent of reports that NF-κB is constitutively activated in some neurons, and that inhibition of NF-κB transcriptional activity triggers neuronal apoptosis characterized by mitochondrial release of cytochrome c, caspase-9 and -3 activation, and poly(ADP-ribose) polymerase- 1 cleavage. These events were reportedly preceded by selective reduction in Bcl-xL, Bcl-2, and A1/Bfl-1 transcription, i.e., NF-κB-dependent antiapoptotic B-cell CLL/lymphoma-2 (Bcl-2) family members (Chiarugi 2002). In our study, we observed a decrease of gadd45β mRNA and protein following inhibition of NF-κB in the organ of Corti (Fig. 5). We were interested in this particular antiapoptotic NF-κB target gene because of reports of negative cross talk between the IKK/NF-κB and JNK pathways mediated by gadd45β (De Smaele et al. 2001; Papa et al. 2004; Tang et al. 2001). c-Jun N-terminal kinase signaling is heavily implicated as one of the key mediators of auditory hair cell death, and our results open up an interesting possibility that NF-κB signaling could function in part to protect hair cells from JNK-mediated apoptosis by ensuring expression of GADD45β. Further research into the role of GADD45β in hair cells is needed to substantiate this hypothesis, however. It is also possible that NF-κB is involved in regulation of the cellular antioxidant program in the cochlea, as it has been proposed for neuronal cells and other cell types that harbor NF-κB in a constitutive activated state (Schütze et al. 1992; Kaltschmidt et al. 1994; Lezoualch'l et al. 1998; Jang and Surh 2004).
In summary, the results presented here are among the first to address the function in the cochlea of one of the most intensely studied eukaryotic transcription factors. Because NF-κB can be found in presumably a constitutively active state in the organ of Corti of p5 animals, and inhibition of its activity results in massive hair cell degeneration, it seems likely that NF-κB may participate in normal hair cell function.
Acknowledgments
We thank Dr. Mathias Höchli (EMZ, University of Zurich) for assistance with confocal imaging and Verena Hoffmann for excellent technical assistance. This work was supported by the Swiss National Science Foundation grant no. 320000-107553.
Abbreviations
- α
antibody
- bp
base pair
- BSA
bovine serum albumin
- CHAPS
3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate
- DMSO
dimethyl sulfoxide
- DTT
dithiothreitol
- EDTA
ethylendiaminetetraacetic acid
- EGTA
ethylenglycol-bis(β-aminoethyl)-N,N,N′,N′-tetraacetic acid
- FITC
fluorescein isothiocyanate
- GADD45β
growth arrest and DNA damage-inducible 45β protein
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase protein
- HEPES
N-2-Hydroxyethylpiperazine- N′-2-ethanesulfonic acid
- IB
immunoblotting
- IHC
inner hair cell
- IκB
inhibitor of κB protein
- INH
Synthetic inhibitor of NF-κB protein
- INH-Mut
synthetic inactive control for the inhibitor of NF-κB protein
- JNK
c-Jun N-terminal kinase
- MIP
multiple image processing
- NF-κB
nuclear factor-κB
- NT
not treated
- OC
organ of Corti
- OHC
outer hair cell
- p5
postnatal day 5
- PBS
phosphate-buffered saline
- PMSF
phenyl–methyl-sulfonyl fluoride
- PNT
parthenolide
- Rel
reticuloendotheliosis viral oncogene homologue
- RT
reverse transcriptase
- SG
spiral ganglion
- SV
stria vascularis
- XIAP
X-linked inhibitor of apoptosis protein
References
- Barrett TQ, Kristiansen LH, Ovesen T. NF-kappaB in cultivated middle ear epithelium. Int. J. Pediatr. Otorhinolaryngol. 67: 895–903, 2003. [DOI] [PubMed]
- Bhakar AL, Tannis LL, Zeindler C, Russo MP, Jobin C, Park DS, MacPherson S, Barker PA. Constitutive nuclear factor-kappa B activity is required for central neuron survival. J. Neurosci. 22: 8466–8475, 2002. [DOI] [PMC free article] [PubMed]
- Bodmer D, Brors D, Pak K, Gloddek B, Ryan AF. Rescue of auditory hair cells from aminoglycoside toxicity by Clostridium difficile toxin B, an inhibitor of small GTPases Rho/Rac/Cdc42. Hear. Res. 172:81–86, 2002. [DOI] [PubMed]
- Bork PM, Schmitz ML, Kuhnt M, Escher C, Heinrich M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett. 40281:85–90, 1997. [DOI] [PubMed]
- Chiarugi A. Characterization of the molecular events following impairment of NF-kappaB-driven transcription in neurons. Brain Res. Mol. Brain Res. 109:179–188, 2002. [DOI] [PubMed]
- Clerici WJ, Hensley K, Dimartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear. Res. 98:116–124, 1996. [DOI] [PubMed]
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature 414:308–313, 2001. [DOI] [PubMed]
- Forman S, KÁš J, Fini F, Steinberg M, Ruml T. The effect of different solvents on the ATP/ADP content and growth properties of HeLa cells. J. Biochem. Mol. Toxicol. 13:11–15, 1999. [DOI] [PubMed]
- Huang T, Cheng AG, Stupak H, Liu W, Kim A, Staecker H, Lefebvre PP, Malgrange B, Kopke R, Moonen G, Van De Water TR. Oxidative stress-induced apoptosis of cochlear sensory cells: otoprotective strategies. Int. J. Dev. Neurosci. 18:259–270, 2000. [DOI] [PubMed]
- Inafuku S, Wu M, Kimura M, Nakayama M, Nakano T, Ishigami H. Immunohistochemical demonstration of inducible nitric oxide and nuclear factor-kappa B with reference to age-related changes in the mouse spiral and vestibular ganglion. Okajimas Folia Anat. Jpn. 77:125–131, 2000. [DOI] [PubMed]
- Jang J-H, Surh Y-J. Bcl-2 attenuation of oxidative cell death is associated with up-regulation of γ-glutamylcysteine ligase via constitutive NF-κB activation. J. Biol. Chem. 279:38779–38786, 2004. [DOI] [PubMed]
- Jiang H, Sha S-H, Schacht J. NF-κB pathway protects cochlear hair cells from aminoglycoside-induced ototoxicity. J. Neurosci. Res. 79:644–651, 2005. [DOI] [PubMed]
- Jin R, De Smaele E, Zazzeroni F, Nguyen DU, Papa S, Jones J, Cox C, Gelinas C, Franzoso G. Regulation of the gadd45beta promoter by NF-kappaB. DNA Cell Biol. 21:491–503, 2002. [DOI] [PubMed]
- Kaltschmidt C, Kaltschmidt B, Neumann H, Wekerle H, Baeuerle PA. Constitutive NF-kappa B activity in neurons. Mol. Cell. Biol. 14:3981–3992, 1994. [DOI] [PMC free article] [PubMed]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat. Rev., Cancer 2: 301–310, 2002. [DOI] [PubMed]
- Korner M, Tarantino N, Debre P. Constitutive activation of NF-κB in human thymocytes. Biochem. Biophys. Res. Commun. 181:80–86, 1991. [DOI] [PubMed]
- Krishnamoorthy RR, Crawford MJ, Chaturvedi MM, Jain SK, Aggarwal BB, Al-Ubaidi MR, Agarwal N. Photo-oxidative stress down-modulates the activity of nuclear factor-kappaB via in„„„volvement of caspase-1, leading to apoptosis of photoreceptor cells. J. Biol. Chem. 274:3734–3743, 1999. [DOI] [PubMed]
- Kucharczak J, Simmons MJ, Fan Y, Gelinas C. To be, or not to be: NF-kappaB is the answer—role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene 22:8961–8982, 2003. [DOI] [PubMed]
- Lang H, Schulte BA, Schmiedt RA. Ouabain induces apoptotic cell death in type I spiral ganglion neurons, but not type II neurons. J. Assoc. Res. Otolaryngol. 6:63–75, 2005. [DOI] [PMC free article] [PubMed]
- Lezoualch'l F, Sagara Y, Holsboer F, Behl C. High constitutive NF-κB activity mediates resistance to oxidative stress in neu„„„ronal cells. J. Neurosci. 18:3224–3232, 1998. [DOI] [PMC free article] [PubMed]
- Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 270: 14255–14258, 1995. [DOI] [PubMed]
- Mattson MP, Culmsee C, Yu Z, Camandola S. Roles of nuclear factor kappaB in neuronal survival and plasticity. J. Neurochem. 74:443–465, 2000. [DOI] [PubMed]
- Nagy I, Bodmer M, Brors D, Bodmer D. Early gene expression in the organ of Corti exposed to gentamicin. Hear. Res. 195: 1–8, 2004. [DOI] [PubMed]
- Nagy I, Bodmer M, Schmid S, Bodmer D. Promyelocytic leukemia zinc finger protein localizes to the cochlear outer hair cells and interacts with prestin, the outer hair cell motor protein. Hear. Res. 204:216–222, 2005. [DOI] [PubMed]
- Pagliari LJ, Perlman H, Liu H, Pope RM. Macrophages require constitutive NF-kappaB activation to maintain A1 expression and mitochondrial homeostasis. Mol. Cell. Biol. 20:8855– 8865, 2000. [DOI] [PMC free article] [PubMed]
- Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, De Smaele E, Tang WJ, D'Adamio L, Franzoso G. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat. Cell Biol. 6:146–153, 2004. [DOI] [PubMed]
- Pivola U, Xing-Qun L, Vikkala J, Saarma M, Murakata C, Camoratto AM, Walton KM, Ylikaski J. Rescue of hearing, auditory hair cells, and neurons by CEP-13471 KT7515, an inhibitor of ’’c-Jun N-terminal kinase activation. J. Neurosci. 20:43–50, 2000. [DOI] [PMC free article] [PubMed]
- Schütze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Krönke M. TNF activates NF-κB by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell 71:765–776, 1992. [DOI] [PubMed]
- Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 46:921–928, 1986. [DOI] [PubMed]
- Sha SH, Schacht J. Formation of reactive oxygen species fol„„„lowing bioactivation of gentamicin. Free Radic. Biol. Med. 26:341–347, 1999. [DOI] [PubMed]
- Sobkowicz HM, Loftus JM, Slapnick SM. Tissue culture of the or„„„„„gan of Corti. Acta Oto-laryngol., Suppl. 502:3–36, 1993. [PubMed]
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature 414:313–317, 2001. [DOI] [PubMed]
- Van De Water TR, Ruben RJ. Organ culture of the mammalian inner ear. Acta Oto-laryngol. 71:303–312, 1971. [DOI] [PubMed]
- Van De Water TR, Ruben RJ. Growth of the inner ear in organ culture. Ann. Otol. Rhinol. Laryngol. 83:1–16, 1974. [DOI] [PubMed]
- Watanabe K, Inai S, Jinnouchi K, Bada S, Hess A, Michel O, Yagi T. Nuclear-factor kappa B (NF-kappa B)-inducible nitric oxide synthase (iNOS/NOS II) pathway damages the stria vascularis in cisplatin-treated mice. Anticancer Res. 22:4081–4085, 2002. [PubMed]