Abstract
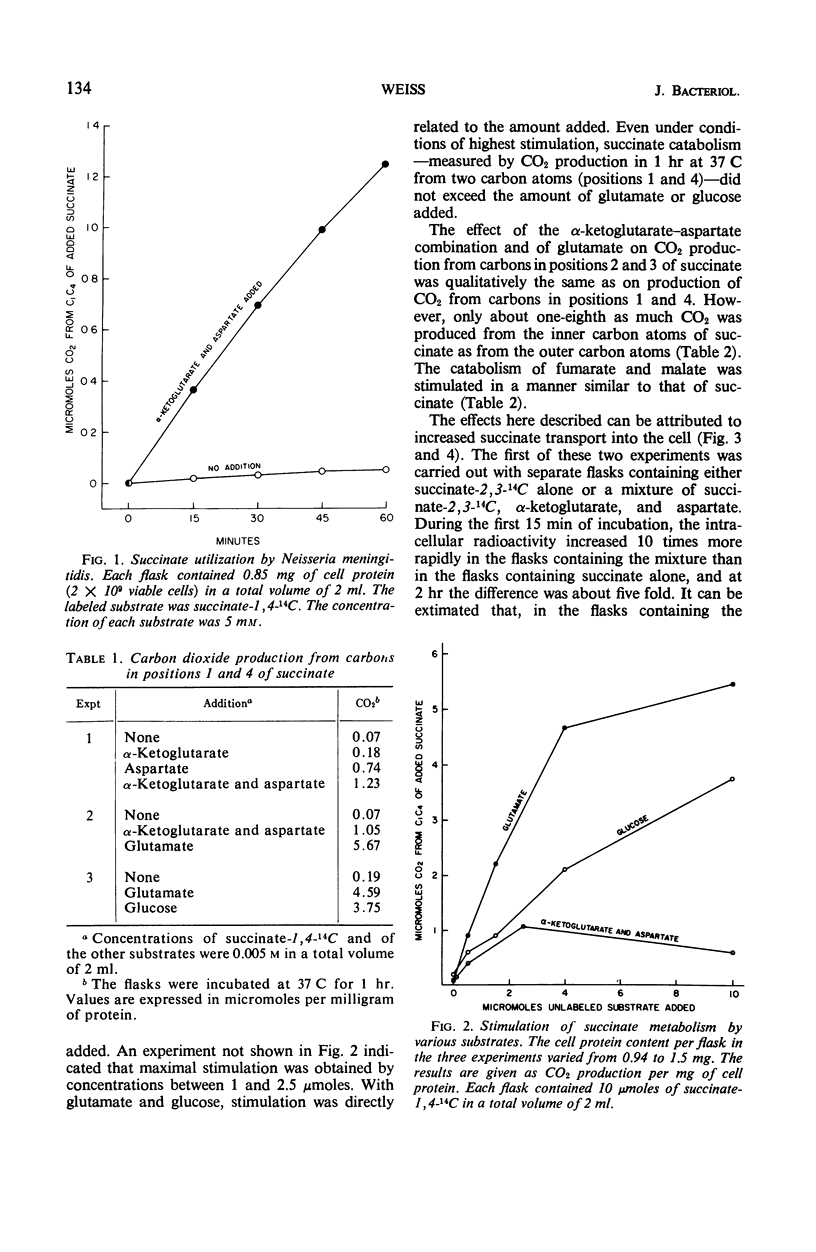
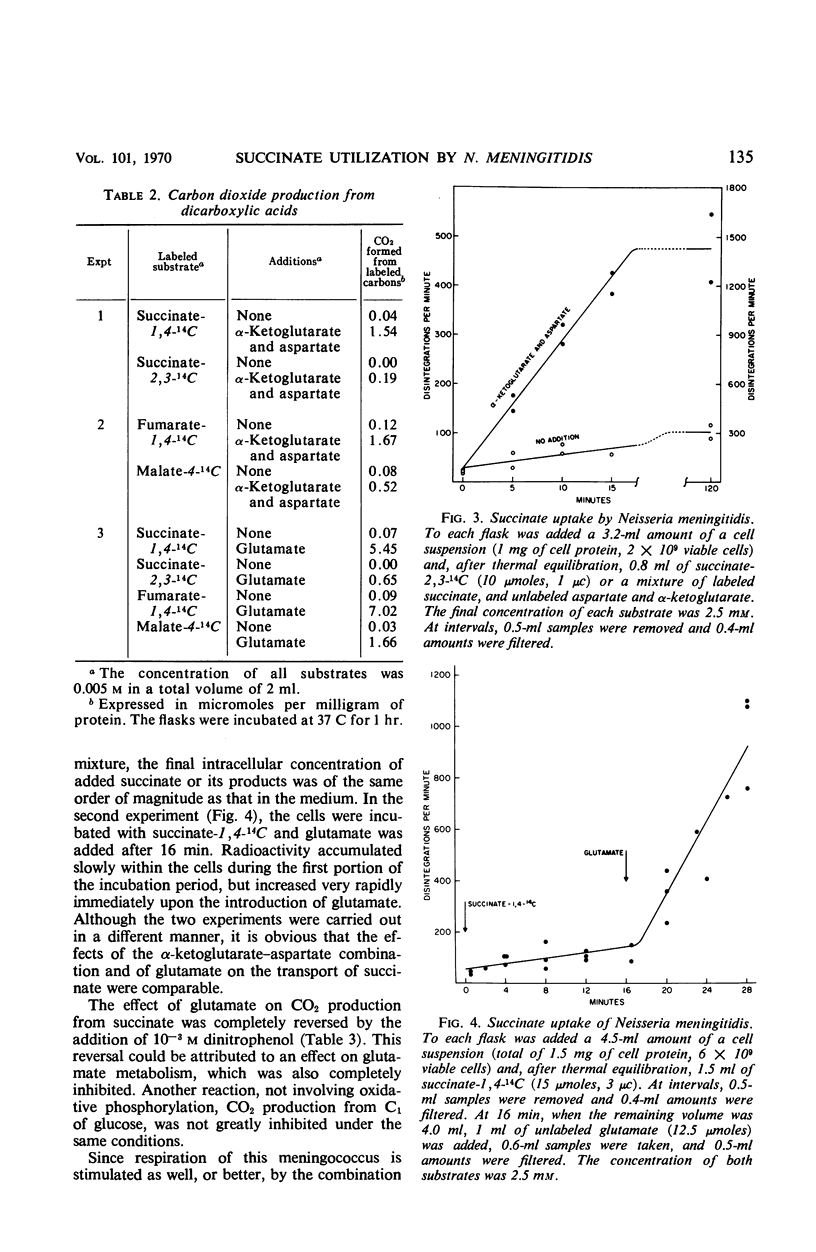
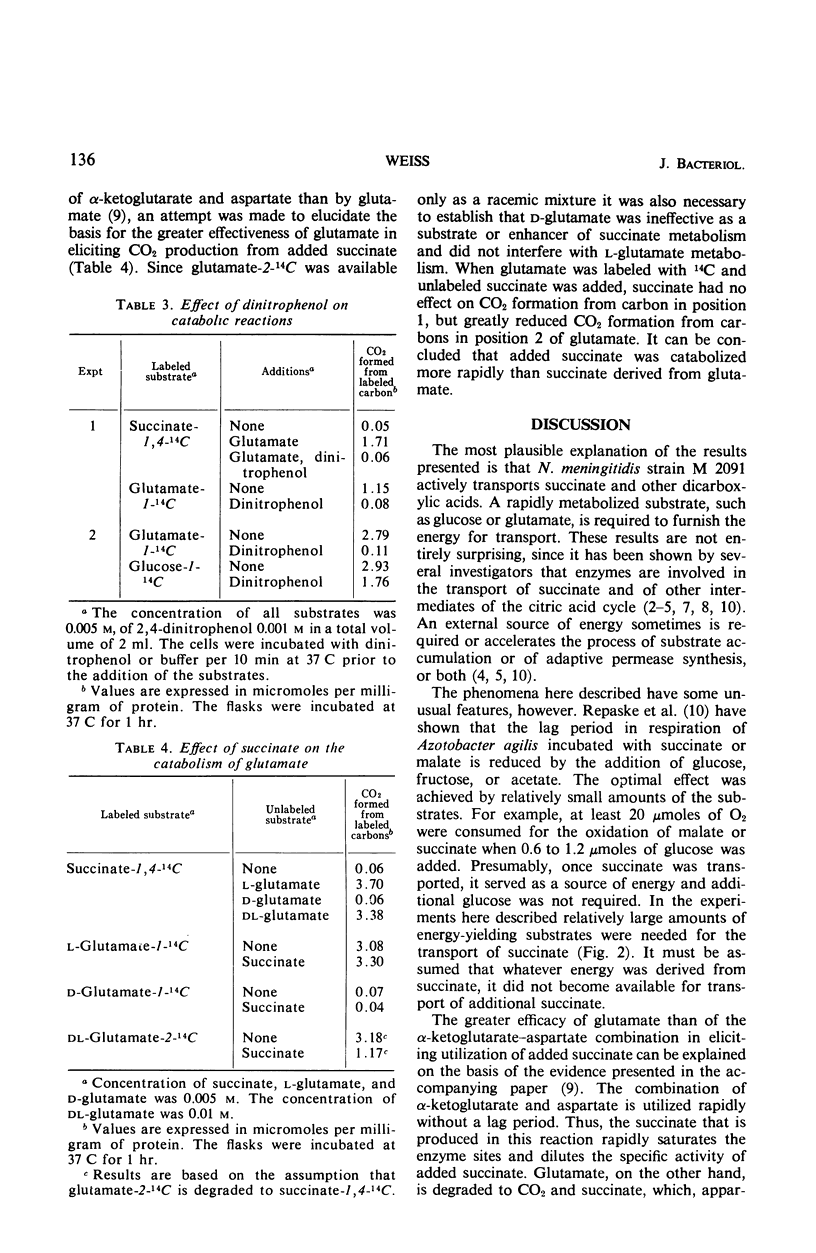
When resting cells of Neisseria meningitidis group B were incubated with either succinate, fumarate, or malate, respiration and CO2 production were not significantly stimulated. These dicarboxylic acids were readily utilized, however, when they were added in association with a combination of α-ketoglutarate and aspartate or with glucose or with glutamate. The amounts of these substrates required for exogenous succinate utilization were relatively large. Both the α-ketoglutarate–aspartate combination and glutamate greatly stimulated succinate uptake into the cells, but glutamate was far more effective than the α-ketoglutarate–aspartate combination in eliciting exogenous succinate utilization. This difference is explained on the basis of evidence reported in another article that succinate derived from the α-ketoglutarate-aspartate mixture is metabolized more rapidly—and thus more rapidly dilutes the specific activity of added succinate—than the succinate derived from glutamate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., SCHNEIDER L. The incorporation of glycine-1-C14 by typhus rickettsiae. J Biol Chem. 1960 Jun;235:1727–1731. [PubMed] [Google Scholar]
- CAMPBELL J. J. R., STOKES F. N. Tricarboxylic acid cycle in Pseudomonas aeruginosa. J Biol Chem. 1951 Jun;190(2):853–858. [PubMed] [Google Scholar]
- CLARKE P. H., MEADOW P. M. Evidence for the occurrence of Permeases for tricarboxylic acid cycle intermediates in Pseudomonas aeruginosa. J Gen Microbiol. 1959 Feb;20(1):144–155. doi: 10.1099/00221287-20-1-144. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN Y. S., EVEN-SHOSHAN A., ARTMAN M. EFFECT OF GLUCOSE ON THE UTILIZATION OF SUCCINATE AND THE ACTIVITY OF TRICARBOXYLIC ACID-CYCLE ENZYMES IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Nov 8;93:228–236. doi: 10.1016/0304-4165(64)90370-8. [DOI] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- KOGUT M., PODOSKI E. P. Oxidative pathways in a fluorescent Pseudomonas. Biochem J. 1953 Dec;55(5):800–811. doi: 10.1042/bj0550800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACQUILLAN A. M., HALVORSON H. O. Physiological changes occurring in yeast undergoing glucose repression. J Bacteriol. 1962 Jul;84:31–36. doi: 10.1128/jb.84.1.31-36.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallavia L. P., Weiss E. Catabolic activities of Neisseria meningitidis: utilization of glutamate. J Bacteriol. 1970 Jan;101(1):127–132. doi: 10.1128/jb.101.1.127-132.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REPASKE R., SHROAT J., ALLMAN D. Permeability of Azotobacter to succinate and malate. J Bacteriol. 1960 Mar;79:394–405. doi: 10.1128/jb.79.3.394-405.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]



